Abstract
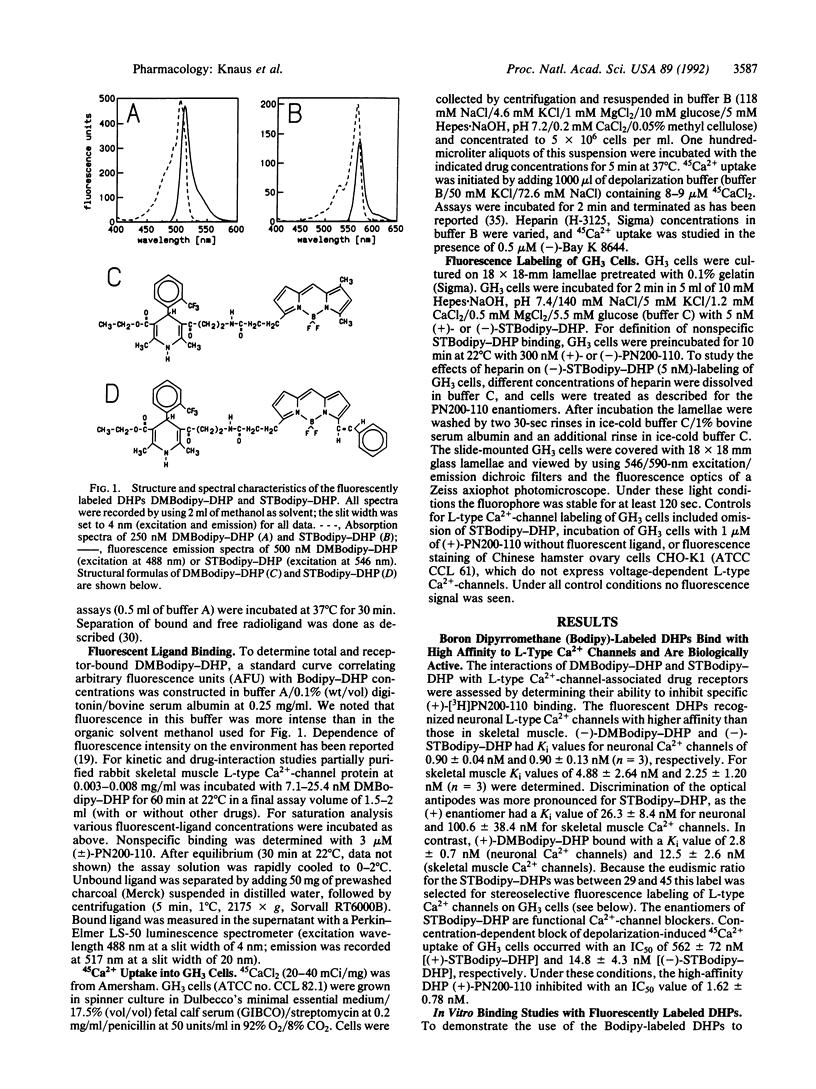
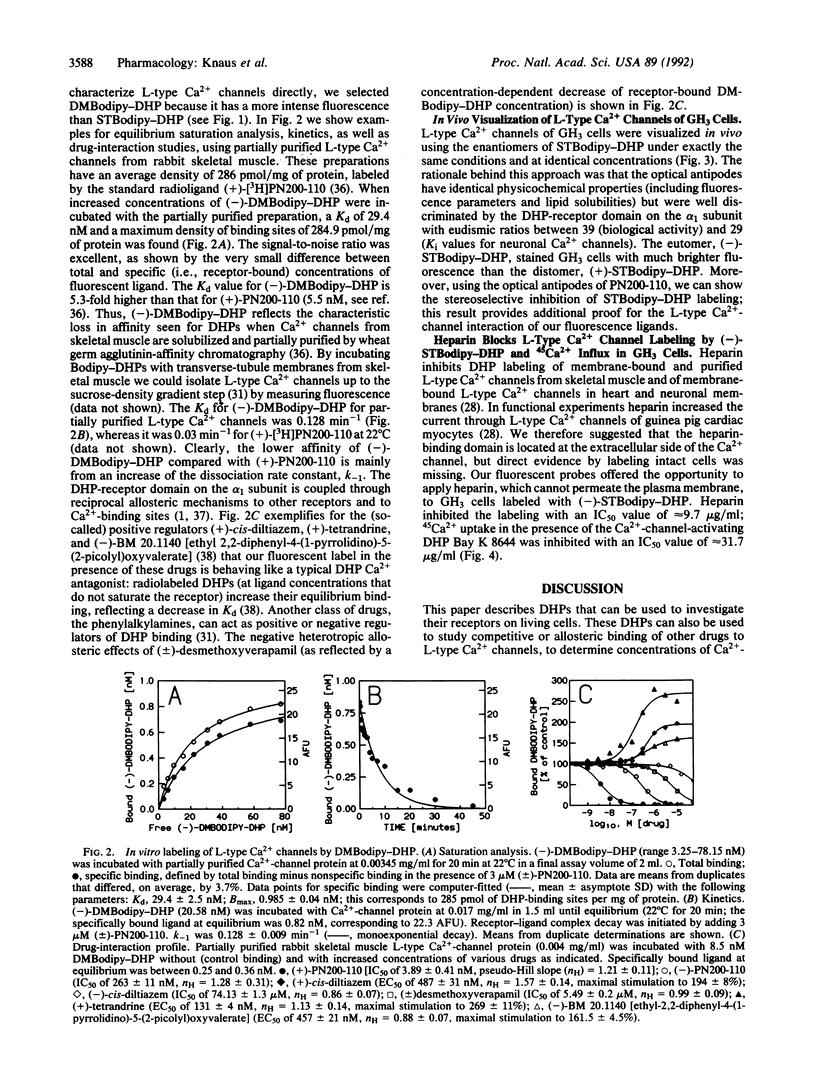
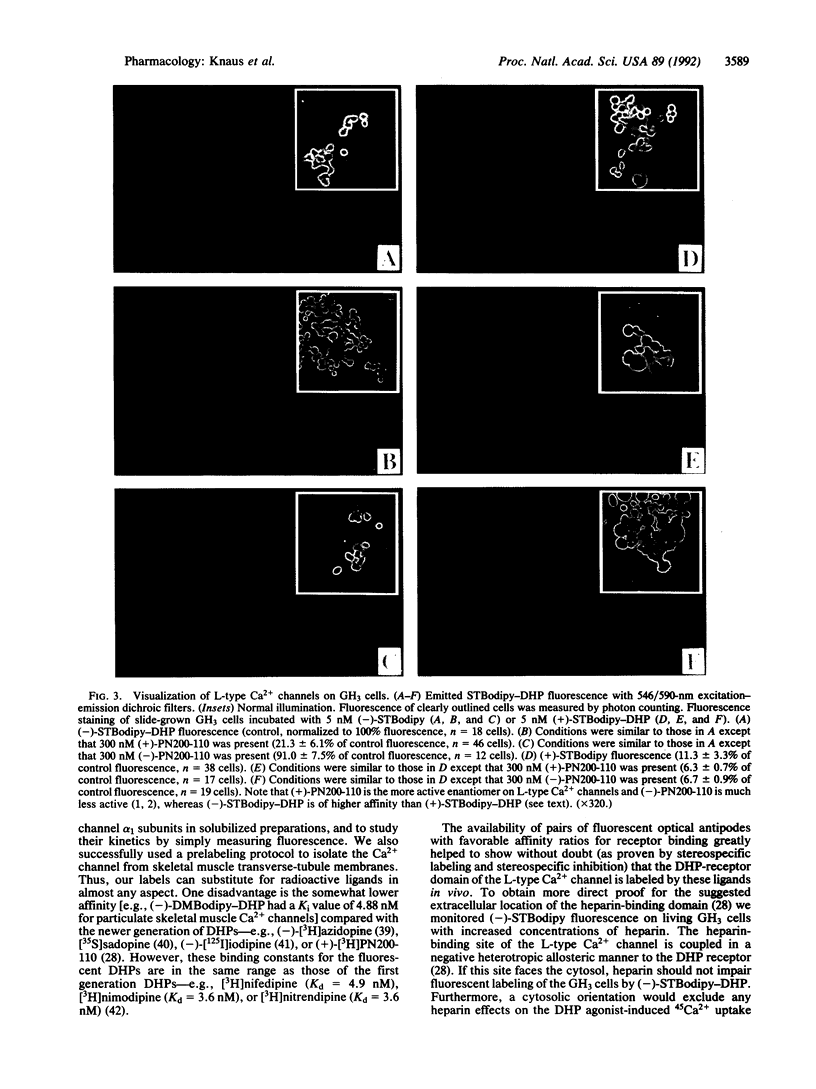
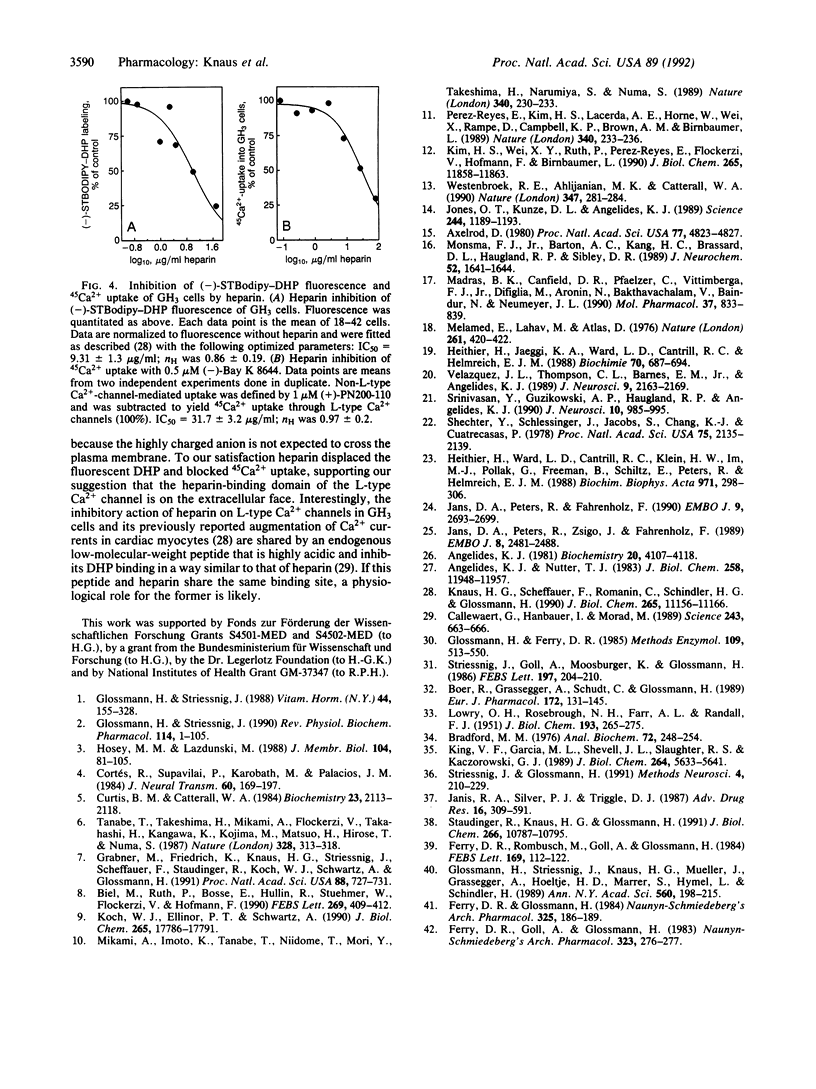
We have synthesized and characterized fluorescently labeled dihydropyridines (DHPs) as probes for L-type Ca2+ channels. Racemic as well as (+)- and (-)-1,4-dihydro- 2,6-dimethyl-4-(2-trifluoromethylphenyl)-3,5-pyridinecarboxylic acid 2-(aminoethyl)ethyl ester hydrochlorides were coupled to boron dipyrromethane (Bodipy) derivatives. (4,4-Difluoro-5,7-dimethyl-4-bora-3a,4a-diaza)-3- (s-indacene)propionic acid (DMBodipy)-DHP and (4,4-difluoro-7-styryl-4-bora-3a,4a-diaza)-3-(s-indacene+ ++)propionic acid (STBodipy)-DHP have Kd values in the nanomolar range for membrane-bound or partially purified skeletal muscle and for neuronal L-type Ca2+ channels. (-)- and (+)-STBodipy-DHPs block 45Ca2+ uptake through L-type Ca2+ channels into GH3 cells with IC50 values of 14.8 and 562 nM, respectively. The measurement of bound fluorescence after removal of free DMBodipy-DHP with charcoal shows that the probes can substitute for radioactive ligands to study the properties (equilibrium binding, kinetics, allosteric regulation) of partially purified L-type Ca2+ channels from skeletal muscle. L-type Ca2+ channels on GH3 cells were steroselectively visualized by using the optical enantiomers of STBodipy-DHP. Heparin inhibited GH3 cell labeling by (-)-STBodipy-DHP with an IC50 value of 9.7 micrograms/ml and blocked L-type Ca(2+)-channel-mediated 45Ca2+ uptake with an IC50 value of 32 micrograms/ml. These findings argue for an extracellular orientation of the heparin-binding domain of the Ca2+ channel that is coupled to the DHP receptor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J. Fluorescent and photoactivatable fluorescent derivatives of tetrodotoxin to probe the sodium channel of excitable membranes. Biochemistry. 1981 Jul 7;20(14):4107–4118. doi: 10.1021/bi00517a025. