Abstract
Nuclear factor of activated T cell (NFAT1, NFATC2) is a transcription factor that binds and positively regulates interleukin-2 expression during T cell activation. NFAT1 has important roles in both innate and adaptive immune responses, but its involvement in cancer is not completely understood. We previously demonstrated that NFAT1 contributes to melanoma growth and metastasis by regulating the autotaxin gene (Enpp2). Here, we report a strong correlation between NFAT1 expression and metastatic potential in melanoma cell lines and tumor specimens. To elucidate the mechanisms underlying NFAT1 overexpression during melanoma progression, we conducted a microarray on a highly metastatic melanoma cell line in which NFAT1 expression was stably silenced. We identified and validated two downstream targets of NFAT1, IL-8 and MMP-3. Accordingly, NFAT1 depletion in metastatic melanoma cell lines was associated with reduced IL-8 and MMP-3 expression, whereas NFAT1 overexpression in a weakly metastatic cell line induced expression of these targets. Restoration of NFAT1 expression recovered IL-8 and MMP-3 expression levels back to baseline, indicating that both are direct targets of NFAT1. Moreover, in vivo studies demonstrated that NFAT1 and MMP-3 promoted melanoma tumor growth and lung metastasis. Collectively, our findings assign a new role for NFAT1 in melanoma progression, underscoring the multifaceted functions that immunomodulatory factors may acquire in an unpredictable tumor microenvironment.
Keywords: Melanoma, Transcription Regulation, Invasion, Metastasis, NFAT1
Introduction
Melanoma is the deadliest and most aggressive form of skin cancer, with an annual death rate higher than for any other skin cancer (1). In the United States, melanoma is the fifth most common form of cancer in men and the seventh in women (2), with 76,100 expected new cases (43,890 in men and 32,210 in women) in 2015 and 9,710 expected deaths (2). Malignant melanoma presents a major clinical challenge albeit recent immune checkpoints treatments.
Nuclear factor of activated T cell (NFAT) family proteins were first identified in T cells as activators of the transcription of interleukin 2 (3,4), and these proteins serve as key regulators of T cell immune responses. All family members have a highly conserved REL-homology domain (RHD), which is a DNA-binding domain. They are also involved in the control of T cell development and T cell differentiation (5). Many years after the discovery of the NFAT gene family, they were found to play multiple roles in other biological systems besides the immune response. Despite their name, proteins from the NFAT family are expressed on cells other than T cells, including epithelial cells, endothelial cells, and other immune cells including dendritic cells and B cells (6,7). Calcium flux, calcineurin, and NFAT kinases are the regulators of NFAT proteins. In recent years, accumulating evidence has suggested that the NFAT family members are also involved in cancer development and metastasis by increasing cell growth, enhancing proliferation, and stimulating angiogenesis (8). We have previously found that one family member, NFAT1, contributes to melanoma progression (9).
NFAT1, also known as NFATc2, was the first member of the NFAT family discovered in T cells. NFAT1 is highly phosphorylated and can be activated through dephosphorylation by calcineurin phosphatase (5). As is the case for other family members, activation of NFAT1 can be blocked using calcineurin inhibitors such as tacrolimus (FK506) or cyclosporine A (10). The dephosphorylation of NFAT1 helps the protein to be relocated into the nucleus and to be active as a transcription factor.
NFAT1 is associated with a wide range of tumor progression events such as invasion, migration, tumor cell survival, and apoptosis. However, in melanoma the majority of the downstream genes are still not identified and the effect of NFAT1 on the melanoma metastatic phenotype still needs to be elucidated. Our laboratory has previously demonstrated that Gal-3 contributes to melanoma growth and metastasis via the regulation of autotaxin and NFAT1 (9).
In the current study, we identified IL-8 and MMP-3 as downstream target genes for NFAT1, thus reavealing new mechanisms by which overexpression of NFAT1 contributes to the malignant melanoma phenotype.
Materials and Methods
Cell lines and cell culture
Human melanoma A375SM cells were derived from nude mice that had been intravenously injected with A375P (parental) cells, from pooled lung metastases collected and grown in culture as described previously (11). The human SB2 melanoma cell line was isolated from a primary cutaneous lesion and is low metastatic and poorly tumorigenic in mice (12). The WM902B human cell line was isolated from a malignant melanoma skin tumor in the vertical growth phase (VGP) (13). DM4 which was derived from lymph node metastasis, is a low tumorigenic melanoma cell line (14). The human embryonic kidney cells (293FT) were used for lentiviral shRNA and overexpression vectors and maintained in DMEM supplemented with 10% FBS. Cell culture conditions and preparation for injections were as previously described (9). All cell lines used in our studies were tested before their usage for authentification by DNA fingerprinting using short tandem repeat (STR) method.
Lentiviral shRNA
NFAT1 targeting shRNA 5′- CTGATGAGCGGATCCTTAA-3′ or MMP-3 targeting shRNA 5′-TCTGAACAAGGTTCATGCT-3′ and non-targeting (NT) shRNA 5′-TTCTCCGAACGTGTCACGT-3′ were designed with a hairpin and inserted into a pSIH-HI-copGFP lentiviral vector. Transfections were performed as previously described (9).
Fluorescence activated cell sorting
A375SM, WM902B, and SB2 cells that were transduced with lentiviral constructs were detached from the flask using 0.05% trypsin EDTA. Cells were centrifuged at 200g, and the supernatant was removed. Cells were than resuspended in 500 μl of PBS and were subjected to FACS.
Protein extraction
Total protein extracts were acquired from 70%-80% confluent cell cultures on a six-well plate or 10-cm dish as previously described (14). Protein concentration was measured using the Bradford assay (Bio-Rad).
Western blot analysis
For detection of NFAT1, 20 μg of whole-cell protein lysate was loaded onto 8% SDS-PAGE and transferred onto 0.45-μm polyvinylidene difluoride (PVDF) membranes (Millipore). To detect expression of MMP-3 protein (a secreted protein), 1 × 106 cells were plated onto a 10-cm dish and then incubated under serum-starvation conditions with 5 ml of serum-free MEM for 48 hours. The supernatant from cell cultures was concentrated to 100 μl. A total of 10 μg of protein from the supernatant was loaded onto 10% SDS-PAGE as previously described (9).
Matrigel invasion assay
Matrigel invasion assays were performed using BioCoat Matrigel invasion chambers (BD Biosciences) as previously described (9).
In vitro proliferation assay
One thousand cells were plated onto 96-well plates (12 replicas for each condition) in MEM medium supplemented with 10% FBS. Every 24 hours, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, a colorimetric assay based on the conversion of MTT to formazan in viable cells, was performed to determine the proliferation rate of the cells (viability) as previously described (9).
Reverse transcription-PCR and Real time PCR
RNA isolation was performed with the RNAqueous kit (Ambion). One microgram of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed with the TaqMan Gene Expression Assay as described previously (15).
