Abstract
Abnormal protein glycosylation is observed in many common disorders like cancer, inflammation, Alzheimer’s disease and diabetes. However, the actual use of this information in clinical diagnostics is still very limited. Information is usually derived from analysis of total serum N-glycan profiling methods, whereas the current use of glycoprotein biomarkers in the clinical setting is commonly based on protein levels. It can be envisioned that combining protein levels and their glycan isoforms would increase specificity for early diagnosis and therapy monitoring. To establish diagnostic assays, based on the mass spectrometric analysis of protein-specific glycosylation abnormalities, still many technical improvements have to be made. In addition, clinical validation is equally important as well as an understanding of the genetic and environmental factors that determine the protein-specific glycosylation abnormalities. Important lessons can be learned from the group of monogenic disorders in the glycosylation pathway, the Congenital Disorders of Glycosylation (CDG). Now that more and more genetic defects are being unraveled, we start to learn how genetic factors influence glycomics profiles of individual and total serum proteins. Although only in its initial stages, such studies suggest the importance to establish diagnostic assays for protein-specific glycosylation profiling, and the need to look beyond the single glycoprotein diagnostic test. Here, we review progress in and lessons from genetic disease, and review the increasing opportunities of mass spectrometry to analyze protein glycosylation in the clinical diagnostic setting. Furthermore, we will discuss the possibilities to expand current CDG diagnostics and how this can be used to approach glycoprotein biomarkers for more common diseases.
Keywords: Congenital disorders of glycosylation, Glycomics, Protein-specific glycosylation, Transferrin
Contents
Background......................................................................5
CDG diagnostics and gene identification........................7
Therapy development for CDG.......................................9
- Novel glycoprotein biomarkers for CDG......................13
-
4.1.The need for glycoprotein biomarkers beyond transferrin.............................................................13
-
4.2.Potential glycoprotein biomarkers for diagnostic application in CDG............................................14
-
4.1.
- Mass spectrometry in glycoprotein analysis.................17
-
5.1.Glycoprotein biomarker discovery.......................19
-
5.2.Glycoprotein biomarker application in clinical diagnostics............................................................21
-
5.1.
Outlook...........................................................................22
References......................................................................24
Background
Protein glycosylation is a non-template driven, dynamic, and post-translational modification on top of protein synthesis. Glycans have important functions in many biological processes such as protein folding, clearance and cell-cell interactions. Considering glycosylation to be the final and top level after DNA and protein synthesis, glycosylation is the most informative level, being a product of the genome and environmental factors together. Therefore, the use of glycomics methods to read this information holds enormous potential for the development of unique biomarkers for human diseases. In several genetic and chronic diseases, like cancer [1–3], inflammation [4], Alzheimer’s disease [5], diabetes [6, 7] and metabolic disorders [8], glycosylation of proteins is changed dramatically. Analysis of glycosylation has the benefit that it provides information about the current status of the patient, which enables early diagnosis of disease onset and therapy monitoring.
Still major technological advances have to be made before glycoprotein biomarkers can be widely used in clinical diagnostics. In addition, clinical validation of potential biomarkers is equally important as well as the knowledge of how to interpret glycosylation abnormalities in the context of a certain disease. Important lessons can be learned by a comparison with a group of well-defined genetic defects, the Congenital Disorders of Glycosylation (CDG). CDG encompasses a group of inherited diseases with abnormal glycan metabolism. According to current nomenclature [9], they are divided into four different biochemical groups, comprising errors in protein N-linked glycosylation, protein O-linked glycosylation, glycolipid and GPI anchor glycosylation, and a group with defects in multiple glycosylation pathways [10]. Over 100 human genetic disorders have been associated with abnormal glycosylation [8, 11], of which at least 50 types are linked to deficient N-glycosylation of serum transferrin, historically referred to as CDG [12]. Because the defective genes are involved in a variety of functionally diverse metabolic pathways, the clinical presentation of CDG subtypes is highly heterogeneous. Clinical phenotypes range from mild to severe and involve multiple or only single affected organs. Examples of clinical symptoms include intellectual disability, seizures, muscle dystrophy, skeletal dysplasia, dysmorphic features, growth retardation, hematological and endocrine abnormalities, which show the widespread requirement of proper glycosylation for the human system [13, 14]. In line with these diverse clinical symptoms, diagnosis can be very challenging. Currently, only transferrin glycosylation analysis is widely used in clinical diagnostics for CDG.
Advanced mass spectrometry (MS) was introduced in CDG research to analyze released glycans from serum proteins [15–17] to gain more detailed insights in the glycan structural abnormalities. The growing contribution of mass spectrometry in the diagnosis of CDG is outlined in a recent review by Sturiale et al. [18]. Intact glycoprotein analysis of transferrin followed after the rapid development of mass spectrometry techniques and has now been added to routine diagnosis [19] and recently to therapy monitoring of CDG [20]. This method for protein-specific analysis has allowed high-resolution annotation of glycan structures, thereby revealing unique glycosylation profiles for specific genetic defects. This shows the potential to include mass spectrometry in specialized diagnostic laboratories and has already improved the diagnostic process by significantly shortening the time to reach a diagnosis.
It can be predicted that more glycosylation disorders will be reported in the near future since at least 2 % of the human genes encodes for proteins involved in glycan biosynthesis and recognition [8], and 5–10 % for proteins involved in Golgi homeostasis with a potential indirect effect on glycan metabolism. In addition, the wide-spread diagnostic application of next-generation sequencing is increasing the speed of CDG gene discovery [21]. The complexity of different glycosylation pathways, and the existence of tissue- and protein- specific glycosylation, indicates that the current use of transferrin as diagnostic marker is only a mere start to apply methods for glycoprotein analysis in a clinical diagnostic setting. In addition, the coming decade will show a merging of monogenic defects with genetic predisposition in more common diseases with abnormal protein glycosylation. Thus, there is a clear need to develop additional glycoprotein biomarkers to grasp the full scope of glycosylation defects in a diagnostic setting, and to learn how to interpret glycosylation abnormalities in the context of more common diseases.
Here, we will first describe the current state of diagnostics and gene identification for CDG. Secondly, we will discuss the potential use of existing plasma glycoprotein biomarkers, and finish with a look to the future in suitable mass spectrometry approaches for analysis of glycoproteins in clinical diagnostics.
CDG diagnostics and gene identification
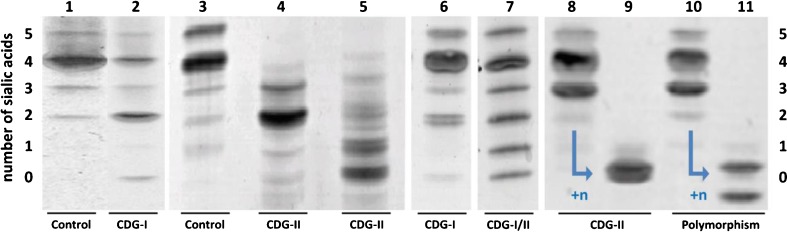
Defects in the N-glycosylation pathway are the best studied types of CDG. They are localized to the cytoplasm and the endoplasmic reticulum (ER) in CDG-I or to the cytoplasm and Golgi apparatus in CDG-II [22]. CDG-I defects are characterized by unoccupied glycosylation sites on proteins, thus lacking complete N-glycans, whereas CDG-II defects show immature, truncated glycans. Currently, the first line screening for these CDG subgroups in the majority of metabolic laboratories world-wide is based on the analysis of transferrin N-glycosylation by isoelectric focusing (TIEF), first introduced in 1984 by Jaeken et al. [23], by HPLC [24] or by capillary electrophoresis (CE) [25, 26]. These techniques allow separation of transferrin isoforms on basis of charge state determined by the number of terminal sialic acid residues (see Fig. 1). Normal plasma transferrin contains two complex type N-glycans with in total four terminal sialic acid residues as most dominant species (lanes 1 and 3). CDG patients show a decrease of this tetrasialo transferrin isoform and an increase of lower sialylated species. Abnormal profiles are classified as either CDG-I or CDG-II, depending on an isolated increase of asialo- and disialotransferrin bands (lane 2, CDG-I) or an additional increase of monosialo- and trisialotransferrin bands (lanes 4, 5 and 6, CDG-II), respectively [27]. Diagnostic interpretation of abnormal profiles can be complicated by the presence of transferrin polymorphisms that co-migrate with abnormally glycosylated transferrin isoforms [28, 29] (example in lane 8, Fig. 1). Treatment of serum transferrin (in vitro) with neuraminidase (sialidase), or alternatively analysis of parental plasma samples, is used to confirm or exclude a transferrin polymorphism [30] (lanes 6 to 9). Secondary causes of abnormal transferrin glycosylation are for example alcohol abuse, resulting in CDG-I profiles, the presence of sialidase in the plasma, or severe liver disease, both resulting in CDG-II profiles. In the majority of cases, CDG-I and -II profiles can be clearly discriminated. However, very mild CDG-I profiles can be interpreted as mild CDG-II (Fig. 1, lane 6) [31], and recently a first genetic defect was reported with a mixed CDG-I/II profile due to a combination of missing and truncated glycans (Fig. 1, lane 7) [20].
