Abstract
We describe a case of a spinal fusion site infection, which was first suggested on plain radiographs due to interval displacement and partial dissolution of the bone graft material. These radiographic findings occurred 4 weeks before the infection became clinically evident. Cultures taken during eventual surgical debridement grew Aspergillus fumigatus. This case emphasizes the importance of noting changes in bone graft material in addition to the routine evaluation of alignment and hardware in patients who have undergone posterior spinal fusion.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging
Introduction
Surgical site infection is an early complication following posterior spinal fusion, and is reported in 1 to 11% of cases [1]. Detection of post-operative infection is usually based on clinical and laboratory evaluation [2, 3]. Although radiographic examinations are often performed in the setting of infection, they are not generally used to initially diagnose surgical site infection. We report a case where conventional radiographs suggested the diagnosis of wound infection before it became clinically apparent.
Case Report
A 57-year-old man with a past surgical history of L4-S1 posterior fusion with a distraction construct 33 years prior presented with increasing back pain, lower extremity pain, and weakness. Past medical history was unremarkable for diabetes or any immunosuppressive disease. A computed tomography (CT) myelogram showed severe stenosis at L3-4 and mild to moderate stenosis at L2-3. An L2-L5 posterior fusion with pedicle screws and bridging rods was performed with L3 and L4 laminectomies. A mixture of morselized bone, Osteoset® pellets (a medical grade of calcium sulfate bone graft substitute), and vancomycin was placed adjacent to decorticated posterior elements from L2 to the sacrum.
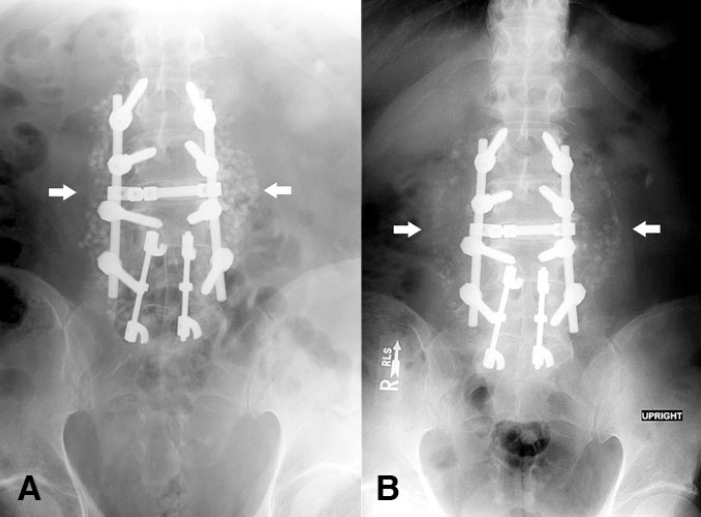
Immediate postoperative radiographs showed the new L2-L5 pedicle screws and rods, the prior L4-S1 distraction rods, and the mixture of bone graft and Osteoset® in expected position without complications (Figure 1A). Early postoperative course was uneventful. Routine radiographs obtained 2 weeks after fusion showed the fusion hardware in the expected position. However, the mixture of morselized bone and Osteoset® was now displaced laterally with more separation of the particles than was seen on the previous radiographs. These findings were thought to reflect a seroma or possibly hematoma (Figure 1B). At this time, the patient was clinically well, with no fever, wound erythema, or drainage, so abscess was considered unlikely.
Figure 1.
A. AP radiograph demonstrates new spinal hardware between L2 and L5 and old spinal hardware between L4 and S1 with no abnormalities. Posterior spinal bone graft is in expected position. B. AP radiograph obtained 2 weeks later demonstrates that the morselized bone and Osteoset® mixture on both sides of the spine has moved laterally and is now much less distinct. [PowerPoint Slide]
Six weeks after fusion, erythema at the inferior aspect of the wound was noted on clinical examination. Aspiration was attempted in the spine clinic without imaging guidance, but no fluid could be initially aspirated. A small incision was then performed over this site, which revealed purulent fluid. The patient next underwent operative debridement and a large amount of purulent fluid was found within the surgical site. Cultures obtained at that time revealed Aspergillus fumigatus. The patient was taken back to the operating room for a second washout with removal of all the loose bone graft and devitalized tissue. The patient was started on a long course of voriconazole and had an uneventful recovery.
Discussion
Posterior spinal fixation is performed for many reasons, including trauma, degenerative disc disease, spinal stenosis, and scoliosis [3]. Conventional radiographs are the most common radiologic examination obtained after spinal fixation. These are useful for evaluating spinal hardware, alignment, and bone graft consolidation [2, 4]. By 6-12 weeks following surgery, progressive consolidation of the bone graft is expected [5], with solidification after 6 to 9 months [6].
Surgical site infection is commonly diagnosed by clinical findings such as increasing back pain, fever and wound erythema, as well as laboratory abnormalities such as increased erythrocyte sedimentation rate, elevated white count, and positive wound cultures [2, 3].