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express Kit (Active Motif) according to the manufacturer's protocol. PCR was performed by using the following primers that can identify both NFAT1 binding sites: NFAT1-F-5′-GCTCAAACTGCCAGCAAAAT-3′ and NFAT1-5′CACAGGGTGTTCACAAATCG-3′. The PCR product was run on a 1.5% agarose gel.
Reporter constructs and luciferase activity analysis
The IL-8 and MMP-3 promoters were cloned from A375SM melanoma cells to encompass 851 (IL-8) or 2682 (MMP-3) base pairs upstream of the transcriptional initiation sites. Direct site mutagenesis of NFAT1 binding sites was performed using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's instructions. After 48 hours, the cells were lysed and luciferase activity was assayed with the Dual Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Immunohistochemistry
Paraffin-embedded tumor specimens were used for IHC staining to determine the expression of IL-8 (Biosource International) (1:100), NFAT1 (SC-7296, Santa Cruz Biotechnology) (1:400), MMP-3, (Abcam) (1:100) and CD31 (1:200) antibody (PharMingen) were used.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was carried out using the DeadEnd Fluoremetric TUNEL System (Promega) with paraffin sections according to manufacturer's instructions.
Animals
Eight- to 10-week-old female athymic BALB/c nude mice (purchased from Taconic Biosciences) were maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and the NIH. All studies were approved and supervised by the Institutional Animal Care and Use Committee (IACUC) of The University of Texas MD Anderson Cancer Center.
In vivo subcutaneous tumor growth
Subcutaneous tumors were produced by injecting 0.5-1 × 106 tumor cells/100 μl PBS into the right flank of each mouse (n = 6-8). Tumor size was monitored twice weekly for 28-40 days. Mice were then sacrificed and tumors were collected. The tumors were processed for immunohistochemistry to detect alterations of IL-8, MMP-3, and CD31. TUNEL assays also were performed to determine the effects on vessel density and apoptosis of tumors.
Experimental lung metastasis assays
For lung metastasis experiments, mice were injected with 0.5-1 × 106 tumor cells in 100 μl of PBS via lateral tail vein injections as previously described (14).
cDNA microarray
Total RNA was isolated from A375SM NT and NFAT1 shRNA melanoma cells using the mirVana Isolation Kit (Life Technologies). Microarray analysis was carried out by Phalanx Biotech Group using the Human OneArray microarray (version HOA 6.1, GEO Platform GPL19137), which contains 31,741 60-mer probes. The hit criteria for genes selected was larger than 2 fold decrease. Data from microarray were deposited to NCBI (accession number: GSE76541).
Statistical analysis
Student's t test was used to analyze the statistical significance of the in vitro data. In the animal studies, the Mann-Whitney U test was used to analyze the tumor growth and lung metastasis results. Values for tumor growth are given as mean volumes ± SEM. P values < 0.05 were considered statistically significant.
TCGA analysis
Statistical analyses were performed utilizing R, version 3.0.1 (http:///www.r-project.org/), with statistical significance defined as P < 0.05. We downloaded and analyzed clinical and mRNA (Level 3 Illumina RNASeqv2) data publicly available from the Cancer Genome Atlas Project (TCGA; http://tcga-data.nci.nih.gov/) for patients with skin cutaneous melanoma (SKCM).
For NFAT1 tumor vs. metastatic comparisons, the Shapiro-Wilk test was used to determine that NFAT1 (log2 reads) did not follow a normal distribution in tumor or metastatic samples. The Mann-Whitney-Wilcoxon nonparametric test was used to compare NFAT1 expression levels between the two groups, and a box-and-whisker plot (box plot representing the first [lower bound] and third [upper bound] quartiles, whiskers representing 1.5 times the interquartile range) was used to visualize the data.
For Kaplan-Meier survival analysis for IL-8 and MMP-3, patients were grouped into percentiles according to mRNA expression. The log-rank test was used to determine the association between mRNA expression and overall survival, and the Kaplan-Meier method was used to generate survival curves. Cut-off points (log-rank test P < 0.05) to significantly split the samples into low and high mRNA groups were recorded. A cut-off to optimally separate the patients into high/low groups (minimum P-value) was chosen.
Results
Analysis of NFAT1 expression in melanoma
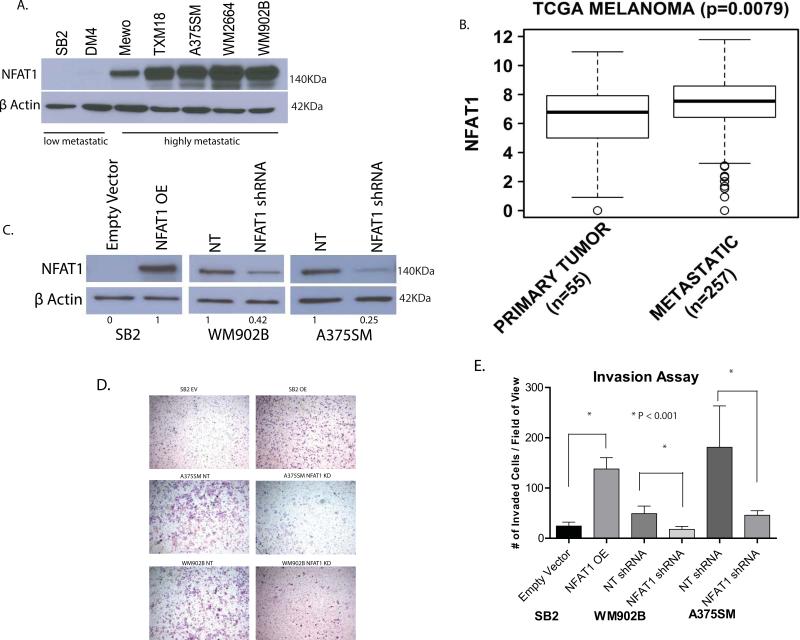
To examine the status of NFAT1 in melanoma progression, we analyzed a panel of melanoma cell lines ranging from low to highly metastatic. Western blot analysis showed that the more metastatic cell lines MeWo, TXM18, A375SM, WM2664, and WM902B expressed higher levels of NFAT1, whereas the low-metastatic cell lines SB2 and DM4 expressed significantly lower levels of NFAT1 (Fig. 1A). To validate this observation in patient specimens, we mined the TCGA (The Cancer Genome Atlas) data for NFAT1 expression. TCGA analysis showed a significant higher expression of NFAT1 in metastatic lesions than in primary melanoma lesions (P = 0.0079) (Fig. 1B). We conclude that NFAT1 is overexpressed in metastatic melanoma cell lines and patient specimens.
Figure 1.