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Nutter T. J. Preparation and characterization of fluorescent scorpion toxins from Leiurus quinquestriatus quinquestriatus as probes of the sodium channel of excitable cells. J Biol Chem. 1983 Oct 10;258(19):11948–11957. [PubMed] [Google Scholar]
- Axelrod D. Crosslinkage and visualization of acetylcholine receptors on myotubes with biotinylated alpha-bungarotoxin and fluorescent avidin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4823–4827. doi: 10.1073/pnas.77.8.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Boer R., Grassegger A., Schudt C., Glossmann H. (+)-Niguldipine binds with very high affinity to Ca2+ channels and to a subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1989 May 11;172(2):131–145. doi: 10.1016/0922-4106(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Hanbauer I., Morad M. Modulation of calcium channels in cardiac and neuronal cells by an endogenous peptide. Science. 1989 Feb 3;243(4891):663–666. doi: 10.1126/science.2536955. [DOI] [PubMed] [Google Scholar]
- Cortés R., Supavilai P., Karobath M., Palacios J. M. Calcium antagonist binding sites in the rat brain: quantitative autoradiographic mapping using the 1,4-dihydropyridines [3H]PN 200-110 and [3H]PY 108-068. J Neural Transm. 1984;60(3-4):169–197. doi: 10.1007/BF01249092. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Glossmann H. 125I-iodipine, a new high affinity ligand for the putative calcium channel. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):186–189. doi: 10.1007/BF00506200. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Glossmann H. Differential labelling of putative skeletal muscle calcium channels by [3H]-nifedipine, [3H]-nitrendipine, [3H]-nimodipine and [3H]-PN 200 110. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jul;323(3):276–277. doi: 10.1007/BF00497674. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Rombush M., Goll A., Glossmann H. Photoaffinity labelling of Ca2+ channels with [3H]azidopine. FEBS Lett. 1984 Apr 9;169(1):112–118. doi: 10.1016/0014-5793(84)80299-9. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Calcium channels. Vitam Horm. 1988;44:155–328. doi: 10.1016/s0083-6729(08)60695-0. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J., Knaus H. G., Müller J., Grassegger A., Höltje H. D., Marrer S., Hymel L., Schindler H. G. Structure of calcium channels. Ann N Y Acad Sci. 1989;560:198–214. doi: 10.1111/j.1749-6632.1989.tb24098.x. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Grabner M., Friedrich K., Knaus H. G., Striessnig J., Scheffauer F., Staudinger R., Koch W. J., Schwartz A., Glossmann H. Calcium channels from Cyprinus carpio skeletal muscle. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):727–731. doi: 10.1073/pnas.88.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heithier H., Jaeggi K. A., Ward L. D., Cantrill R. C., Helmreich E. J. Synthesis and characterization of CGP-12177-NBD: a fluorescent beta-adrenergic receptor probe. Biochimie. 1988 May;70(5):687–694. doi: 10.1016/0300-9084(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Heithier H., Ward L. D., Cantrill R. C., Klein H. W., Im M. J., Pollak G., Freeman B., Schiltz E., Peters R., Helmreich E. J. Fluorescent glucagon derivatives. I. Synthesis and characterisation of fluorescent glucagon derivatives. Biochim Biophys Acta. 1988 Oct 7;971(3):298–306. doi: 10.1016/0167-4889(88)90145-0. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Jans D. A., Peters R., Fahrenholz F. Lateral mobility of the phospholipase C-activating vasopressin V1-type receptor in A7r5 smooth muscle cells: a comparison with the adenylate cyclase-coupled V2-receptor. EMBO J. 1990 Sep;9(9):2693–2699. doi: 10.1002/j.1460-2075.1990.tb07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans D. A., Peters R., Zsigo J., Fahrenholz F. The adenylate cyclase-coupled