Fig. 1.
Serum transferrin isoelectric focusing (TIEF) gels with the number of terminal sialic acid residues indicated at the left and right side (0 to 5). Lanes 1 and 3: normal pattern; lane 2: CDG-I pattern (elevated asialo- and disialotransferrin bands); lanes 4, 5, 7 and 8: CDG-II pattern (additional increase of monosialo- and trisialotransferrin bands). Lane 6: mild CDG-I profile, resembles mild CDG-II pattern. Lane 7: combined CDG-I and -II profile. Lanes 8 to 11: transferrin before and after neuraminidase treatment (+n) for CDG-II defect MAN1B1-CDG and a polymorphism of transferrin, respectively. Two bands instead of one band will become visible when treating a transferrin polymorphism with neuraminidase
Conventionally, the follow up in case of CDG-I profiles consists of enzyme analysis in fibroblasts or leukocytes to diagnose PMM2-CDG, which is the most common type [32], or PMI-CDG. If excluded, the next step is analysis of dolichol- or lipid-linked oligosaccharides (LLO) in fibroblasts [33]. Nowadays, this is being replaced by whole exome sequencing using a filter for CDG-I genes [34], or targeted sequencing of a CDG-I gene panel. Alternatively, direct application of whole exome sequencing without prior CDG screening increasingly results in the identification of CDG defects, which need to be confirmed with Sanger sequencing. A combination of Capillary Electrophoresis [26] or LC [19] with mass spectrometry as a quick and sensitive test could functionally confirm the genetic defect. Also for confirmation of mild CDG-I profiles, mixed CDG-I/II profiles and even for further subtyping of CDG-I defects (such as ALG1-CDG, own data), high-resolution mass spectrometry of intact transferrin can be used.
The standard procedure for CDG-II patterns includes a follow up by isoelectric focusing of plasma apolipoprotein C-III (apoC-III-IEF) to discriminate between isolated N-glycosylation defects and a combined disorder of N- and mucin type O-glycosylation [35]. In addition, two-dimensional electrophoresis with immunoblotting was reported to separate and detect the isoforms of apoC-III, alpha-antitrypsin, alpha-1 acid glycoprotein, haptoglobin and transferrin [36]. Since CDG-II is characterized by truncated glycans, mass spectrometry methods can provide additional insights into the underlying genetic defect. Several methods have been reported for the analysis of transferrin glycopeptides [15, 37] and analysis of whole serum N-glycans [16, 38, 39] by MALDI-TOF mass spectrometry. With respect to O-glycans, mass spectrometry analysis of apoC-III [40] and combined mass spectrometry of released serum N- and O-glycans [41] have been reported. Recently, mass spectrometry analysis of apoC-III O-glycosylation was compared with two-dimensional electrophoresis by Yen-Nicolaÿ et al. and appeared to be a promising technique for analysis of defects in mucin O-glycosylation [42]. Application of high-resolution QTOF mass spectrometry for analysis of intact transferrin allowed to accurately annotate glycan structures on the level of the intact protein [31, 43] and proved highly efficient in subgrouping of CDG-II patients. For example, highly characteristic profiles can be observed for MAN1B1-CDG, PGM1-CDG with a characteristic combined CDG-I/II profile, and SLC35A2-CDG. The method has been validated for use in a clinical metabolic laboratory. The successful identification of gene defects directly from a characteristic glycoprofile of transferrin, has placed this method as second test immediately following CDG screening (diagnostic flowchart shown in [31]).
Currently, mass spectrometry of total serum N-glycans and of transferrin N-glycans has been included in only a few metabolic diagnostic laboratories. However, the high sensitivity and high specificity have already resulted in a significant shortening of the time to reach a genetic diagnosis in CDG, thereby showing the advantage to use mass spectrometry more broadly in CDG diagnostics.
Therapy monitoring in CDG
Although many new glycosylation disorders have been identified in the last decade, only little progress has been made in development of new therapies. Different future therapeutic approaches have been proposed for the most common CDG type, PMM2-CDG, in a very comprehensive review by Freeze [44]. These include gene therapy, enzyme enhancement, enzyme replacement, bypassing the metabolic pathway and substrate supplementation. Most of these therapeutic strategies would be applicable to other CDG types as well, but are still under development. Several challenges have to be overcome, like the lack of appropriate model systems, high costs, small number of patients and the limited bioavailability of the drugs to reach their target, especially in regard to pass the blood–brain-barrier [44]. Only in four CDG subtypes, namely MPI-CDG, PGM1-CDG, SLC35C1-CDG and PIGM-CDG, a mechanism-based treatment has been applied [20, 45–48].
MPI-CDG is caused by a mutation in the cytosolic enzyme phosphomannose isomerase (MPI). The enzyme catalyzes the conversion of fructose-6-phosphate to mannose-6-phosphate [49], and therefore the disease is characterized by decreased mannose-6-phosphate. Mannose supplementation increased the mannose-6-phosphate levels and improved the clinical features in patients [45]. Despite mannose therapy, patients can develop progressive liver failure at a later stage of the disease. One first patient was successfully treated by liver transplantation, resulting in immediate recovery of most severe clinical symptoms [50]. As expected in view of the hepatic origin of transferrin, intact transferrin glycosylation profiles completely normalized after the transplantation. However, the disease status in other organs cannot be followed adequately by analysis of transferrin alone. This clearly shows the need for additional biomarkers for CDG therapy monitoring, including glycoproteins that are synthesized by other tissues and cell types.
PGM1-CDG is caused by a mutation in the phosphoglucomutase 1 enzyme (PGM1). The enzyme catalyzes the interconversion of glucose-6-phosphate and glucose-1-phosphate [51]. The disease was identified by a highly characteristic transferrin glycosylation profile with high-resolution mass spectrometry, which showed a combination of CDG-I and CDG-II. The spectra showed truncated glycans lacking galactose. Based on the spectra and known metabolic pathways, supplementation of patients with galactose resulted in almost complete normalization of transferrin glycosylation [20] (Fig. 2). The normalization of the truncated glycans upon galactose treatment can be explained based on known metabolic pathways. However, the normalization of the complete glycans (CDG-I) cannot be fully explained. More detailed insight into the dynamics and regulation of sugar metabolism, and its link with protein glycosylation, would help us to better understand the glycosylation machinery, further optimize therapy and develop new therapies for other CDG patients. In addition, monitoring of the glycosylation of additional proteins is required to learn if other tissues apart from liver respond equally well to galactose treatment.
Fig. 2.
Effects of Dietary Galactose on Glycosylation. High resolution mass spectrometry showing glycan structures of transferrin before (a) and after (b) 2 weeks intake of supplementary galactose and corresponding patterns of transferrin isoelectric focusing (IEF). The protein backbone is symbolized by a brown horizontal line. The unoccupied positions are indicated by open arrows ( ) and define the CDG-I-component of this phenotype. The yellow arrow (
) and define the CDG-I-component of this phenotype. The yellow arrow ( ) demonstrates the absence of galactose on one of the truncated glycans, which define the CDG type-II component. The number of sialic acids is indicated above each structure, and the insets at the right show respective IEF results
) demonstrates the absence of galactose on one of the truncated glycans, which define the CDG type-II component. The number of sialic acids is indicated above each structure, and the insets at the right show respective IEF results
SLC35C1-CDG is caused by a mutation in the GDP-fucose transporter in the Golgi-membrane, resulting in hypofucosylation of N-glycopeptides [52]. Patients typically lack sialyl-Lewis X (sLex) structures on the cell surface of neutrophils, which is required for their rolling on endothelial cells before extravasation [53]. This CDG type is therefore also known as leukocyte adhesion deficiency II (LAD II) and leads to infectious complications. Depending on the nature of the mutation, some patients respond to L-fucose application [46, 54]. However, no proper glycoprotein marker is currently available to monitor therapy response and disease progression.