The status of NFAT1 expression in melanoma progression. (A) The low metastatic SB2 and DM4 melanoma cell lines expressed significantly less NFAT1 than did the more tumorigenic and metastatic MeWo, TXM-18, A375SM, WM2664, and WM902B cell lines. (B) TCGA data for the expression of NFAT1 in melanoma patients. A significant overexpression of NFAT1 in metastatic lesions (n = 257) compared with primary melanoma lesions (n = 55) (P = 0.0079). (C) Overexpression of NFAT1 in SB2 cells and silencing of NFAT1 in WM902B cells (60%) and in A375SM cells (75%). (D) Overexpression of NFAT1 in SB2 cells resulted in increased invasion through Matrigel-coated filters, while silencing of NFAT1 in both A375SM and WM902B cell lines resulted in reduced invasion. (E) Quantitation summary of invasion assay presented in Figure 2D (NT, non-targeting; OE, overexpression; EV, empty vector). A significant increase in SB2 cells and decreases in A375SM and WM902B cells were observed (P < 0.001).
Silencing and overexpression of NFAT1 in melanoma cells
To establish the role of NFAT1 in melanoma growth and metastasis, we chose to stably silenced NFAT1 in two metastatic melanoma cell lines with high levels of NFAT1 expression (WM902B, A375SM) and to overexpress NFAT1 in the low-metastatic SB2 cell line (with low levels of NFAT1). WM902B and A375SM, were stably transduced with non-targetable (NT) or NFAT1 shRNA packaged lentivirus. Western blots and densitometry analyses revealed that the WM902B and A375SM cell lines had 58% and 75% knock down of NFAT1, respectively, compared with the NT shRNA control (Fig. 1C). In SB2 cells, NFAT1 was overexpressed several-fold compared with the empty vector–transduced SB2 cells showing lack of NFAT1 expression. These three cell lines were used throughout the study.
In vitro invasive phenotype of melanoma cells following NFAT1 silencing/overexpression
We next analyzed the ability of these cells to invade through Matrigel-coated filters. A significant reduction in the number of invading melanoma cells was observed after silencing NFAT1 in both WM902B and A375SM cell lines (P < 0.001) (Fig. 1 D, E). We observed a greater than 2-fold reduction in WM902B cells and more than 3-fold reduction in A375SM. A significant increase (more than 5-fold) in the number of invading cells was observed after overexpression of NFAT1 in SB2 cells. These data show that NFAT1 is critical for the invasive phenotype of melanoma cells. We found no changes in the viability (doubling time) of A375SM and WM902B after NFAT1 silencing compared with the NT. Similar results were observed in SB2 cells after overexpression of NFAT1 (Supplementary Fig. 1). We conclude that the changes in the invasion assay were not due to differences in cell viability but rather to NFAT1 genetic manipulations that influenced the invasive phenotype.
In vivo effect of NFAT1 on tumor growth and metastasis
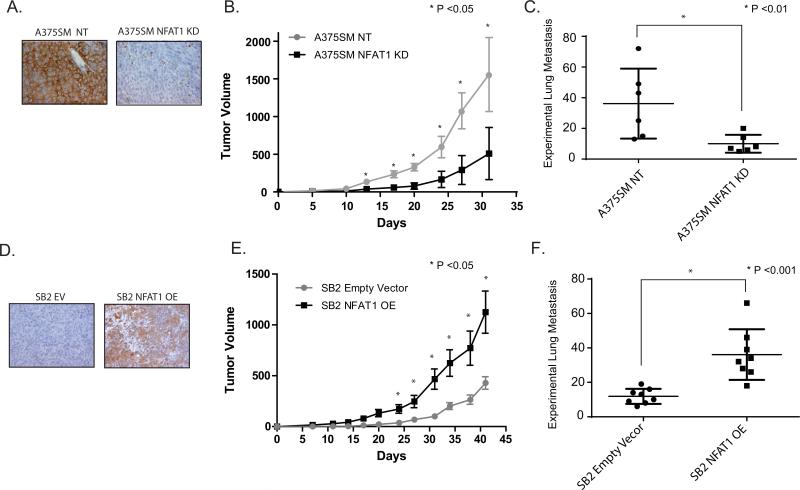
We next analyzed the role of NFAT1 on melanoma growth and metastasis in vivo. To that end, we used highly metastatic A375SM cells transduced with NFAT1 shRNA compared with NFAT1 NT shRNA transduced cells and the low-metastatic SB2 after NFAT1 overexpression compared with SB2 EV (empty vector) (Fig. 2A and D). Cells were injected subcutaneously into the right flank of nude mice (six mice per group) for tumor growth experiments or intravenously into the tail vein of the mice (six mice per group) for experimental lung metastases. At the end of the experiment the tumor volumes were 1557 mm3 for the A375SM NT group and 509 mm3 for the A375SM cells transduced with NFAT1 shRNA (Fig. 2B). At day 41, SB2 EV cells had a mean tumor volume of 428 mm3 compared with 1125 mm3 for SB2 NFAT1 OE cells (Fig. 2E). We observed a significant decrease in tumor growth after silencing NFAT1 and a significant increase in tumor growth after overexpressing NFAT1 in melanoma cells. Because we had already demonstrated that there were no changes in the doubling time of these cell lines, we conclude that the differences observed in tumor growth rates were due to NFAT1 expression.
Figure 2.
Role of NFAT1 in tumor growth and metastasis in vivo. Silencing NFAT1 in A375SM melanoma cells reduced tumor growth and experimental lung metastases in nude mice, while overexpression of NFAT1 in SB2 increased both tumor growth and metastasis. (A) IHC staining for NFAT1 from in vivo tumors, demonstrating NFAT1 silencing in the A375SM NFAT1 KD injected mice. (B) Tumor growth was significantly reduced after silencing NFAT1 in A375SM cells; (*P < 0.05). (C) Experimental lung metastasis: silencing NFAT1 significantly reduced the number of experimental lung metastases; (*P < 0.01). (D) IHC staining for NFAT1 from in vivo tumors, demonstrating NFAT1 overexpression in SB2 OE injected mice. (E) Tumor growth was significantly increased after overexpressing NFAT1 in SB2 cells; (*P < 0.05). (F) Experimental lung metastasis: overexpressing NFAT1 significantly increased the number of experimental lung metastasis in SB2 cells; (*P < 0.001).
For the experimental lung metastases, the same cell lines were injected intravenously. The number of lung metastases was significantly decreased after silencing of NFAT1 (mean of 10 metastases) in the highly metastatic A375SM cells compared with the A375SM NT group (mean of 36, P < 0.01) (Fig. 2 C). Conversely, the number of lung metastases was significantly increased in the SB2 NFAT1 OE group (mean of 36 metastases) compared with SB2 NFAT1 EV (mean of 11 metastases, P < 0.001 (Fig. 2F).
Taken together, with the limitations of the experimental lung metastasis assay used, our findings indicate that NFAT1 silencing in A375SM cells reduced their tumorigenicity and metastatic capabilities whereas overexpression of NFAT1 in SB2 cells increased these features, thus supporting a role for NFAT1 in melanoma growth and metastasis.
