Abstract
Nodular fasciitis is a benign fibroblastic lesion that was historically misdiagnosed as a malignant neoplasm. Patients present with pain and swelling of relatively brief duration. The clinical presentation is suggestive of an aggressive lesion, usually occurring in muscle fascia. Histologic features can cause it to be mistaken for sarcoma. After the diagnosis is established histologically, observation is the suggested treatment. We present the case of a patient who had a large soft-tissue tumor in the upper arm with a clinical picture indicative of sarcoma, which ultimately was diagnosed as nodular fasciitis. The patient was treated with anti-inflammatory agents and observation. Within 7 months, the mass almost completely resolved, as documented by magnetic resonance imaging.
Abbreviations: MRI, magnetic resonance imaging
Introduction
Nodular (pseudosarcomatous) fasciitis is a benign proliferation of fibroblasts frequently mistaken as a sarcoma because of its rapid growth, rich cellularity, and mitotic activity. Although nodular fasciitis is well accepted as a self-limited reactive process and not a true neoplasm, its precise cause is unknown [1, 2, 3, 4, 5]. Most lesions are solitary and occur in adults 20 to 40 years of age [1, 6, 7, 8]. Nodular fasciitis affects both men and women with equal frequency. It is found most commonly in the forearm (27%-29%), back or chest wall (15%-18%), and upper arm (12%) [1, 6, 7, 8]. In the forearm, it usually presents on the flexor surface [1, 6, 7, 8]. Most patients have a history of a painful, rapidly growing mass or nodule that has been present only a few weeks. Patients may develop numbness or paresthesias if the mass exerts pressure on a peripheral nerve.
Case Report
A 34-year-old, right-hand-dominant man presented for evaluation of a proximal lesion in his right arm. Six weeks earlier, the patient had noted pain in the deltoid region of the right arm after he assembled and moved some furniture. Two weeks later, the patient noticed a lump over the posterior aspect of his upper arm. He had constant discomfort in his arm, exacerbated somewhat by physical activity. The patient reported that even typing produced a dull ache over the lesion. He denied any proximal or distal radiation of pain. He had no history of fever, sweats, chills, or weight change.
Physical examination showed that the patient had full symmetric range of motion in the shoulders, elbows, wrists and digits. He showed no lags in flexion or extension. Inspection revealed obvious swelling along the posteromedial aspect of the upper arm just beyond the axilla. On palpation, the lesion was found to be about 5 cm wide at its greatest diameter. It appeared to be located deep within the musculature of the triceps, just posterior to the major neurovascular structures and near the axillary fold. It was tender when palpated. Palpation produced no paresthesia. It was minimally mobile both medial to lateral and proximal to distal, and it did not seem affixed to bone. Tinel sign was absent over the brachial plexus and the major nerves immediately adjacent to the lesion. The axillary and brachial pulses were easily palpable, and the vascular bundle was mobile relative to the lesion. There was no axillary or supraclavicular lymphadenopathy.
Radiographs showed nonspecific soft tissue fullness in the posterior aspect of the upper arm. A chest radiograph was normal. An MRI demonstrated a 5.8(4.9(3.2-cm mass located between the heads of the triceps muscle (Fig 1). The mass showed heterogeneous increased T1 and T2-weighted signal with intense inhomogeneous enhancement. T2-weighted images were degraded by patient motion. The mass was predominantly well-circumscribed but had less well-circumscribed borders distally, adjacent to the deep brachial artery and radial nerve. There was no surrounding soft tissue edema. The initial radiologic interpretation favored a soft-tissue sarcoma. The proximity of the lesion to the radial nerve raised the possibility of peripheral nerve sheath tumor.
Figure 1.