PIGM-CDG is a defect in GPI-anchor biosynthesis and is caused by a mutation in the glycosylphosphatidylinositol glycan (class M) (PIGM) promoter. PIGM transfers the first mannose to the GPI anchor at the luminal side of the endoplasmic reticulum [55]. Mutation in the promoter causes histone hypoacetylation, resulting in defective GPI anchored proteins on blood cells [47]. A patient was therefore treated with sodium phenylbutyrate, which is a histone deacetylase inhibitor. This increased PIGM transcription, resulting in improved GPI anchored glycoproteins: CD59 (MAC-inhibitory protein) on erythrocytes and CD59 and CD24 (heat stable antigen) on granulocytes (FACS analysis) [55]. Clinically, the patient improved and was able to walk again and had no more seizures [48].
Currently, CDG therapy monitoring is mainly based on clinical features, transferrin analysis or flow cytometry of several glycoprotein surface markers on blood cells. The main advantage of using transferrin as a biomarker for CDG is its high abundance in blood, allowing fast, detailed and sensitive detection of N-glycosylation in small sample volumes. Also for treatment of fructosemia, a common secondary cause of CDG, the loss of intact glycans can be monitored sensitively by high resolution mass spectrometry of transferrin [31]. However, therapy monitoring in clinical diagnostics is very limited with the analysis of transferrin, and additional biomarkers are essential for the development and monitoring of new and current therapies.
Novel glycoprotein biomarkers for CDG
The need for glycoprotein biomarkers beyond transferrin
Although transferrin is a very useful diagnostic biomarker for most CDG types with deficient N-glycosylation, several defects in the N-glycosylation pathway are known with a normal transferrin profile. These include both CDG-I subtypes (TUSC3-CDG, ALG13-CDG, ALG14-CDG), Golgi glycosylation defects (GCS1-CDG, SLC35A3-CDG and SLC35C1-CDG), as well as defects in sugar metabolism (GNE, PGM3 and GFPT1). The growing list of these type of defects with normal transferrin glycosylation indicates that additional proteins have to be included for correct diagnosis. Since glycans can be expressed in a tissue-specific way [56–58], defects in their synthesis will be missed when analyzing only hepatocyte-derived transferrin. Furthermore, analysis of defects in the synthesis of glycan structures not or hardly present on transferrin (such as fucose in SLC35C1-CDG), will require inclusion of additional proteins. Additionally, recent studies indicate the existence of regulatory processes in protein-specific glycosylation that we don’t yet understand. For example, apart from the normal glycosylation of transferrin, glycosylation of IgG was abnormal in GCS1-CDG patients [59], although GCS1 is ubiquitously expressed. Cohen syndrome due to mutations in VPS13B shows normal transferrin glycosylation as well, however, Duplomb et al. found abnormal N-glycan structures by mass spectrometry of total serum glycans [60]. They identified a decrease in sialylation and galactosylation of total serum N-glycans, which indicates deficient Golgi N-glycosylation of specific proteins. They propose to analyze the highly glycosylated protein intercellular cell adhesion molecule 1 (ICAM-1) and lysosome-associated membrane protein 2 (LAMP-2) in blood cells as a pre-screening test for Cohen syndrome.
In addition to defects in the N-glycosylation pathway, there are many other genetic glycosylation defects (e.g., in O- and lipid-glycosylation, and synthesis of glycosaminoglycans and glycophosphatidylinositol (GPI) anchors) for which specific markers need to be developed, which would be of similar value as transferrin for defects in the N-glycosylation pathway. In conclusion, there is a clear need for additional glycoprotein biomarkers, in addition to transferrin, for efficient diagnostics of the full scope of genetic glycosylation defects.
Potential glycoprotein biomarkers for diagnostic application in CDG
An extensive number of glycoprotein biomarkers is already described in the literature for diagnosis of other types of diseases, particularly for diabetes mellitus [61], Alzheimer’s disease [62], rheumatoid arthritis or other inflammatory diseases [63, 64], and cancer [65]. These proteins could be a potential glyco-marker for CDG as well, because they are readily measurable in biological matrices, and they have different type of glycosylation, or are from other origin compared to transferrin.
The analysis of glycoprotein levels of some cancer biomarkers is FDA approved as prognostic or diagnostic screening [66–68], indicated by an asterisk in the table below. The detection of most glycoproteins is still mainly based on immunoassays and electrophoresis techniques, although the application of mass spectrometric screening is starting to be explored as well. To highlight the extent of today’s glycoproteomic biomarker research, Table 1 provides an overview of some key examples of candidate glycoprotein biomarkers from recent literature for the previously mentioned diseases.
Table 1.
Overview of some key examples of serum glycoprotein biomarkers from recent literature for diseases that present with altered glycoprotein levels and glycosylation abnormalities
| Glycoprotein Biomarker | Disease | Detection on protein levels or altered glycoforms | Detection technique | Reference |
|---|---|---|---|---|
| Transferrina | – CDG | – Glycosylation abnormalities | – IEF, QTOF | [50] |
| – Sepsis | – Decrease in sialylation | – ELLA | [93] | |
| – Acute pancreatitis | – Increased fucosylation | – LC-MS/MS | [94] | |
| – Ovarian cancer | – Increase of concentration | – ELISA | [95]a | |
| α1-antitrypsin | – CDG | – Altered glycosylation | – 2DE | [36] |
| – Chronic obstructive pulmonary disease (COPD) | – Decreased concentration | – ELISA | [96] | |
| – Liver cirrhosis and liver cancer | – Core and outer arm fucosylation | – 2DE, MALDI-TOF – FLISA |
[97] | |
| – Lung cancer | – Altered glycosylation | – Lectin microarray, ELISA | [98] | |
| Haptoglobin | – CDG | – Altered glycosylation | – 2DE | [36] |
| – Long cancer | – sLeX increase | – HILIC, WAX-HPLC | [99] | |
| – Acute phase ovarian cancer | – sLeX increase | – 2DE, NP-HPLC | [100] | |
| – Stomach cancer | – sLeX increase | – 2DE, LC-MS/MS – ELISA – HILIC |
[101] | |
| – Pancreatic cancer | – Fucosylation | – MALDI-TOF – Immunoblot |
[102, 103] | |
| – Rheumatoid arthritis | – Reduced mannosylation | – 2DE, MALDI-TOF – WAX-HPLC – ELISA |
[104] | |
| – Pancreatic cancer | – Changes in sialylation | – LC-MS/MS | [94] | |
| – Gastric cancer | – Increase of concentration | – ELISA – MRM |
[105] | |
| Fetuin | – Hepatocellular carcinoma (HCC) | – Fucosylation | – LC-MS/MS – FLISA |
[106] |
| – Chronic pancreatitis, pancreatic cancer | – sLeX increase | – 2DE, WAX-HPLC, NP-HPLC, LC-MS/MS, MALDI-TOF | [107] | |
| α-1-acid glycoprotein | – CDG | – Altered glycosylation | – 2DE | [36] |
| – Acute phase ovarian cancer | – sLeX increase | – 2DE, NP-HPLC | [100] | |
| – Chronic pancreatitis, pancreatic cancer | – Increased branching and sLeX | – 2DE, WAX-HPLC, NP-HPLC, LC-MS/MS, MALDI-TOF | [107] | |
| – Rheumatoid arthritis | – Altered mannosylation | – 2DE, MALDI-TOF – WAX-HPLC – ELISA |
[104] | |
| Immunoglobulin G (IgG) | – Acute phase ovarian cancer | – sLeX increase | – 2DE, NP-HPLC | [100] |
| – Advanced ovarian cancer | – Reduced galactosylation and sialylation levels | – 2DE, NP-HPLC | [100] | |
| – Stomach cancer | – Core fucosylation | – 2DE, LC-MS/MS | [101] | |
| α1-antichymotrypsin | – Acute phase ovarian cancer | – sLeX increase | – 2DE, NP-HPLC | [100] |
| – Alzheimer’s disease | – Concentration | – Immunoassay | [108] | |
| α2-macroglobulin | – Sjögren’s syndrome | Abnormal glycosylation | Immunoblot | [109] |
| α-fetoproteina | – Liver diseases | – Core fucosylation, concentration | – 2DE, immunoblot | [110] |
| – Liver cancera | – Concentration | – Anti-body (liquid phase binding assay) | [67]a | |
| des-γ-carboxypro-thrombin | Liver diseases, liver cancer | Core fucosylation | – 2DE, immunoblot | [110] |
| Ferritin | Still’s disease, hemophagocytic syndrome | Altered glycosylation | Immunoassay | [111] |
| Ceruloplasmin | – CDG | – Low levels, glycosylation abnormalities | – 2DE | [50] |
| – Hepatocellular carcinoma (HCC) | – Upregulated, core fucosylation | – LC-MS/MS – 2DE, MALDI-TOF |
[112, 113] | |
| – Pancreatic cancer | – sLeX increase | – LC-MS/MS – Immunoblot |
[114] | |
| Thyroglobulina | – CDG | – Low levels, glycosylation abnormalities | – 2DE | [50] |