Identification of IL-8 and MMP-3 as downstream target genes
To identify potential downstream targets of NFAT1, an Illumina microarray was performed. We focused our attention on genes that were downregulated after silencing of NFAT1, because they were likely to be tumor-promoter genes. The top identified genes were then further selected for their relevance to cancer and are depicted in Supplementary Table 1. Among the genes downregulated after NFAT1 silencing were follistatin (FST), which has been reported as a contributor to bone metastasis (16), and placenta-specific 8 (PLAC8), whose overexpression has been reported to protect cancer cells from apoptosis (17-19). Downregulation was also observed in the frizzeld family receptor 4 (FZD4) and the nicotinamide N-methyltransferase (NNMT) genes. We have already demonstrated that autotaxin is regulated by NFAT1 (9). Note that our gene expression array confirmed that after NFAT1 silencing, autotaxin was reduced by almost 3-fold as we previously reported (9). Of the potential genes shown in Supplementary Table 1, we decided to focus our research on interleukin 8 (IL-8/CXCL8) and matrix metallopeptidase 3 (MMP-3, also known as stromalysin-1), which were downregulated by 2.4-fold and 3.22-fold, respectively.
IL-8 plays important roles in the progression and metastasis of several different cancers, including melanoma (20,21). Our laboratory previously demonstrated that overexpression of IL-8 is associated with increasing tumor stage, disease progression, and recurrence in human melanoma. Furthermore, we have shown a direct correlation between high levels of IL-8 and tumor angiogenesis and metastasis in melanoma nude mouse xenograft models (20,22,23). Therefore, we hypothesized that NFAT1 may contribute to melanoma tumor growth and metastasis through the regulation of IL-8. The second gene that we decided to study as a downstream target of NFAT1 is MMP-3. MMP-3 contributes to several pathologies such as asthma, rheumatoid arthritis, and cancer (24). In melanoma, the role of MMP-3 is yet to be elucidated, and hence the focus of the current studies.
IL-8 and MMP-3 expressions are reduced in melanoma cells after silencing NFAT1 and increased after overexpressing NFAT1
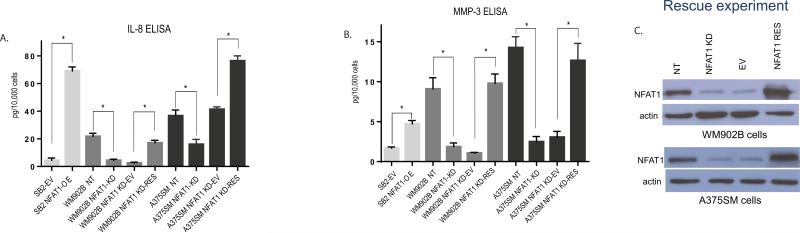
To validate our gene expression microarray, qRT-PCR and ELISA assays were performed. As shown in Fig. 3A, silencing NFAT1 significantly reduced the amount of the secreted IL-8 within the supernatant by approximately 4-fold in WM902B cells and approximately 2.5-fold in A375SM cells. Also, IL-8 secreted protein levels were significantly increased by more than 10-fold in SB2 cells after NFAT1 overexpression compared with the EV control (Fig. 3A). These results were also supported at the mRNA levels as demonstrated by qPCR (Supplementary Fig. 2). Considering all these results, we conclude that IL-8 is regulated by NFAT1 at both the mRNA and protein levels.
Figure 3.
Effect of NFAT1 on IL-8 and MMP-3 expression and secretion. (A) ELISA assay for the secreted IL-8 protein demonstrating that NFAT1 silencing reduced the secretion of IL-8 in both A375SM and WM902B melanoma cell lines. NFAT1 rescue reverted the secretion of IL-8. NFAT1 overexpression in SB2 cells resulted in upregulation of IL-8 secretion. (B) Rescue of NFAT1 reverted the protein levels of secreted MMP-3. NFAT1 silencing in A365SM and WM902B cells reduced the secretion of MMP-3. Rescuing NFAT1 reverted MMP-3 secretion in both A375SM and WM902B melanoma cell lines. Overexpression of NFAT1 in SB2 cells caused upregulation of MMP-3 secretion. (C) Rescue of NFAT1 in A375SM and WM902B KD cells. In both cell lines, more than a 5-fold rescue of NFAT1 was observed compared with β-actin expression (NFAT1 140 kDa, β-actin 42 kDa). (* P<0.05)
To ascertain that IL-8 regulation by NFAT1 is not due to an off-target effect of the shRNA, we rescued the expression of NFAT1 in both of the NFAT1-KD (shRNA) cell lines (Fig. 3C). Rescue of NFAT1 in these cells reverted the expression and secretion of IL-8 (Fig. 3A), thus confirming IL8 regulation by NFAT1. The same experiments were performed for MMP-3. The array data showed more than a 3-fold reduction in MMP-3 expression after NFAT1 silencing (Supplementary Table 1). Because MMP-3 is a secreted protein, we performed an ELISA after silencing NFAT1 in A375SM and WM903B as well as after overexpressing NFAT1 in SB2 cells (Fig. 3B). ELISA results demonstrated a significant reduction of MMP-3 secretion after NFAT1 silencing in both A375SM and WM902B cells. After NFAT1 rescue, there was a complete rescue of the MMP-3, where the protein secretion levels returned to NT levels in both A375SM and WM902B cell lines. In SB2 cells, a significant increase in MMP-3 secretion levels after NFAT1 overexpression was observed (Fig. 3B). Next, we performed qRT-PCR assays, which demonstrated similar results at the mRNA level (Supplementary Fig. 2). Immunohistochemical staining, confirmed that IL-8 and MMP-3 expressions were decreased in vivo in A375SM cells after NFAT1 silencing and increased after overexpressing NFAT1 in SB2 cells (Supplementary Fig. 3). We have previously demonstrated that IL-8 acts as an angiogenic factor in melanoma. To verify the angiogenic role of IL-8, immunohistochemical staining was performed on both A375SM and SB2 xenograft tumors with anti-CD31, a widely used endothelial cell marker that detects blood vessels (angiogenesis). A375SM NFAT1 NT shRNA melanoma cells presented a high number of blood vessels compared with NFAT1 shRNA. The overexpression of NFAT1 in SB2 cells showed a phenotype similar to that of the highly metastatic line A375SM, and it was clearly noticeable that the number of blood vessels was higher after NFAT1 overexpression compared with the empty vector SB2-EV. Apoptosis was also analyzed in the tumor sections from our xenograft model using the TUNEL assay. The number of positively stained (apoptotic) tumor cells was significantly increased in NFAT1-silenced A375SM tumors. The overexpression of NFAT1 in SB2 cells reduced the number of apoptotic cells (Supplementary Fig. 3).