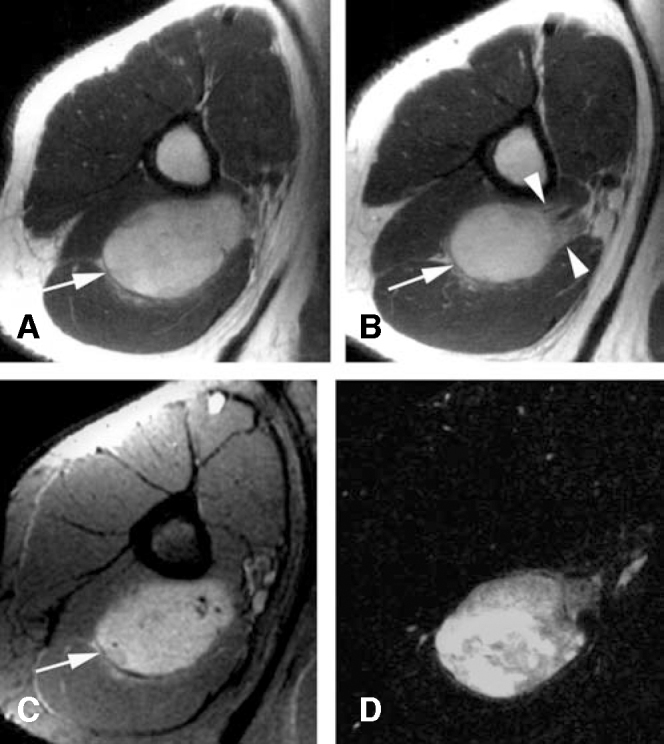
Initial MRI of the upper right arm. A-B, Axial T1-weighted images through the proximal (A) and distal (B) aspects of the mass demonstrate a heterogeneous mass of predominantly high signal. The mass (arrow) has well-defined borders proximally that become ill-defined distally (arrowheads), adjacent to the neurovascular bundle. C, Axial T1-weighted, fat-suppressed image acquired after IV gadolinium demonstrates inhomogeneous enhancement. D, Post-processed subtraction image confirms intense enhancement. [PowerPoint Slide]
A biopsy specimen taken with a Tru-Cut needle (Baxter Healthcare Corp, Deerfield, Illinois) was interpreted as nodular fasciitis. An open incisional biopsy was performed to confirm the diagnosis. The surgical approach took into consideration the clinical likelihood of sarcoma, thus avoiding the potential for limb loss in the event of an inaccurate core needle biopsy diagnosis. The open biopsy produced samples of firm pink-and-yellow tissue. Histopathologic findings again demonstrated nodular fasciitis. Microscopically, the tumor consisted mostly of plump, immature-looking fibroblasts in short irregular bundles and fascicles accompanied by a dense reticulin meshwork containing a small amount of mature collagen (Fig. 2). Among the fibroblasts and erythrocytes were lymphoid cells, lipid macrophages, and multinucleated giant cells.
Figure 2.
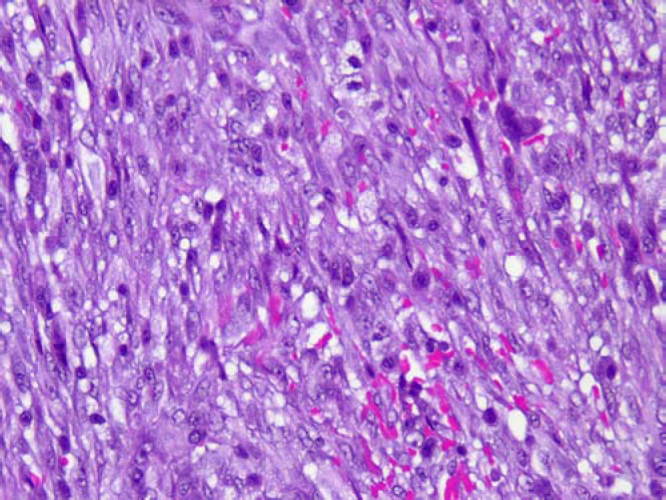
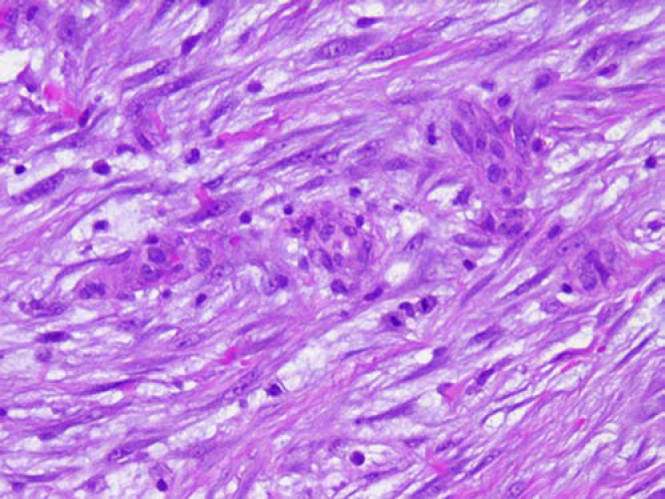
A, Microscopic appearance of the proliferation of cellular spindle cells in short fascicles (Hematoxylin-eosin; x10). B, High-power view of fusiform cells shows uniform nuclei, admixed with extravasated erythrocytes and keloid-type collagen (Hematoxylin-eosin; x40). [PowerPoint Slide]
After the diagnosis of nodular fasciitis was confirmed with open biopsy, surgical excision was not recommended. Instead, the patient was treated with ibuprofen (600 mg, 3 times daily for 6 weeks) and observation. In the ensuing weeks, he reported progressive decrease in the size of the mass and in the concomitant pain. At 11-week follow-up, the patient was asymptomatic, and the mass was no longer clinically palpable despite the presence of slightly thickened tissue at the site from the open biopsy. A repeat MRI seven months after the incisional biopsy demonstrated near resolution of the mass. An irregular sub-centimeter region of abnormal increased T1 and T2-weighted signal remained present in the region of the prior 5.8 cm mass (Fig. 3).