| – Thyroid cancer | – Increase of concentration | – Immunoassay | [115], [68]a | |
| Thyrotropin, Thyroid stimulating hormone | – CDG | – Low levels, altered glycosylation | – IEF, serum levels | [50, 116, 117] |
| – Thyroid function | – Increase of glycoprotein concentration | – Immunoassay | [118] | |
| Hemopexin | Hepatocellular carcinoma (HCC) | Fucosylation | – LC-MS/MS – FLISA |
[106] |
| Clusterin | – Stomach cancer | – Smaller N-glycans | – 2DE, LC-MS/MS | [101] |
| – Gastric cancer | – Decrease of concentration | – ELISA – MRM |
[105] | |
| – Clear cell renal cell carcinoma | – Decreased (core fucosylated) biantannary glycans | – SDS-PAGE, LC-MS/MS – Immunoblot |
[119] | |
| Leucine rich-α2-glycoprotein | Stomach cancer | Upregulation, altered glycosylation | 2DE, LC-MS/MS | [101, 120] |
| α-dystroglycan | Walker-Warburg syndrome | Hypoglycosylation | Immunoassay | [121] |
| Kininogen | – Colorectal cancer (CRC) | – Elevated sialylation and fucosylation | – LC-MS/MS – Lectin microarray |
[122] |
| – Colorectal cancer (CRC) | – Increase of concentration | – MALDI-TOF – ELISA – Immunostaining |
[123] | |
| Kallistatin | Liver cirrhosis | Increase of concentration | ELISA | [124] |
| Afamin | – Metabolic syndrome | – Increase of concentration | – ELISA | [125] |
| – Gastric cancer | – Decrease of concentration | – ELISA – MRM |
[105] | |
| Prostate Specific Antigen (PSA)a | Prostate cancer | Increase of concentration | Immunoassay | [126], [68]a |
| Human chorionic gonadotrophin (hCG) | – Ovarian tumors – Testicular tumors |
Increase of concentration | Immunoassay | [68], [66]a |
| Apolipoprotein (A-1)a | Ovarian cancer | Decrease of concentration | ELISA | [95]a |
| Transthyretin, prealbumina | Ovarian cancer | Decrease of concentration | ELISA | [95]a |
| β2-microglobulina | Ovarian cancer | Increase of concentration | ELISA | [95]a |
| Cancer antigen 125 (CA125) or MUC16a | Ovarian cancer | Increase of concentration | ELISA | [67, 95]a |
| Carbohydrate antigen 19–9 (CA19-9)a | – Pancreatic cancer Ovarian cancer | sLea on mucin glycoproteins | ELISA | [64, 68]a |
| Cancer antigen 15–3 (CA15-3)a | Breast cancer | Sialylated O-linked oligosaccharide on MUC1 | ELISA | [67, 68]a |
| CA27-29a (MUC1) | Breast cancer | Protein concentration | ELISA | [67, 68]a |
2DE 2D-electrophoresis; ELLA enzyme-linked lectin assay; FLISA Lectin-Fluorophore-linked Immunosorbent Assay; HILIC Hydrophilic Interaction Liquid Chromatography; sLe a Sialyl Lewis A structures; sLe X Sialyl Lewis X structures; WAX-HPLC weak anion exchange high performance liquid chromatography
aFDA approved
As can be seen from this overview, the number of glycoproteins as diagnostic biomarker is growing for multiple diseases, see Table 1 for details and references. For some of these glycoproteins, altered glycosylation has already been investigated in more detail using mass spectrometry. In particular core or sLeX fucosylation seems to be a frequent significant change observed. It should also be noted that the glycoprotein level is often the diagnostic parameter. So ideally, both protein levels and glycosylation analysis should be included in one assay. It could be envisioned that combination of several plasma proteins with a more heterogeneous glycan composition and increased fucosylation as compared to transferrin, can be used in a panel to further increase the diagnostic efficiency for CDG-I and CDG-II. Apart from selections based on biological aspects as discussed in section 4.1, an additional challenge is the mass spectrometric compatibility and the different approaches for analysis, which will be discussed in the next paragraph.
Mass spectrometry in glycoprotein analysis
A growing number of protein biomarkers are analyzed in the clinics by mass spectrometry. Mass spectrometry is ideally suited for the clinical laboratory, because the technique is sensitive, compatible with biological matrices, suitable for high-throughput analyses and it provides detailed structural information. The technique has proven itself for CDG diagnostics through the routine analysis of serum transferrin, which will pave the way in the search for additional glycoprotein biomarkers. In Fig. 3, an overview is depicted of the different levels of glycosylation analysis by mass spectrometry, better known as Glycomics. Intact glycoprotein analysis provides quantitative glycan structural information of the intact glycoprotein. Quantification is possible with the assumption that ionization of the molecules dominated by the amino acid backbone. Loss of complete glycans can therefore accurately be detected. Intact protein analysis is limited by size and hydrophobicity of the protein, and limited by the number of glycosylation sites for correct interpretation of the spectra. Glycopeptide analysis provides detailed site-specific information on glycan forms, and enzymatic digestions increase the chance to measure large or hydrophobic proteins of interest. However, the ionization efficiency is influenced by the type of glycan, which hampers any quantitative comparison between different glycoforms. Analysis of free glycans allows sensitive detection of very low abundant glycan structures, however information on the corresponding protein/peptide attachment site is lost. Therefore, each detection technique has its own strengths and weaknesses depending on the intended goal of the analysis. Below, we discuss the use of mass spectrometry in biomarker discovery and for its potential use as a diagnostic test.
Fig. 3.
Schematic representation of the different types of glycoprotein analysis using mass spectrometry
Glycoprotein biomarker discovery
The most widely used methods for biomarker discovery within the field of glycomics includes free N-glycan profiling of a biological fluid, such as serum, plasma or cerebrospinal fluid. Many groups have published on the discovery of altered glycosylation in a wide variety of diseases, and convincingly showed that glycans have potential as biomarkers, being highly sensitive to pathological changes [69–73]. Thus far, only a very limited number of potential biomarkers based on free glycan profiling is further developed and used in a real clinical diagnostic test, one example being the GlycoCirrhoTest [74] for distinguishing cirrhotic from non-cirrhotic chronic liver disease patients.
Two major limitations of total serum N-glycan analysis create the basis for improvement: First, the information to which proteins glycans were attached, is lost in total serum N-glycan analysis. Secondly, alterations in glycosylation are biased by fluctuations in the dynamic N-glycoproteome, which are dependent on environmental factors such as an immune response [75–78]. Ideally, profiling methods need to be used that take into account the protein to which the altered glycans are attached, such as profiling of intact glycopeptides from complex biological samples. Recent technical developments and improved databases seem to make this challenge feasible. A combination of collision induced dissociation (CID) and electron-transfer dissociation (ETD) fragmentation techniques is preferred to characterize glycopeptides [79, 80]. While CID provides information related to the composition and structure of glycan moieties attached to a peptide backbone, ETD fragments mainly the peptide backbone and leaves the glycan intact, permitting de novo sequencing of the peptide moiety [81, 82]. Due to the relative low abundance and low ionization efficiency of glycopeptides, glycoproteins need to be enriched, digested and chromatographically separated for this type of analyses. Since the glycan moiety makes the glycopeptide generally more polar, HILIC is a commonly used enrichment strategy for glycopeptides [83]. Other usual approaches are immune-affinity (reactive beads) [84, 85] or lectin-affinity purifications [86, 87]. Furthermore, commercial kits are available to deplete serum samples from the most abundant serum proteins, although that might cause potential biomarkers (see Table 1) to be lost. Combining these techniques enables unambiguous in-depth identification of both the peptide and the glycan. However, the data analysis software is not yet sophisticated enough to enable automated sequencing of peptides and combining search results from both fragmentation techniques for identification. Therefore, intact glycopeptide analysis from complex biological samples is still far from being a routine diagnostic analysis. Within a research environment, glycopeptide profiling is highly promising for the identification of new glycoprotein biomarkers.