NFAT1 enhances the promoter activities of IL-8 and MMP3
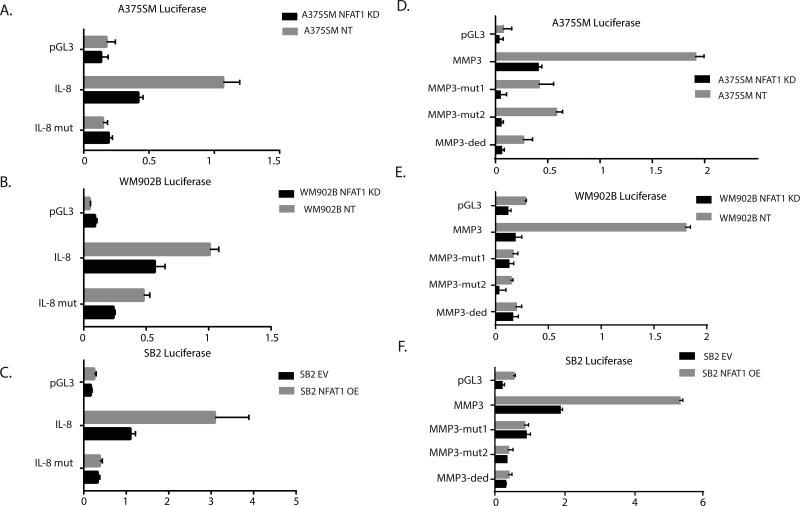
To determine whether NFAT1 regulates IL-8 at the transcriptional level, we used a dual luciferase reporter gene promoter assay designed so that the IL-8 promoter was cloned in front of the luciferase. The promoter was designed with and without mutations in the IL-8 promoter NFAT1-binding site at location −32 from the transcription initiation site (TIS) (Supplementary Fig. 4A). Silencing NFAT1 resulted in a reduction of luciferase activity of approximately 40% compared with the wild-type promoter in A375SM and WM902B cells (Fig. 4 A-B). When the mutated promoter was inserted into NT shRNA melanoma cells, the luciferase activity was reduced by approximately 50% compared with the wild-type promoter. The mutation also had an effect on luciferase activity in NFAT1-silenced melanoma cells compared with the wild-type promoter (Fig. 4 A-B). Therefore, we conclude that the reduced promoter activity (after silencing NFAT1) is a direct result of reduced binding of NFAT1 protein to the IL-8 promoter. Overexpression of NFAT1 in SB2 cells significantly increased IL-8 promoter activity (Fig. 4C).
Figure 4.
Effect of NAFT1 on IL-8 and MM-3 promoter activities. (A, B) Silencing NFAT1 in both A375SM and WM902B cells significantly reduced the luciferase activity driven by the wild-type IL-8 promoter by approximately 75% compared with NT shRNA. Mutating NFAT1 binding sites (each one separately or both) resulted in reduced promoter activity compared with the wild-type promoter (P < 0.05). (A) A375SM melanoma cells. (B) WM902B melanoma cells after silencing NFAT1. (C) SB2 melanoma cells after overexpressing NFAT1. (D, E) Silencing NFAT1 in both A375SM and WM902B cells significantly reduced the luciferase promoter activity of the wild-type MMP-3 promoter by approximately 50% compared with NT shRNA (P < 0.05). Mutating the NFAT1-binding site at location −32 from the TIS resulted in reduced promoter activity to approximately 50% of the wild-type promoter (P < 0.05). (F) NFAT1 overexpression in SB2 cells increased luciferase promoter activity.
For the MMP-3 promoter, it was not clear which binding site was more significant and whether the binding of NFAT1 would result in transcriptional activation of MMP-3 (Supplementary Fig. 4B). We therefore cloned the MMP-3 promoter (−1500 to the TIS) in front of the luciferase reporter gene. The luciferase activity driven by the MMP-3 promoter was significantly decreased after NFAT1 silencing by approximately 4-fold in A375SM cells and by approximately 7-fold in WM902B cells (Fig. 4D-E). In NFAT1-overexpressing SB2 cells, the promoter activity of MMP-3 was significantly increased (approximately 3-fold) after NFAT1 overexpression (Fig. 4F). These results indicate that NFAT1 regulates MMP-3 at the transcriptional level.
To further demonstrate that NFAT1 directly binds to the MMP-3 promoter, we generated three luciferase constructs with mutations at different binding sites: 1) MMP-3-mut1 had a mutation in site −414, 2) MMP-3-mut2 had a mutation in binding site −1291, and 3) MMP-3-ded (‘ded’ standing for double edited, mutations in both sites) had mutations in both binding sites. When the mutations were inserted, there was a significant decrease in luciferase activity compared with the control samples (A375SM NT, WM902B NT). Interestingly, both mutations had approximately the same effect (no significant different was observed) on the promoter activity and the dual mutation had no additive effect (Fig. 4D-F). These results suggest that both sites are equally important for the transcriptional activation of MMP-3.
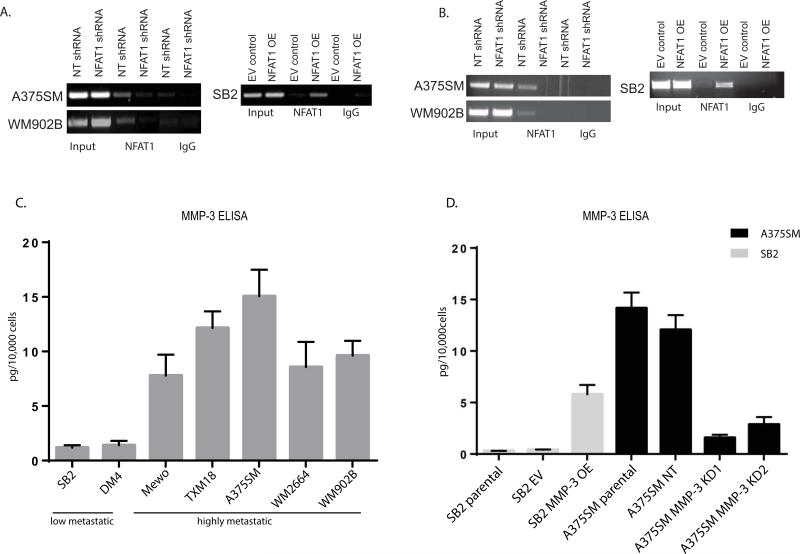
We next performed chromatin immunoprecipitation (ChIP) assays to verify the binding of NFAT1 to IL-8 and MMP-3 promoters. First, we identified a binding site of NFAT1 on the IL-8 promoter at position −32 bp from TIS (Supplementary Fig. 4A). To that end, we tested whether NFAT1 binds to the IL-8 promoter and if silencing or overexpressing NFAT1 affects the binding. As shown in Figure 5A, NFAT1 bound to the promoter of IL-8 in both the A375SM and WM902B cell lines. When NFAT1 was silenced, no binding to the IL-8 promoter was detected in either melanoma line. Also, when NFAT1 was overexpressed in SB2 cells, we observed a strong band demonstrating the binding of NFAT1 to the binding site (Fig. 5A). We also utilized the ChIP assay for the binding of NFAT1 to the MMP-3 promoter, located at −414 bp from the transcription initiation site (Supplementary Fig. 4B). NFAT1 bound to the promoter of MMP-3 in both A375SM and WM902B melanoma cells. When NFAT1 was silenced (NFAT1 shRNA), no binding to the MMP-3 promoter was observed in either cell line (Fig. 5B). In SB2 cells, the binding of NFAT1 was observed after overexpression, which was not observed in the empty vector control cells (Fig. 5B). The ChIP assay confirmed that NFAT1 binds to the MMP-3 promoter and that binding is lost after NFAT1 silencing.