Figure 3.
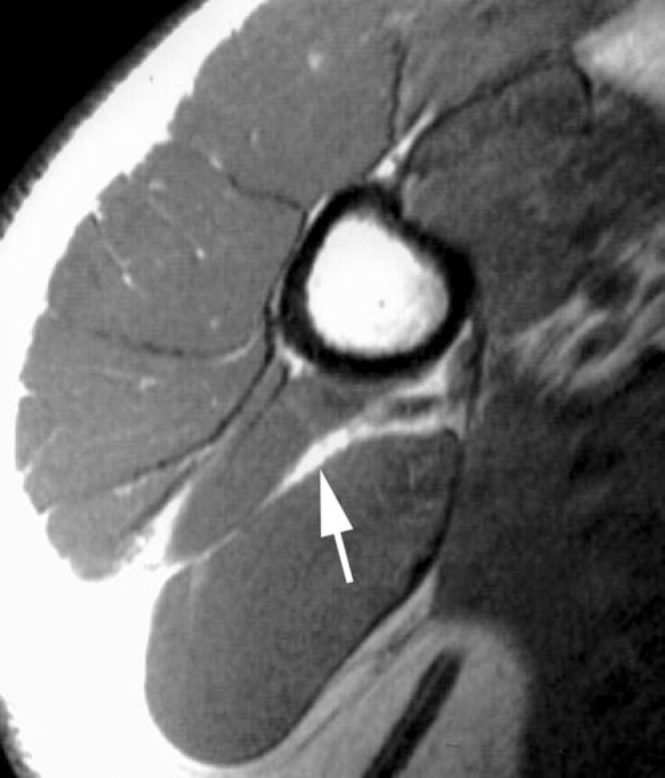
Axial T1-weighted MRI performed seven months later shows near complete resolution of the mass. A residual sub-centimeter lesion (arrow) has high signal similar to the original mass. [PowerPoint Slide]
Discussion
Nodular fasciitis, with characteristic pseudosarcomatous features, is considered a benign, reactive fibroblastic growth. Konwaler et al [9] were the first to report cases of this lesion, and they suggested that it be considered in a distinct class of its own. Further studies showed that the lesions were a benign proliferation of fibroblasts and myofibroblasts of unknown causes [2, 3, 4, 10]. The histologic features of nodular fasciitis vary. Shimizu et al [4] classified the lesions into 3 subgroups and reviewed the relationship between their clinical and histologic features, thus focusing attention on the chronological transition of changes in nodular fasciitis. In their study, they analyzed 250 cases that occurred primarily in adults who were in their 40s or 50s. Other authors have found persons 30 to 50 years of age at highest risk. Both men and women were equally affected. The most common locations for nodular fasciitis were forearm (27%), thigh (17%), or upper arm (12%). The lesions had a mean diameter of 1.5 cm and frequently were accompanied by pain. The three histologic subgroups are myxoid, cellular, and fibrous, with the mitotically active myxoid form having the shortest clinical history and the fibrous type the longest. The cellular group was of intermediate duration. In a review of more than 1,000 patients with nodular fasciitis, Enzinger and Weiss [1] found solitary lesions only, although previous series described multiple lesions.
Pathogenesis
The pathogenesis of nodular fasciitis is unclear, but these lesions frequently are associated with a history of trauma to the affected region [1, 7]. Nodular fasciitis could be caused by a local inflammatory reaction in the fibrous connective tissue. Pain is a common complaint in more than half the patients. Neurologic symptoms are present if the mass compresses a nearby nerve. Most cases originate in the superficial fascia, but some can occur in intermuscular or deep fascia. The masses tend to grow quickly, and patients frequently seek treatment within the first few weeks. Various authors have reported a time from first detection to surgical intervention of less than 2 months [4, 7].