One first example of glycopeptide analysis using the combination of CID and ETD fragmentation, is a very recent study of Medzihradszky et al. on tissue-specific glycosylation [58, 88]. They compared the glycosylation patterns of mouse liver with brain tissue and not only found that these patterns differ by the cellular location of the glycoprotein (e.g., ER, lysosomal or transmembrane), but also showed tissue-specific differences for the same protein.
Glycoprotein biomarker application in clinical diagnostics
Diagnostic assays are required to be fast, robust and suitable for high-throughput measurements, while in addition the interpretation in a clinical setting should be straightforward. Free N-glycan analysis meets the technical requirements, however, diagnostic interpretation is highly complicated due to the many causes that influence N-glycan profiles. For some well-defined CDG subtypes with a clear genetic defect, such as B4GALT1-CDG and MGAT2-CDG, interpretation is clear. However, abnormalities in free N-glycan profiles for other defects are more subtle or overlapping with multiple defects. Examples include PGM1-CDG, where the lack of complete glycans is not found in total serum N-glycan profiles and only minimal loss of galactose is not sufficiently specific to reach a diagnosis.
Diagnostic application of glycopeptides is of particular interest, because multiple identified biomarkers could subsequently be used for diagnostic high-throughput screening by means of multiple/serial reaction monitoring (MRM/SRM), similar as is increasingly used for the detection of protein biomarkers in clinical diagnostics. In this sensitive targeted analysis, specific (transitions of) predefined precursor ions are sequentially selected for MS/MS detection in a quantitative and reproducible manner [89]. For instance, Hong et al. developed an MRM analysis to analyze the IgG free glycans in whole serum. By normalizing protein glycosylation to absolute protein concentrations they were able to examine quantitative changes in glycosylation at site-specific level [90], which is crucial to distinguish between altered glycosylation and change in protein levels. Hulsmeier et al. published a MRM method to quantify the N-glycosylation site occupancy for transferrin and α1-antitrypsin, and found out that the site occupancy correlated well with the severity of the disease [91]. Moreover, they observed preferences for specific occupation sites. The method could in principle easily be extended to multiple glycoproteins.
Very recently, Sun et al. proposed a new method to measure absolute protein glycosylation stoichiometry in complex biological samples [92]. They use the relative ratios between the N-glycosylated peptide, the peptide without the glycans and the complete glycoprotein from the same type of biological sample to profile the glycosylation occupancy and glycoform stoichiometry and determined 117 absolute N-glycosylation occupancies in OVCAR-3 cells. Application to CDG has not been tested yet, but might be promising in biomarker discovery.
A specific set of intact glycoproteins would have several advantages over glycopeptide mixtures, mainly improving on the relative quantification. One needs to consider that transferrin is a relative simple glycoprotein to analyze as it has only two glycosylation sites, whereas other proteins might go up to multiple glycosylation sites and increased glycan heterogeneity. Each additional glycosylation site will complicate annotation [83]. In addition, an antibody would be required to affinity-purify the protein from the biological complex sample. The antibody would need to have its epitope outside of any glycosylation motif, to ensure glycosylation-independent purification of all glycoforms.
Overall, each method has its strengths and weaknesses. Targeted, MRM based mass spectrometry of glycopeptides is fast, robust and sensitive, allowing complex mixtures to be analyzed. With intact glycoprotein analysis, a limited number of proteins can be analyzed. However, all glycoforms of one protein are measured simultaneously in the mass spectrometer, which allows relative quantification of glycoforms and easy comparison of both CDG type-I and CDG type-II defects in one spectrum.
Outlook
The ultimate goal in clinical diagnostics of CDG would be to cover the complete field of glycosylation disorders in a single or only few assays. This would not only benefit diagnostics of monogenic, inherited disorders of glycosylation, but also the more common, complex genetic disorders like cancer, Alzheimer or inflammatory diseases. Several diseases show abnormal glycosylation, and an increasing number of glycosylation disorders is identified by next generation sequencing techniques. More and more defects within the newly discovered disorders are found outside the pathway of protein N-glycosylation, for example defects in energy metabolism, or GPI-defects. To include these defects, the repertoire of glycoprotein biomarkers should be expanded, by analyzing a well-defined set of multiple, intact biomarker glycoproteins.
Table 1 provides an overview of glycoprotein biomarkers, which are worth considering for clinical diagnostics of CDG. It lists candidates which are already applied in clinical laboratories for other types of diseases, so addition of these proteins should be feasible. Our preference is to include several proteins in one assay, either as glycopeptides or as intact proteins. The intact transferrin spectra already show the potential to develop a unique classification system for CDG. The spectra provide such a characteristic profile that clinical diagnostics is possible at a glance [20, 43].
When glycoproteins are digested, housekeeping proteins or non-glycosylated peptides of a targeted glycoprotein are necessary for normalization and quantification purposes. In addition, improved ionization and enhanced separation for low abundant glycopeptides might be required. When followed by high-resolution detection and adequate databases for automated searches, automated MRM analyses can be used as a fast diagnostic test. Until then, we propose that intact glycoprotein profiling of well-defined protein mixtures will have the best potential for CDG diagnostics and therapy monitoring in the near future.
In conclusion, simultaneous analysis of multiple glycoproteins would highly increase our knowledge of the glycosylation machinery in different types of cells, and improve our diagnostic power to sensitively and specifically monitor disease in CDG patients. A highly detailed glycosylation read-out of individual proteins would work in synergy with Next Generation Sequencing techniques in the identification of new inherited disorders. Also, the diagnostics of more common disorders shall benefit from the lessons that will be learned from the analysis of complex glycoprotein mixtures.
Acknowledgments
This work was supported by the Dutch Organisation for Scientific Research, NWO (Medium Investment Grant 40-00506-98-9001 and VIDI Grant 91713359 to DL).
Abbreviations
- 2DE
2D-electrophoresis
- apoC-III
apolipoprotein CIII
- CA125
cancer antigen 125
- CA15-3
cancer antigen 15–3
- CA19-9
carbohydrate antigen 19–9
- CA27-29
cancer antigen 27–29
- CD24
cluster of differentiation 24 or heat stable antigen CD24 (HSA)
- CD59
MAC-inhibitory protein (MAC-IP)
- cdg
Congenital Disorders of Glycosylation
- CDG-I
Congenital Disorders of Glycosylation type I
- CDG-II
Congenital Disorders of Glycosylation type II
- CID
collision induced energy
- COPD
Chronic obstructive pulmonary disease
- CRC
Colorectal cancer
- ELISA
Enzyme Linked Immunosorbent Assay
- ELLA
enzyme-linked lectin assay
- ETD
electron transfer dissociation
- FACS
fluorescence activated cell sorting
- FDA
US Food and Drug Administration
- FLISA
Lectin-Fluorophore-linked Immunosorbent Assay
- FRU
fructose
- FUC
fucose
- GAL
galactose
- GDP
guanosine diphosphate
- GFPT1
Glutamine-Fructose-6-Phosphate Transaminase 1
- GLC
glucose
- GNE
UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase
- gpi
glycosylphosphatidylinositol
- HCC
Hepatocellular carcinoma
- hCG
Human chorionic gonadotrophin
- HILIC
Hydrophilic Interaction LIquid Chromatography
- ICAM-1
intercellular cell adhesion molecule 1
- IEF
Iso Electric Focusing
- IgG
Immunoglobulin G
- LAD-II
leukocyte adhesion deficiency II
- LAMP-2
lysosome-associated membrane protein 2
- LC
Liquid Chromatography
- LC-MS
Liquid Chromatography - mass spectrometry
- LLO
Lipid linked oligosaccharide
- MAC
membrane attack complex
- MALDI-TOF
matrix assisted laser desorption ionisation - Time-of-Flight
- MAN
Mannose
- MRM
multiple reaction monitoring