Figure 5.
Binding of NFAT1 to IL-8 and MMP-3 promoters and positive correlation between the metastatic potential with MMP-3 expression. (A) ChIP of NFAT1 on the IL-8 promoter. Binding of NFAT1 was lost when NFAT1 was silenced in A375SM and WM902B cells, while the binding was increased when NFAT1 was overexpressed in SB2 cells (B) NFAT1 binds to the promoter region of MMP-3. Binding was lost when NFAT1 was silenced in A375SM and WM902B cells, while binding was increased when NFAT1 was overexpressed in SB2 cells. (C) ELISA assay for MMP-3 secretion in a panel of melanoma cell lines with different metastatic capabilities. The less-tumorigenic SB2 and DM4 melanoma cell lines secreted significantly less MMP-3 than the more tumorigenic and metastatic MeWo, TXM-18, A375SM, WM2664, and WM902B cell lines. These results correlate with the western blot analysis for NFAT1 expression in the same panel of cells presented in Figure 1A. (D) MMP-3 ELISA verified the silencing of MMP-3 in A375SM cells and overexpression in SB2 cell lines. ELISA assay for the secreted MMP-3 demonstrated a successful silencing of MMP-3 with both targets (KD1, KD2) and a significant overexpression of MMP-3 in SB2 cells (*P < 0.05).
MMP-3 expression is positively correlated with metastatic potential in melanoma cell lines
To our knowledge, the correlation between MMP-3 and metastatic potential had not yet been established for melanoma. To investigate whether MMP-3 expression is correlated with the metastatic potential of the cell lines, we used ELISA to measure MMP-3 secretion from the same panel of melanoma cell lines depicted in Figure 1A. The results from the ELISA demonstrated a direct correlation between the metastatic potential of the cell lines and MMP-3 secretion (Fig. 5C).
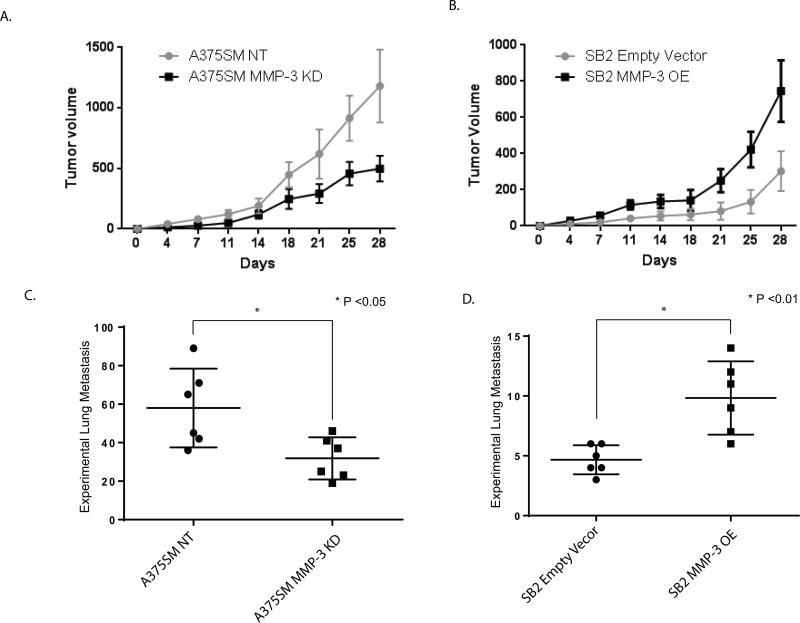
Overexpression of MMP-3 contributes to melanoma tumor growth and metastasis
To study the role of MMP-3 in melanoma growth and metastasis, we decided to stably overexpress MMP-3 in the low-metastatic SB2 cell line, and to stably silence MMP-3 expression in A375SM cells. The expression of the MMP-3 was validated using the ELISA assay, as presented in Figure 5D. These cells (A375SM MMP-3 KD1 and MMP-3 OE) were then injected both subcutaneously and intravenously into nude mice to investigate tumor growth and experimental lung metastases, respectively. Tumor volume was measured for 28 days. As shown in Figure 6, the in vivo studies demonstrated that MMP-3 is an important player in melanoma tumorigenicity. Indeed, 3 weeks after injections, there was a significant difference in the tumor size. In A375SM, the control group (A375SM NT) had a higher tumor volume than A375SM MMP-3 shRNA. At day 28, the mean tumor size was 1100 mm3 for the control group but only 450 mm3 after silencing of MMP-3 (Fig. 6A). The SB2 MMP-3 overexpression group had a mean size of 700 mm3 at day 28 compared with 200 mm3 for the empty vector group (Fig. 6B), hence overexpression of MMP-3 in SB2 cells increased their tumor growth. The results for the experimental lung metastases also supported the hypothesis that high levels of MMP-3 contribute to a higher number of experimental lung metastases. The mean number of lung metastases derived from A375SM NT was significantly higher than for the A375SM MMP-3 shRNA group (mean of 56 compared with 25, respectively, P < 0.05) (Fig. 6C). The differences in the number of lung metastases were also significant in the SB2 group. The mean number of lung metastases was higher after overexpressing MMP-3 than for the empty vector control group (9 compared with 4, P < 0.01) (Fig. 6D). We conclude that MMP-3 contributes to the malignant phenotype of melanoma.
Figure 6.
Role of MMP-3 on tumor growth and metastasis in vivo. Silencing MMP-3 in A375SM melanoma cells inhibited tumor growth and experimental lung metastases in nude mice, while overexpression of MMP-3 in SB2 cells increased both tumor growth and metastasis. (A) Tumor growth of A375SM cells was significantly reduced after silencing MMP-3; (*P < 0.05). (B) Tumor growth of SB2 cells was significantly increased after overexpressing MMP-3; (*P < 0.05) (C) Six weeks after intravenous injections of A375SM cells, silencing MMP-3 significantly reduced the number of experimental lung metastases; (*P < 0.05). (D) In SB2 cells, overexpressing MMP-3 significantly increased the number of experimental lung metastases; (*P < 0.01).