Gross pathology and histology
The gross pathologic findings of nodular fasciitis are not unique enough to differentiate it from other tumors [1, 6, 8]. The mass usually is oval or round and not encapsulated or well circumscribed. Nodular fasciitis is tan to grayish white and has a slimy consistency because of its myxoid component. Most tumors are about 2 cm in diameter; however, larger ones, such as that we report here, can occur. Larger tumors, in particular, require careful evaluation and follow-up, because fibrosarcoma is known to have regions that may closely resemble nodular fasciitis [1, 6, 8]. Four histologic features of nodular fasciitis can help facilitate the pathologic diagnosis: 1) spindle-shaped fibroblasts, 2) clefts separating fibroblasts, 3) extravasated erythrocytes, and 4) interstitial mucoid material [1, 8].
The histologic findings are similar to those observed with sarcoma but are differentiated by plump, immature-looking fibroblasts in short irregular bundles and fascicles. These fibroblasts are accompanied by a dense reticulin meshwork containing a small amount of mature collagen. Lymphoid cells, lipid macrophages, and multinucleated giant cells are among the fibroblasts and erythrocytes. An important diagnostic criterion is the presence of a loosely textured “feathery” pattern of an intervening ground substance composed of mucopolysaccharide [1, 6, 7, 8]. Numerous authors have reported that ablative surgical treatment has been performed unnecessarily for nodular fasciitis misdiagnosed as sarcoma [2, 3, 4, 6, 9].
Differential diagnosis
Pathologic diagnosis of nodular fasciitis has been problematic, as noted in numerous studies in which as many as half the tumors were confused with fibrosarcoma or another malignant neoplasm [1, 6, 7] and patients were treated unnecessarily by excision [1]. Tumors that are pathologically similar to nodular fasciitis include fibrosarcoma, fibroma, fibrous histiocytoma, and desmoids. Fibrosarcoma differs in appearance from nodular fasciitis in that fibrosarcoma cells have a monomorphic appearance, often with a herringbone pattern. In contrast, fasciitis has a random arrangement of cells. Fibromas are characterized by lower cellularity, deeply staining collagenous fibers, and clear circumscription of the tumor. Fibrous histiocytomas or dermatofibromas occur in the dermis and are accompanied by storiform patterns, histiocytes, and hemosiderin. Desmoids form large tumors, usually in muscle or rarely in subcutaneous tissue. Bernstein and Lattes [6] report that recurrence of a lesion originally diagnosed as nodular fasciitis should result in immediate and careful reappraisal of the pathologic findings, because retrospective review has often shown such lesions to have been misdiagnosed.
Nodular fasciitis has a nonspecific appearance on MRI. Most commonly, they are isointense to hyperintense on T1-weighted images and hyperintense on T2-weighted images [11, 12, 13, 14]. Lesions typically enhance, although to a varying degree. The variability in signal and enhancement is likely due to differing histologic, predominantly myxoid and fibrous, components of the lesion [15].
Treatment
Nodular facsciitis is a self-limiting process that does not require surgical intervention [16, 17]. Medical intervention is usually in the form of pain control. A single case report in the literature describes intralesional treatment of nodular fasciitis with steroids [18].
Conclusion
This case illustrates the potential for resolution of even quite large tumors diagnosed as nodular fasciitis. As documented by MRI of our patient, the clinical course is one of spontaneous resolution. There is a need for awareness not only of this entity, which mimics malignancy, but also of the importance of nonsurgical care after diagnosis. Such care avoids the potential morbidity associated with a surgical procedure, particularly if the mass is in the proximity of major neurovascular structures. The potential inaccuracy of histologic diagnosis must be taken into consideration before proceeding with nonsurgical care. Close clinical follow-up is mandatory.
Footnotes
Competing Interests: The authors declare that no competing interests exist.