- MUC16
mucin 16 or ovarian cancer-related tumor marker CA125
- NP-HPLC
normal phase - high pressure liquid chromatography
- PSA
prostate specific antigen
- QTOF
quadrupole time of flight
- SRM
single reaction monitoring
- sLeX
Sialyl Lewis X structures
- sLea
Sialyl Lewis A structures
- tief
transferrin isoelectric focusing
- UDP
uridine diphosphate
- UPLC-MS
ultra high pressure liquid chromatography- mass spectrometry
- WAX-HPLC
weak anion exchange high performance liquid chromatography
- ᅟ
List of human genes
- ALG13
subunit of UDP-N-acetylglucosamine transferase complex
- ALG14
subunit of UDP-N-acetylglucosamine transferase complex
- ALG1
mannosyltransferase 1
- ALG8
glucosyltransferase 2
- DOLK
dolichol kinase
- GCS1
mannosyl-oligosaccharide glucosidase
- MAN1B1
alpha-1,2-mannosidase
- MPI
mannose phosphate isomerase
- PGM1
phosphoglucomutase 1
- PGM3
phosphoglucomutase 3
- PIGM
glycosylphosphatidylinositol
- PMI
Phosphomannose isomerase
- PMM2
phosphomannomutase
- SLC35A2
UDP-galactose transporter
- SLC35A3
UDP-N-acetylglucosamine transporter
- SLC35C1
GDP-fucose transmembrane transporter
- TUSC3
Oligosaccharyltransferase TUSC3 subunit
- VPS13B
Vacuolar Protein Sorting 13 Homolog B
References
- 1.Mechref Y, et al. Identifying cancer biomarkers by mass spectrometry-based glycomics. Electrophoresis. 2012;33(12):1755–1767. doi: 10.1002/elps.201100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miwa HE, et al. The bisecting GlcNAc in cell growth control and tumor progression. Glycoconj. J. 2012;29(8–9):609–618. doi: 10.1007/s10719-012-9373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büll C, et al. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013;12(10):1935–1946. doi: 10.1158/1535-7163.MCT-13-0279. [DOI] [PubMed] [Google Scholar]
- 4.Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Dis. Markers. 2008;25(4–5):267–278. doi: 10.1155/2008/493289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schedin‐Weiss S, Winblad B, Tjernberg LO. The role of protein glycosylation in Alzheimer disease. FEBS J. 2014;281(1):46–62. doi: 10.1111/febs.12590. [DOI] [PubMed] [Google Scholar]
- 6.Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- 7.Thanabalasingham G, et al. Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes. 2013;62(4):1329–1337. doi: 10.2337/db12-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeze HH, et al. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 2014;94(2):161–175. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeken J. CDG nomenclature: time for a change! Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(9):825–826. doi: 10.1016/j.bbadis.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaeken, J., van den Heuvel L.: Congenital disorders of glycosylation, in physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Springer. p. 483–512 (2014)
- 11.Hennet T. Diseases of glycosylation beyond classical congenital disorders of glycosylation. Biochimica et Biophysica Acta (BBA)-General Subjects. 2012;1820(9):1306–1317. doi: 10.1016/j.bbagen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Scott K, et al. Congenital disorders of glycosylation: new defects and still counting. J. Inherit. Metab. Dis. 2014;37(4):609–617. doi: 10.1007/s10545-014-9720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grünewald S, Matthijs G, Jaeken J. Congenital disorders of glycosylation: a review. Pediatr. Res. 2002;52(5):618–624. doi: 10.1203/00006450-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe LA, Krasnewich D. Congenital disorders of glycosylation and intellectual disability. Developmental disabilities research reviews. 2013;17(3):211–225. doi: 10.1002/ddrr.1115. [DOI] [PubMed] [Google Scholar]
- 15.Wada Y. Mass spectrometry for congenital disorders of glycosylation, CDG. J. Chromatogr. B. 2006;838(1):3–8. doi: 10.1016/j.jchromb.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Morelle W, Michalski J-C. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007;2(7):1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 17.Guillard M, et al. Automated measurement of permethylated serum N-glycans by MALDI–linear ion trap mass spectrometry. Carbohydr. Res. 2009;344(12):1550–1557. doi: 10.1016/j.carres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Sturiale L, Barone R, Garozzo D. The impact of mass spectrometry in the diagnosis of congenital disorders of glycosylation. J. Inherit. Metab. Dis. 2011;34(4):891–899. doi: 10.1007/s10545-011-9306-8. [DOI] [PubMed] [Google Scholar]
- 19.Lacey JM, et al. Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin. Chem. 2001;47(3):513–518. [PubMed] [Google Scholar]
- 20.Tegtmeyer LC, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N. Engl. J. Med. 2014;370(6):533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthijs G, et al. Approaches to homozygosity mapping and exome sequencing for the identification of novel types of CDG. Glycoconj. J. 2013;30(1):67–76. doi: 10.1007/s10719-012-9445-7. [DOI] [PubMed] [Google Scholar]
- 22.Jaeken J. Congenital disorders of glycosylation. Ann. N. Y. Acad. Sci. 2010;1214(1):190–198. doi: 10.1111/j.1749-6632.2010.05840.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaeken J, et al. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin. Chim. Acta. 1984;144(2):245–247. doi: 10.1016/0009-8981(84)90059-7. [DOI] [PubMed] [Google Scholar]
- 24.Helander A, Husa A, Jeppsson J-O. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin. Chem. 2003;49(11):1881–1890. doi: 10.1373/clinchem.2003.023341. [DOI] [PubMed] [Google Scholar]
- 25.Carchon HA, et al. Diagnosis of congenital disorders of glycosylation by capillary zone electrophoresis of serum transferrin. Clin. Chem. 2004;50(1):101–111. doi: 10.1373/clinchem.2003.021568. [DOI] [PubMed] [Google Scholar]
- 26.Sanz‐Nebot V, et al. Characterization of transferrin glycoforms in human serum by CE-UV and CE-ESI-MS. Electrophoresis. 2007;28(12):1949–1957. doi: 10.1002/elps.200600648. [DOI] [PubMed] [Google Scholar]
- 27.Lefeber DJ, Morava E, Jaeken J. How to find and diagnose a CDG due to defective N-glycosylation. J. Inherit. Metab. Dis. 2011;34(4):849–852. doi: 10.1007/s10545-011-9370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albahri Z, et al. Genetic variants of transferrin in the diagnosis of protein hypoglycosylation. J. Inherit. Metab. Dis. 2005;28(6):1184–1188. doi: 10.1007/s10545-005-0113-y. [DOI] [PubMed] [Google Scholar]
- 29.Zühlsdorf A, et al. Transferrin variants: Pitfalls in the diagnostics of congenital disorders of glycosylation. Clin. Biochem. 2015;48(1):11–13. doi: 10.1016/j.clinbiochem.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Kühnl P, Spielmann W. Transferrin: evidence for two common subtypes of the TfC allele. Hum. Genet. 1978;43(1):91–95. doi: 10.1007/BF00396483. [DOI] [PubMed] [Google Scholar]
- 31.van Scherpenzeel, M., et al.: High-resolution Qtof-MS glycoprofiling of intact transferrin for diagnosis and subtype identification in the congenital disorders of glycosylation. Translational Res, (2015) [DOI] [PubMed]
- 32.Jaeken J. Congenital disorders of glycosylation (CDG): it’s (nearly) all in it! J. Inherit. Metab. Dis. 2011;34(4):853–858. doi: 10.1007/s10545-011-9299-3. [DOI] [PubMed] [Google Scholar]
- 33.Gao N, Lehrman MA. Analyses of dolichol pyrophosphate–linked oligosaccharides in cell cultures and tissues by fluorophore-assisted carbohydrate electrophoresis. Glycobiology. 2002;12(5):353–360. doi: 10.1093/glycob/12.5.353. [DOI] [PubMed] [Google Scholar]
- 34.Timal S, et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum. Mol. Genet. 2012;21(19):4151–4161. doi: 10.1093/hmg/dds123. [DOI] [PubMed] [Google Scholar]
- 35.Wopereis S, et al. Transferrin and apolipoprotein C-III isofocusing are complementary in the diagnosis of N-and O-glycan biosynthesis defects. Clin. Chem. 2007;53(2):180–187. doi: 10.1373/clinchem.2006.073940. [DOI] [PubMed] [Google Scholar]
- 36.Bruneel A, et al. Two-dimensional gel electrophoresis of apolipoprotein C-III and other serum glycoproteins for the combined screening of human congenital disorders of O-and N-glycosylation. PROTEOMICS-Clinical Applications. 2007;1(3):321–324. doi: 10.1002/prca.200600777. [DOI] [Google Scholar]
- 37.Wada Y, Tajiri M, Yoshida S. Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal. Chem. 2004;76(22):6560–6565. doi: 10.1021/ac049062o. [DOI] [PubMed] [Google Scholar]
- 38.Guillard M, et al. Plasma N-glycan profiling by mass spectrometry for congenital disorders of glycosylation type II. Clin. Chem. 2011;57(4):593–602. doi: 10.1373/clinchem.2010.153635. [DOI] [PubMed] [Google Scholar]