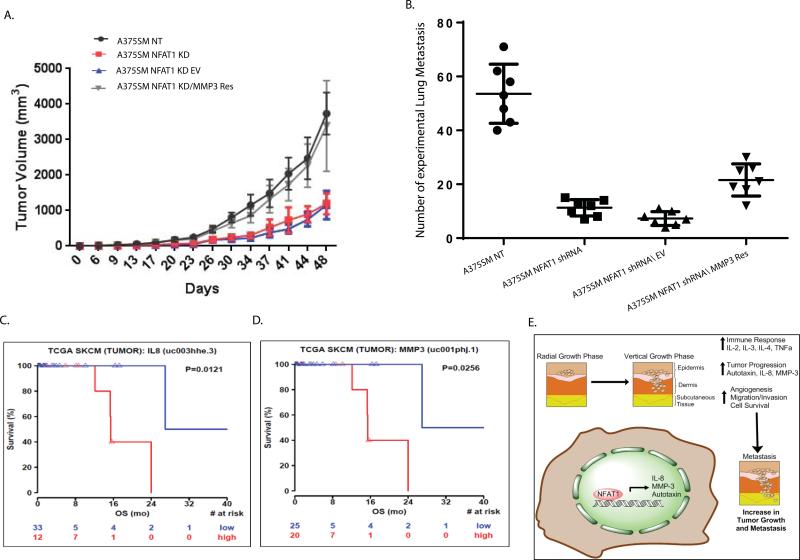
Rescue of MMP-3 in NFAT1 shRNA cells restores tumor growth and metastasis in vivo
To study whether NFAT1 regulates melanoma progression in part by modulating MMP-3 expression and activity, we stably re-expressed MMP-3 in NFAT1-silenced melanoma cells and injected them subcutaneously and intravenously into nude mice (Fig. 7 A, B). Re-expression of MMP-3 rescued tumor growth compared with NFAT1 shRNA melanoma cells transduced with an empty vector (P < 0.05, Fig. 7A) Rescuing MMP-3 in A375SM cells resulted in a slight difference in the number of experimental lung metastases. When compared with NT shRNA A375SM, significantly fewer cells metastasized to the lungs after silencing of NFAT1 (median of 53 compared with 11, P < 0.05). When we re-expressed MMP-3, the number of metastatic colonies significantly increased compared with results for empty vector, with median values of 20 and 8, respectively (P < 0.05, Fig. 7B). The number of experimental lung metastases increased but did not return completely to the level of A375SM NT, which suggests that more pathways are involved.
Figure 7.
The role of IL-8 and MMP-3 in patient survival. (A) Induced re-expression of MMP-3 completely rescued tumor growth as compared with an empty vector control in A375SM NFAT1-KD melanoma cells (*P < 0.05). (B) The number of experimental lung metastases formed after MMP-3 was rescued in NFAT1-KD cells. The number was significantly increased after MMP-3 rescue (*P < 0.05). (C) TCGA data demonstrating a correlation between survival of patients and the levels of IL-8 and MMP-3 expression. Patients with higher IL-8 expression had lower overall survival (in red) than did patients with low IL-8 expression (in blue). (D) Patients with higher MMP-3 expression had lower overall survival (in red) than did patients with low MMP-3 expression (in blue). (E) Proposed mechanism for the role of NFAT1 in melanoma progression. The transition from radial growth phase (RGP) to VGP is associated with increased expression of NFAT1. This, in turn, activates the expression/secretion of autotaxin, IL-8, and MMP-3, contributing to melanoma angiogenesis, invasion, tumor growth, and metastasis.
TCGA analysis for the expression of IL-8 and MMP-3 and survival
To further validate the relevance of the identified downstream target genes for patient outcome, we used the TCGA data to investigate whether there is a correlation between expression levels of IL-8 and MMP-3 and patient survival. The analysis was based on 45 patients. The results showed that patients with higher expressions of these genes had a significantly worse overall survival than patients with low expressions of these proteins (P = 0.0121 and P = 0.0256 for Il-8 and MMP-3, respectively; Fig. 7C, D). It should be noted that the low and high groups for each IL-8 and MMP-3 belong to different melanoma stages.
Discussion
Although the role of NFAT1 in the immune response as a T cell activator is very well established, its role in cancer—and particularly in melanoma—is less documented. Our current study had several novel findings that further elucidate the role of NFAT1 in melanoma progression. We demonstrated that NFAT1 expression positively correlated with the metastatic melanoma phenotype in both patient specimens as well as a panel of cell lines with different metastatic potentials. TCGA data from melanoma patients demonstrated that NFAT1 expression was significantly higher in metastatic lesions than in primary tumors, albeit, no associations were found between NFAT1 expression and BRAF (V600E), or p16 deletion classifications. In cell lines, NFAT1 expression increased with the metastatic potential.
To further establish the contribution of NFAT1 in melanoma metastasis, we silenced NFAT1 expression by lentiviral shRNA in two metastatic melanoma cell lines, A375SM and WM902B. In vivo, silencing of NFAT1 reduced the tumor growth and metastatic potential of A375SM melanoma cells, whereas overexpression of NFAT1 in the low-metastatic SB2 cells increased their in vivo metastatic properties. We found that NFAT1 positively regulated the expression of IL-8 and MMP-3 at the transcriptional level. No correlations were found in the TCGA data between NFAT1 and IL-8 or MMP-3 expression levels. However, IL-8 levels were found to be increased in the serum of patients with metastatic melanoma (25). Our own ELISA analyses demonstrated a direct correlation between MMP-3 expression and metastatic potential in melanoma cell lines (Fig 5C). Promoter analyses and ChIP assays demonstrated that NFAT1 binds to the promoter of both IL-8 and MMP-3 and promotes their transcription. Rescue of NFAT1 expression in NFAT1-silenced cells restored the protein expression of both IL-8 and MMP-3. This confirms that our results were not an off-target effect of the NFAT1 shRNA used in the studies.
The role of IL-8 in melanoma progression and metastasis has been previously established in our laboratory (21). We therefore decided to concentrate our efforts on elucidating the role of MMP-3 in promoting the metastatic melanoma phenotype and how NFAT1 regulates its expression. In vivo, silencing of MMP-3 reduced tumor growth and the metastatic potential of A375SM melanoma cells, whereas overexpression of MMP-3 in SB2 cells significantly increased their tumor growth and metastatic potential. Using the TCGA data, we found that patients with low expression of IL-8 or MMP-3 had a significantly better survival rate than did patients with high expression of these proteins. Taken together, these studies assign a previously undescribed role for NFAT1 in the melanoma metastatic phenotype by regulating IL-8 and MMP-3.
Earlier reports have described the correlation between NFAT1 and melanoma. One such published work demonstrated that the absence of NFAT1 expression in the microenvironment caused a significant difference in the ability of the B16F10 murine melanoma cell line to grow (26). NFAT1-deficient mice had less experimental lung metastasis colonization after B16F10 melanoma cell injections than did wild-type mice (26).
In addition to NFAT1's role on the tumor cells themselves, NFAT1 has a major role in host immune response. Indeed, NFAT1 has been shown to support tumor-induced anergy of CD4+ T cells (27) and regulates a set of genes responsible for helper T-cell (CD8+) anergy (28). Data from a recent publication demonstrated that NFAT1 increased CTLA-4 promoter activity in CD4+ T cells compared with CD8+ T cells. The expression of CTLA-4 mediated by NFAT1 in CD4+ can potentially be important for anti–CTLA-4 therapy (29). Our laboratory has previously demonstrated that Gal-3 regulates autotaxin through NFAT1 and that high levels of Gal-3 support melanoma growth and metastasis (9).