Published: January 28, 2006
References
- 1.Mentzel T, Fletcher CD. Lipomatous tumours of soft tissues: an update. Virchows Arch. 1995;427:353–363. doi: 10.1007/BF00199383. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433–1466. doi: 10.1148/rg.245045120. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Knierim DS, Wacker M, Peckham N, Bedros AA. Lumbosacral intramedullary myolipoma. Case report. J Neurosurg. 1987;66:457–459. doi: 10.3171/jns.1987.66.3.0457. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Michal M. Retroperitoneal myolipoma. A tumour mimicking retroperitoneal angiomyolipoma and liposarcoma with myosarcomatous differentiation. Histopathology. 1994;25:86–88. doi: 10.1111/j.1365-2559.1994.tb00603.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Imamura T, Irie H. Myolipoma of the retroperitoneum. Pathol Int. 2004;54:460–463. doi: 10.1111/j.1440-1827.2004.01646.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Meis JM, Enzinger FM. Myolipoma of soft tissue. Am J Surg Pathol. 1991;15:121–125. doi: 10.1097/00000478-199102000-00003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Lehrman BJ, Nisenbaum HL, Glasser SA, Najar D, Rosen M. Uterine myolipoma. Magnetic resonance imaging, computed tomographic, and ultrasound appearance. J Ultrasound Med. 1990;9:665–668. doi: 10.7863/jum.1990.9.11.665. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Ekici E, Vicdan K. Uterine myolipoma: diagnosis by ultrasound. Int J Gynaecol Obstet. 1993;42:167–171. doi: 10.1016/0020-7292(93)90632-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Villanueva AJ, Martinez-Noguera A, Perez C, Clotet M, Oliva E. Uterine myolipoma: CT and US. Abdom Imaging. 1993;18:402–403. doi: 10.1007/BF00201793. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Aguilar S, Saint-Aubain N, Dargent JL, Fayt I, Noel JC. Myolipoma of soft tissue: an unusual tumor with expression of estrogen and progesterone receptors. Report of two cases and review of the literature. Acta Obstet Gynecol Scand. 2002;81:1088–1090. [PubMed] [PubMed] [Google Scholar]
- 11.Sonobe H, Ohtsuki Y, Iwata J, Furihata M, Ido E, Hamada I. Myolipoma of the round ligament: report of a case with a review of the English literature. Virchows Arch. 1995;427:455–458. doi: 10.1007/BF00199397. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Sharara N, Lee WR, Weir C. Myolipoma of the eyelid. Graefes Arch Clin Exp Ophthalmol. 1998;236:630–634. doi: 10.1007/s004170050133. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Ben-Izhak O, Elmalach I, Kerner H, Best LA. Pericardial myolipoma: a tumour presenting as a mediastinal mass and containing oestrogen receptors. Histopathology. 1996;29:184–186. doi: 10.1046/j.1365-2559.1996.d01-504.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.McGregor DK, Whitman GJ, Middleton LP. Myolipoma of the breast: mammographic, sonographic, and pathologic correlation. Breast J. 2004;10:259–260. doi: 10.1111/j.1075-122X.2004.21317.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Luijendijk RW, Jeekel J, Storm RK. The low transverse Pfannenstiel incision and the prevalence of incisional hernia and nerve entrapment. Ann Surg. 1997;225:365–369. doi: 10.1097/00000658-199704000-00004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yahchouchy-Chouillard E, Aura T, Picone O, Etienne JC, Fingerhut A. Incisional hernias. I. Related risk factors. Dig Surg. 2003;20:3–9. doi: 10.1159/000068850. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Franchi M, Ghezzi F, Buttarelli M, Tateo S, Balestreri D, Bolis P. Incisional hernia in gynecologic oncology patients: a 10-year study. Obstet Gynecol. 2001;97:696–700. doi: 10.1016/s0029-7844(01)01192-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Delpero JR, Lieutaud R, Dominguez C, Guerinel G, Carcassonne Y. Primary pedunculated fibrosarcoma of the peritoneum: a case report. J Surg Oncol. 1984;25:178–185. doi: 10.1002/jso.2930250309. [PubMed] [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 19.Marshall RM, Gould VE, King ME, Jensik S, Chejfec G. Multicystic abdominal peritoneal tumor presenting as an enlarging incisional hernia. Ultrastruct Pathol. 1985;8:249–256. doi: 10.3109/01913128509142157. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Sataloff DM, La Vorgna KA, McFarland MM. Extrapelvic endometriosis presenting as a hernia: clinical reports and review of the literature. Surgery. 1989;105:109–112. [PubMed] [PubMed] [Google Scholar]
- 21.Huber-Buchholz MM, Buchholz NP. Late recurrence of fallopian tube carcinoma in an incisional hernia. Arch Gynecol Obstet. 1994;255:55–56. doi: 10.1007/BF02390677. [PubMed] [DOI] [PubMed] [Google Scholar]