vasopressin V2-receptor is highly laterally mobile in membranes of LLC-PK1 renal epithelial cells at physiological temperature. EMBO J. 1989 Sep;8(9):2481–2488. doi: 10.1002/j.1460-2075.1989.tb08384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T., Kunze D. L., Angelides K. J. Localization and mobility of omega-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989 Jun 9;244(4909):1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Wei X. Y., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L. Studies on the structural requirements for the activity of the skeletal muscle dihydropyridine receptor/slow Ca2+ channel. Allosteric regulation of dihydropyridine binding in the absence of alpha 2 and beta components of the purified protein complex. J Biol Chem. 1990 Jul 15;265(20):11858–11863. [PubMed] [Google Scholar]
- King V. F., Garcia M. L., Shevell J. L., Slaughter R. S., Kaczorowski G. J. Substituted diphenylbutylpiperidines bind to a unique high affinity site on the L-type calcium channel. Evidence for a fourth site in the cardiac calcium entry blocker receptor complex. J Biol Chem. 1989 Apr 5;264(10):5633–5641. [PubMed] [Google Scholar]
- Knaus H. G., Scheffauer F., Romanin C., Schindler H. G., Glossmann H. Heparin binds with high affinity to voltage-dependent L-type Ca2+ channels. Evidence for an agonistic action. J Biol Chem. 1990 Jul 5;265(19):11156–11166. [PubMed] [Google Scholar]
- Koch W. J., Ellinor P. T., Schwartz A. cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J Biol Chem. 1990 Oct 15;265(29):17786–17791. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madras B. K., Canfield D. R., Pfaelzer C., Vittimberga F. J., Jr, Difiglia M., Aronin N., Bakthavachalam V., Baindur N., Neumeyer J. L. Fluorescent and biotin probes for dopamine receptors: D1 and D2 receptor affinity and selectivity. Mol Pharmacol. 1990 Jun;37(6):833–839. [PubMed] [Google Scholar]