Conventional radiographs are often performed in the setting of a wound infection to evaluate the underlying bone, spinal hardware, and alignment, but are not generally used to initially diagnose infection [2]. Ultrasound, computed tomography (CT), and magnetic resonance (MR) imaging are useful in demonstrating fluid collections in the operative site, and may be helpful in diagnosing a hematoma. However, these modalities are not as useful in differentiating infected from sterile fluid collections [7, 8]. CT and MR images are also often limited by artifact from the metal hardware [8]. Fluid aspiration or surgical exploration of the site is ultimately required for diagnosis [7, 8], although these procedures can be performed with imaging guidance. In our case, the partial interval dissolution of the bone graft increased the likelihood that the fluid collection represented a postoperative abscess.
Aspergillus species are ubiquitous, occurring most frequently in soil, water and decaying vegetation [9]. Invasive aspergillosis is usually caused by Aspergillus fumigatus, and to a lesser extent, by Aspergillus flavus, Aspergillus niger and Aspergillus terreus [10]. Most Aspergillus infections are acquired through the respiratory tract and most cases of Aspergillus infection involve the lungs [11]. The most common extrapulmonary sites for aspergillosis are the brain, heart, kidney and gastrointestinal tract [12].
Aspergillus osteomyelitis is rare, and its most common location is in the spine, particularly in the lumbar spine. Vertebral osteomyelitis may occur from either direct extension of a pulmonary focus or hematogenous spread from a distant site. About two thirds of these cases occur in patients with predisposing conditions for opportunistic infections, such as impaired granulocyte function, chemotherapy for malignancy and organ transplantation, previous pulmonary infection, and corticosteroid usage [10, 12, 13]. However, the remaining one third, including the patient we describe, have no known predisposing conditions.
Aspergillus osteomyelitis is primarily treated medically with antibiotics alone. Voriconazole currently offers the best prospect for success and tolerance as a first-line treatment for aspergillosis [13]. Second-line therapies include lipid formulations of amphotericin B, caspofungin, or intravenous itraconazole. Patients such as ours with large paravertebral abscesses may also require surgical drainage and debridement. A literature review by Vinas et. al. [12] reported an overall recovery rate of 68.3%, with a mortality rate of 26.8%.
In our case, the follow-up postoperative radiographs showed lateral displacement and separation of the morselized bone and Osteoset® mixture when compared to the initial postoperative film. This movement suggested the possibility of a fluid collection displacing the graft material – in this case an abscess. We have been unable to find prior reports of this finding. The development of partial dissolution of the bone graft material provided evidence suggestive of infection. This case demonstrates an atypical finding on plain films that suggested the diagnosis before it was clinically evident, and emphasizes the importance of noting changes in bone graft material in addition to the routine evaluation of alignment and hardware in patients who have undergone posterior spinal fusion.
Footnotes
Competing Interests: The authors declare that no competing interests exist.
Published: January 29, 2006
References
- 1.Banco SP, Vaccaro AR, Blam O. Spine infections: variations in incidence during the academic year. Spine. 2002;27:962–965. doi: 10.1097/00007632-200205010-00016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Rubayi S. Wound management in spinal infection. Orthop Clin North Am. 1996;27:137–153. [PubMed] [PubMed] [Google Scholar]
- 3.Theiss SM, Lonstein JE, Winter RB. Wound infections in reconstructive spine surgery. Orthop Clin North Am. 1996;27:105–110. [PubMed] [PubMed] [Google Scholar]
- 4.Slone RM, MacMillan M, Montgomery WJ. Spinal fixation. Part 3. Complications of spinal instrumentation. Radiographics. 1993;13:797–816. doi: 10.1148/radiographics.13.4.8356269. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Hilibrand AS, Dina TS. The use of diagnostic imaging to assess spinal arthrodesis. Orthop Clin North Am. 1998;29:591–601. doi: 10.1016/s0030-5898(05)70033-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Foley MJ, Calenoff L, Hendrix RW, Schafer MF. Thoracic and lumbar spine fusion: postoperative radiologic evaluation. AJR Am J Roentgenol. 1983;141:373–380. doi: 10.2214/ajr.141.2.373. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Korge A, Fischer R, Kluger P, Puhl W. The importance of sonography in the diagnosis of septic complications following spinal surgery. Eur Spine J. 1994;3:303–307. doi: 10.1007/BF02200141. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Rothman SL. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am. 1996;27:15–31. [PubMed] [PubMed] [Google Scholar]
- 9.Garber G. An overview of fungal infections. Drugs. 2001;61(Suppl 1):1–12. doi: 10.2165/00003495-200161001-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56(Suppl 1):i5–i11. doi: 10.1093/jac/dki218. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Patterson TF, Kirkpatrick WR, White M. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Vinas FC, King PK, Diaz FG. Spinal aspergillus osteomyelitis. Clin Infect Dis. 1999;28:1223–1229. doi: 10.1086/514774. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Wingard JR, Leather H. A new era of antifungal therapy. Biol Blood Marrow Transplant. 2004;10:73–90. doi: 10.1016/j.bbmt.2003.09.014. [PubMed] [DOI] [PubMed] [Google Scholar]