- 39.Xia B, et al. Serum N-glycan and O-glycan analysis by mass spectrometry for diagnosis of congenital disorders of glycosylation. Anal. Biochem. 2013;442(2):178–185. doi: 10.1016/j.ab.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Wada Y, Kadoya M, Okamoto N. Mass spectrometry of apolipoprotein C-III, a simple analytical method for mucin-type O-glycosylation and its application to an autosomal recessive cutis laxa type-2 (ARCL2) patient. Glycobiology. 2012;22(8):1140–1144. doi: 10.1093/glycob/cws086. [DOI] [PubMed] [Google Scholar]
- 41.Faid V, et al. A rapid mass spectrometric strategy for the characterization of N-and O-glycan chains in the diagnosis of defects in glycan biosynthesis. Proteomics. 2007;7(11):1800–1813. doi: 10.1002/pmic.200600977. [DOI] [PubMed] [Google Scholar]
- 42.Yen-Nicolaÿ, S., et al.: MALDI-TOF MS applied to apoC-III glycoforms of patients with congenital disorders affecting O-glycosylation. Comparison with two-dimensional electrophoresis. PROTEOMICS-Clinical Applications, (2015) [DOI] [PubMed]
- 43.Van Scherpenzeel M, et al. Diagnostic serum glycosylation profile in patients with intellectual disability as a result of MAN1B1 deficiency. Brain. 2014;137(4):1030–1038. doi: 10.1093/brain/awu019. [DOI] [PubMed] [Google Scholar]
- 44.Freeze HH. Towards a therapy for phosphomannomutase 2 deficiency, the defect in CDG-Ia patients. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(9):835–840. doi: 10.1016/j.bbadis.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Lonlay P, Seta N. The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(9):841–843. doi: 10.1016/j.bbadis.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Marquardt T, et al. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94(12):3976–3985. [PubMed] [Google Scholar]
- 47.Almeida A, Layton M, Karadimitris A. Inherited glycosylphosphatidyl inositol deficiency: a treatable CDG. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(9):874–880. doi: 10.1016/j.bbadis.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Almeida AM, et al. Targeted therapy for inherited GPI deficiency. N. Engl. J. Med. 2007;356(16):1641–1647. doi: 10.1056/NEJMoa063369. [DOI] [PubMed] [Google Scholar]
- 49.Freeze HH, Aebi M. Molecular basis of carbohydrate-deficient glycoprotein syndromes type I with normal phosphomannomutase activity. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1999;1455(2):167–178. doi: 10.1016/S0925-4439(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 50.Janssen MC, et al. Successful liver transplantation and long-term follow-up in a patient with MPI-CDG. Pediatrics. 2014;134(1):e279–e283. doi: 10.1542/peds.2013-2732. [DOI] [PubMed] [Google Scholar]
- 51.Quick CB, Fisher RA, Harris H. A kinetic study of the isozymes determined by the three human phosphoglucomutase loci PGM1, PGM2 and PGM3. Eur. J. Biochem. 1974;42(2):511–517. doi: 10.1111/j.1432-1033.1974.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 52.Etzioni A, et al. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am. J. Med. Genet. 2002;110(2):131–135. doi: 10.1002/ajmg.10423. [DOI] [PubMed] [Google Scholar]
- 53.Marquardt T, et al. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J. Pediatr. 1999;134(6):681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cagdas D, et al. A novel mutation in leukocyte adhesion deficiency type II/CDGIIc. J. Clin. Immunol. 2014;34(8):1009–1014. doi: 10.1007/s10875-014-0091-7. [DOI] [PubMed] [Google Scholar]
- 55.Maeda Y, et al. PIG-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. The EMBO journal. 2001;20(1–2):250–261. doi: 10.1093/emboj/20.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varki, A., et al.: Essentials of glycobiology. 2nd ed. Chapter 13: Cold Spring (2009)
- 57.Zielinska DF, et al. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell. 2010;141(5):897–907. doi: 10.1016/j.cell.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Medzihradszky KF, Kaasik K, Chalkley RJ. Tissue-specific glycosylation at the glycopeptide level. Mol. Cell. Proteomics. 2015;M115 doi: 10.1074/mcp.M115.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadat MA, et al. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N. Engl. J. Med. 2014;370(17):1615–1625. doi: 10.1056/NEJMoa1302846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duplomb, L., et al.: Cohen syndrome is associated with major glycosylation defects. Human molecular genetics, p. ddt630 (2013) [DOI] [PubMed]
- 61.Testa R, et al. N-Glycomic changes in serum proteins in type 2 diabetes mellitus correlate with complications and with metabolic syndrome parameters. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitazume S. Glycosylation of amyloid β precursor protein. Glycoscience: Biology and Medicine; 2015. pp. 1283–1288. [Google Scholar]
- 63.Albrecht S, et al. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark. 2014;14:17–28. doi: 10.3233/CBM-130373. [DOI] [PubMed] [Google Scholar]
- 64.Angata T, et al. Integrated approach toward the discovery of glyco-biomarkers of inflammation-related diseases. Ann. N. Y. Acad. Sci. 2012;1253(1):159–169. doi: 10.1111/j.1749-6632.2012.06469.x. [DOI] [PubMed] [Google Scholar]
- 65.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochimica et Biophysica Acta (BBA)-General Subjects. 2012;1820(9):1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer. 2005;5(11):845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 67.Shinohara, Y., Furukawa, J.-I., Miura Y.: Glycome as biomarkers. General Methods Biomarker Res their Appl, p. 111–140 (2015)
- 68.Kirwan, A., et al.: Glycosylation-based serum biomarkers for cancer diagnostics and prognostics. BioMed Res Int, 2015. (2015) [DOI] [PMC free article] [PubMed]
- 69.Chu CS, et al. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9(7):1939. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song T, Aldredge D, Lebrilla CB. A method for in-depth structural annotation of human serum glycans that yields biological variations. Anal. Chem. 2015;87(15):7754–7762. doi: 10.1021/acs.analchem.5b01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leymarie N, Zaia J. Effective use of mass spectrometry for glycan and glycopeptide structural analysis. Anal. Chem. 2012;84(7):3040–3048. doi: 10.1021/ac3000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wuhrer M. Glycomics using mass spectrometry. Glycoconj. J. 2013;30(1):11–22. doi: 10.1007/s10719-012-9376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoeckmann, H., et al.: Ultra-high throughput, ultrafiltration-based N-glycomics platform for ultra-performance liquid chromatography (ULTRA3). Analytical Chem, (2015) [DOI] [PubMed]
- 74.Callewaert N, et al. Noninvasive diagnosis of liver cirrhosis using DNA sequencer–based total serum protein glycomics. Nat. Med. 2004;10(4):429–434. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 75.Ota H, et al. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433(5):419–426. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 76.Tangvoranuntakul P, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. 2003;100(21):12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knezevic A, et al. Variability, heritability and environmental determinants of human plasma N-glycome. J. Proteome Res. 2008;8(2):694–701. doi: 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- 78.Butler M, et al. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology. 2003;13(9):601–622. doi: 10.1093/glycob/cwg079. [DOI] [PubMed] [Google Scholar]
- 79.Alley WR, Mechref Y, Novotny MV. Characterization of glycopeptides by combining collision-induced dissociation and electron-transfer dissociation mass spectrometry data. Rapid Commun. Mass Spectrom. 2009;23(1):161–170. doi: 10.1002/rcm.3850. [DOI] [PubMed] [Google Scholar]
- 80.Mechref, Y.: Use of CID/ETD mass spectrometry to analyze glycopeptides. Current Protocols Protein Sci: p. 12.11. 1–12.11. 11 (2012) [DOI] [PMC free article] [PubMed]
- 81.Wuhrer M, et al. Glycoproteomics based on tandem mass spectrometry of glycopeptides. J. Chromatogr. B. 2007;849(1):115–128. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 82.Ma, B., Johnson R.: De novo sequencing and homology searching. Mol. Cell. Proteomics. 11(2): p. O111. 014902 (2012) [DOI] [PMC free article] [PubMed]
- 83.Khatri K, et al. Confident assignment of site-specific glycosylation in complex glycoproteins in a single step. J. Proteome Res. 2014;13(10):4347–4355. doi: 10.1021/pr500506z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whiteaker, J.R., et al.: Sequential multiplexed analyte quantification using peptide immunoaffinity enrichment coupled to mass spectrometry. Molecular & Cellular Proteomics. 11(6): p. M111. 015347 (2012) [DOI] [PMC free article] [PubMed]