- Melamed E., Lahav M., Atlas D. Direct localisation of beta-adrenoceptor sites in rat cerebellum by a new fluorescent analogue of propranolol. Nature. 1976 Jun 3;261(5559):420–422. doi: 10.1038/261420a0. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Barton A. C., Kang H. C., Brassard D. L., Haugland R. P., Sibley D. R. Characterization of novel fluorescent ligands with high affinity for D1 and D2 dopaminergic receptors. J Neurochem. 1989 May;52(5):1641–1644. doi: 10.1111/j.1471-4159.1989.tb09220.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Schlessinger J., Jacobs S., Chang K. J., Cuatrecasas P. Fluorescent labeling of hormone receptors in viable cells: preparation and properties of highly fluorescent derivatives of epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1978 May;75(5):2135–2139. doi: 10.1073/pnas.75.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y., Guzikowski A. P., Haugland R. P., Angelides K. J. Distribution and lateral mobility of glycine receptors on cultured spinal cord neurons. J Neurosci. 1990 Mar;10(3):985–995. doi: 10.1523/JNEUROSCI.10-03-00985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger R., Knaus H. G., Glossmann H. Positive heterotropic allosteric regulators of dihydropyridine binding increase the Ca2+ affinity of the L-type Ca2+ channel. Stereoselective reversal by the novel Ca2+ antagonist BM 20.1140. J Biol Chem. 1991 Jun 15;266(17):10787–10795. [PubMed] [Google Scholar]
- Striessnig J., Goll A., Moosburger K., Glossmann H. Purified calcium channels have three allosterically coupled drug receptors. FEBS Lett. 1986 Mar 3;197(1-2):204–210. doi: 10.1016/0014-5793(86)80327-1. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Velazquez J. L., Thompson C. L., Barnes E. M., Jr, Angelides K. J. Distribution and lateral mobility of GABA/benzodiazepine receptors on nerve cells. J Neurosci. 1989 Jun;9(6):2163–2169. doi: 10.1523/JNEUROSCI.09-06-02163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]