In the current studies, we identified a novel mechanism for melanoma progression in which NFAT1 regulates IL-8 and MMP-3 and promotes the malignant phenotype. This modus operandi is depicted in Figure 7E. Targeting NFAT1 could be a potential therapeutic tool. Therapy directed at NFAT1 is clinically feasible, as shown by the use of cyclosporine A to inhibit T cell–mediated organ transplant rejection (30). In melanoma, however, immunotherapy and immune surveillance promoted by T cells are considered methods for melanoma treatment. Therefore, systemic therapy directed towards NFAT1 in melanoma could be counterintuitive because of the reduction of T-cell activity. The multiple roles of NFAT1 within the tumor microenvironment (7,31) create a “double-edged sword” in regards to its therapeutic potential in melanoma. Therefore, directing therapy towards the downstream targets IL-8 and MMP-3 instead of NFAT1 could be a better choice.
These findings can translate to the clinic by improving treatment options for metastatic melanoma. Results suggest that targeting IL-8 and/or MMP-3 is a possible therapeutic modality, either alone or in combination with immunotherapy or chemotherapy. Treatment options focused on targeting IL-8 and MMP-3 can be taken in several directions, including finding the connection between IL-8 expression and BRAF inhibitor resistance in melanoma cells. Indeed, BRAFi- resistant melanoma cells express higher levels of IL-8, and combination treatment with BRAFi plus neutralizing antibody to IL-8 rendered these cells to become more sensitive to BRAF inhibitors (data not shown).
Our previous data on autotaxin regulation by Gal-3 through NFAT1 (9), along with the current data, implicate NFAT1 as a major player in modulating the tumor microenvironment to support melanoma growth and metastasis (Figure 7E). Taken together, our findings establish a novel mechanism by which NFAT1 contributes to melanoma growth and metastasis through the regulation of IL-8 and MMP-3.
Supplementary Material
Acknowledgements
We thank the department of scientific publication at MDACC for the editing of this manuscript.
These studies are supported by SINF, an MDACC grant and NIH skin cancer SPORE p50 CA 093549
Footnotes
The Role of NFAT1 in Melanoma Metastasis
All authors declare no conflict of interests.
References
- 1.Ward EM, Thun MJ, Hannan LM, Jemal A. Interpreting cancer trends. Annals of the New York Academy of Sciences. 2006;1076:29–53. doi: 10.1196/annals.1371.048. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241(4862):202–5. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 4.Durand DB, Shaw JP, Bush MR, Replogle RE, Belagaje R, Crabtree GR. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Molecular and cellular biology. 1988;8(4):1715–24. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 6.Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer. 2009;9(11):810–20. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–56. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 8.Pan MG, Xiong Y, Chen F. NFAT gene family in inflammation and cancer. Current molecular medicine. 2013;13(4):543–54. doi: 10.2174/1566524011313040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braeuer RR, Zigler M, Kamiya T, Dobroff AS, Huang L, Choi W, et al. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res. 2012;72(22):5757–66. doi: 10.1158/0008-5472.CAN-12-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattila PS, Ullman KS, Fiering S, Emmel EA, McCutcheon M, Crabtree GR, et al. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990;9(13):4425–33. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Price JE, Fan D, Zhang RD, Bucana CD, Fidler IJ. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. Journal of the National Cancer Institute. 1989;81(18):1406–12. doi: 10.1093/jnci/81.18.1406. [DOI] [PubMed] [Google Scholar]
- 12.Verschraegen CF, Giovanella BC, Mendoza JT, Kozielski AJ, Stehlin JS., Jr. Specific organ metastases of human melanoma cells injected into the arterial circulation of nude mice. Anticancer Res. 1991;11(2):529–35. [PubMed] [Google Scholar]
- 13.Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7(9):2876–83. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L, et al. Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. J Biol Chem. 2009;284(38):26194–206. doi: 10.1074/jbc.M109.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer research. 2008;68(21):9078–86. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo-Saito C. FSTL1 promotes bone metastasis by causing immune dysfunction. Oncoimmunology. 2013;2(11):e26528. doi: 10.4161/onci.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourtada-Maarabouni M, Watson D, Munir M, Farzaneh F, Williams GT. Apoptosis suppression by candidate oncogene PLAC8 is reversed in other cell types. Current cancer drug targets. 2013;13(1):80–91. [PubMed] [Google Scholar]
- 18.Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, et al. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. The Journal of clinical investigation. 2014;124(5):2172–87. doi: 10.1172/JCI71103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsey C, Balakrishnan V, O'Dell MR, Huang JL, Newman L, Whitney-Miller CL, et al. Plac8 links oncogenic mutations to regulation of autophagy and is critical to pancreatic cancer progression. Cell reports. 2014;7(4):1143–55. doi: 10.1016/j.celrep.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161(1):125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melnikova VO, Bar-Eli M. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res. 2006;19(5):395–405. doi: 10.1111/j.1600-0749.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 22.Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, et al. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9(8):3167–75. [PubMed] [Google Scholar]
- 23.Mills L, Tellez C, Huang S, Baker C, McCarty M, Green L, et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res. 2002;62(17):5106–14. [PubMed] [Google Scholar]
- 24.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. Journal of neurochemistry. 2012;123(2):203–16. doi: 10.1111/j.1471-4159.2012.07900.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20(22):5697–707. doi: 10.1158/1078-0432.CCR-13-3203. [DOI] [PubMed] [Google Scholar]
- 26.Werneck MB, Vieira-de-Abreu A, Chammas R, Viola JP. NFAT1 transcription factor is central in the regulation of tissue microenvironment for tumor metastasis. Cancer immunology, immunotherapy : CII. 2011;60(4):537–46. doi: 10.1007/s00262-010-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe BT, Shin DS, Mocholi E, Macian F. NFAT1 supports tumor-induced anergy of CD4(+) T cells. Cancer research. 2012;72(18):4642–51. doi: 10.1158/0008-5472.CAN-11-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe BT, Macian F. Uncovering the mechanisms that regulate tumor-induced T-cell anergy. Oncoimmunology. 2013;2(2):e22679. doi: 10.4161/onci.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan DV, Gibson HM, Aufiero BM, Wilson AJ, Hafner MS, Mi QS, et al. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes and immunity. 2014;15(1):25–32. doi: 10.1038/gene.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fidler IJ. Critical determinants of melanoma metastasis. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 1996;1(2):203–8. [PubMed] [Google Scholar]
- 31.Vaeth M, Bauerlein CA, Pusch T, Findeis J, Chopra M, Mottok A, et al. Selective NFAT targeting in T cells ameliorates GvHD while maintaining antitumor activity. Proc Natl Acad Sci U S A. 2015;112(4):1125–30. doi: 10.1073/pnas.1409290112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.