- 85.Ongay S, et al. Glycopeptide enrichment and separation for protein glycosylation analysis. J. Sep. Sci. 2012;35(18):2341–2372. doi: 10.1002/jssc.201200434. [DOI] [PubMed] [Google Scholar]
- 86.Yang Z, Hancock WS. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J. Chromatogr. A. 2004;1053(1):79–88. doi: 10.1016/S0021-9673(04)01433-5. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Wu S-l, Hancock WS. Approaches to the study of N-linked glycoproteins in human plasma using lectin affinity chromatography and nano-HPLC coupled to electrospray linear ion trap—Fourier transform mass spectrometry. Glycobiology. 2006;16(6):514–523. doi: 10.1093/glycob/cwj091. [DOI] [PubMed] [Google Scholar]
- 88.Trinidad JC, et al. N-and O-glycosylation in the murine synaptosome. Mol. Cell. Proteomics. 2013;12(12):3474–3488. doi: 10.1074/mcp.M113.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Picotti P, Aebersold R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods. 2012;9(6):555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 90.Hong Q, et al. Absolute quantitation of immunoglobulin G and its glycoforms using multiple reaction monitoring. Anal. Chem. 2013;85(18):8585–8593. doi: 10.1021/ac4009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hülsmeier AJ, Paesold-Burda P, Hennet T. N-glycosylation site occupancy in serum glycoproteins using multiple reaction monitoring liquid chromatography-mass spectrometry. Mol. Cell. Proteomics. 2007;6(12):2132–2138. doi: 10.1074/mcp.M700361-MCP200. [DOI] [PubMed] [Google Scholar]
- 92.Sun, S., Zhang H.: Large-scale measurement of absolute protein glycosylation stoichiometry. Analytical Chem, (2015) [DOI] [PMC free article] [PubMed]
- 93.Gornik O, et al. Change of transferrin sialylation differs between mild sepsis and severe sepsis and septic shock. Intern. Med. 2011;50(8):861–869. doi: 10.2169/internalmedicine.50.4704. [DOI] [PubMed] [Google Scholar]
- 94.Kontro H, et al. Comparison of sialylated N-glycopeptide levels in serum of pancreatic cancer patients, acute pancreatitis patients, and healthy controls. Proteomics. 2014;14(15):1713–1723. doi: 10.1002/pmic.201300270. [DOI] [PubMed] [Google Scholar]
- 95.Abraham J. OVA1 test for preoperative assessment of ovarian cancer. Commun. Oncol. 2010;7(6):249–250. doi: 10.1016/S1548-5315(11)70565-4. [DOI] [Google Scholar]
- 96.Stockley RA, Turner AM. α-1-Antitrypsin deficiency: clinical variability, assessment, and treatment. Trends Mol. Med. 2014;20(2):105–115. doi: 10.1016/j.molmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Comunale, M.A., et al.: Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. (2010) [DOI] [PMC free article] [PubMed]
- 98.Liang, Y., et al.: Differentially expressed glycosylated patterns of alpha-1-antitrypsin as serum biomarkers for the diagnosis of lung cancer. Glycobiology: p. cwu115 (2014) [DOI] [PubMed]
- 99.Arnold JN, et al. Novel glycan biomarkers for the detection of lung cancer. J. Proteome Res. 2011;10(4):1755–1764. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 100.Saldova R, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17(12):1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 101.Bones J, et al. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J. Proteome Res. 2010;10(3):1246–1265. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 102.Okuyama N, et al. Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer. 2006;118(11):2803–2808. doi: 10.1002/ijc.21728. [DOI] [PubMed] [Google Scholar]
- 103.Miyoshi E, Nakano M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures. Proteomics. 2008;8(16):3257–3262. doi: 10.1002/pmic.200800046. [DOI] [PubMed] [Google Scholar]
- 104.Saroha A, et al. Altered glycosylation and expression of plasma alpha-1-acid glycoprotein and haptoglobin in rheumatoid arthritis. J. Chromatogr. B. 2011;879(20):1839–1843. doi: 10.1016/j.jchromb.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 105.Humphries JM, et al. Identification and validation of novel candidate protein biomarkers for the detection of human gastric cancer. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2014;1844(5):1051–1058. doi: 10.1016/j.bbapap.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 106.Comunale MA, et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J. Proteome Res. 2008;8(2):595–602. doi: 10.1021/pr800752c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarrats A, et al. Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. PROTEOMICS-Clinical Applications. 2010;4(4):432–448. doi: 10.1002/prca.200900150. [DOI] [PubMed] [Google Scholar]
- 108.Lieberman J, et al. Serum α1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol. Aging. 1995;16(5):747–753. doi: 10.1016/0197-4580(95)00056-K. [DOI] [PubMed] [Google Scholar]
- 109.Saso L, et al. Abnormal glycosylation of α2-macroglobulin, a non-acute-phase protein, in patients with autoimmune diseases. Inflammation. 1993;17(4):465–479. doi: 10.1007/BF00916586. [DOI] [PubMed] [Google Scholar]
- 110.Mehta A, Block TM. Fucosylated glycoproteins as markers of liver disease. Dis. Markers. 2008;25(4–5):259–265. doi: 10.1155/2008/264594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fardet L, et al. Low glycosylated ferritin, a good marker for the diagnosis of hemophagocytic syndrome. Arthritis & Rheumatism. 2008;58(5):1521–1527. doi: 10.1002/art.23415. [DOI] [PubMed] [Google Scholar]
- 112.Yin H, et al. Mass-selected site-specific core-fucosylation of ceruloplasmin in alcohol-related hepatocellular carcinoma. J. Proteome Res. 2014;13(6):2887–2896. doi: 10.1021/pr500043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Comunale MA, et al. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J. Proteome Res. 2006;5(2):308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 114.Balmaña M, et al. Identification of potential pancreatic cancer serum markers: increased sialyl-Lewis X on ceruloplasmin. Clin. Chim. Acta. 2015;442:56–62. doi: 10.1016/j.cca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 115.Baudin E, et al. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. The Journal of Clinical Endocrinology & Metabolism. 2003;88(3):1107–1111. doi: 10.1210/jc.2002-021365. [DOI] [PubMed] [Google Scholar]
- 116.Mohamed M, et al. Thyroid function in PMM2-CDG: diagnostic approach and proposed management. Mol. Genet. Metab. 2012;105(4):681–683. doi: 10.1016/j.ymgme.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Macchia PE, et al. Thyroid function tests and characterization of thyroxine-binding globulin in the carbohydrate-deficient glycoprotein syndrome type I. The Journal of Clinical Endocrinology & Metabolism. 1995;80(12):3744–3749. doi: 10.1210/jcem.80.12.8530628. [DOI] [PubMed] [Google Scholar]
- 118.Ross DS. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol. Metab. Clin. N. Am. 2001;30(2):245–264. doi: 10.1016/S0889-8529(05)70186-9. [DOI] [PubMed] [Google Scholar]
- 119.Gbormittah, F.O., et al.: Clusterin glycopeptide variant characterization reveals significant site-specific glycan changes in the plasma of clear cell renal cell carcinoma. J Proteome Res, (2015) [DOI] [PubMed]
- 120.Kakisaka T, et al. Plasma proteomics of pancreatic cancer patients by multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis (2D-DIGE): up-regulation of leucine-rich alpha-2-glycoprotein in pancreatic cancer. J. Chromatogr. B. 2007;852(1):257–267. doi: 10.1016/j.jchromb.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Reeuwijk J, et al. POMT2 mutations cause α-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J. Med. Genet. 2005;42(12):907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu Y, et al. Plasma glycoprotein profiling for colorectal cancer biomarker identification by lectin glycoarray and lectin blot. J. Proteome Res. 2008;7(4):1693–1703. doi: 10.1021/pr700706s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang J, et al. Identification of kininogen-1 as a serum biomarker for the early detection of advanced colorectal adenoma and colorectal cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng, Z., et al.: Kallistatin, a new and reliable biomarker for the diagnosis of liver cirrhosis. Acta Pharmaceutica Sinica B, (2015) [DOI] [PMC free article] [PubMed]
- 125.Dieplinger H, Dieplinger B. Afamin—A pleiotropic glycoprotein involved in various disease states. Clin. Chim. Acta. 2015;446:105–110. doi: 10.1016/j.cca.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 126.Gilgunn S, et al. Aberrant PSA glycosylation—a sweet predictor of prostate cancer. Nature Reviews Urology. 2013;10(2):99–107. doi: 10.1038/nrurol.2012.258. [DOI] [PubMed] [Google Scholar]