Abstract
Hypertensive disorders of pregnancy (HDP) comprise a spectrum of syndromes that range in severity from gestational hypertension and pre-eclamplsia (PE) to eclampsia, as well as chronic hypertension and chronic hypertension with superimposed PE. HDP occur in 2% to 10% of pregnant women worldwide, and impose a substantial burden on maternal and fetal/infant health. Cardiovascular disease (CVD) is the leading cause of death in women. The high prevalence of non-obstructive coronary artery disease and the lack of an efficient diagnostic workup make the identification of CVD in women challenging. Accumulating evidence suggests that a previous history of PE is consistently associated with future CVD risk. Moreover, PE as a maladaptation to pregnancy-induced hemodynamic and metabolic stress may also be regarded as a “precision” testing result that predicts future cardiovascular risk. Therefore, the development of PE provides a tremendous, early opportunity that may lead to changes in maternal and infant future well-being. However, the underlying pathogenesis of PE is not precise, which warrants precision medicine-based approaches to establish a more precise definition and reclassification. In this review, we proposed a stage-specific, PE-targeted algorithm, which may provide novel hypotheses that bridge the gap between Big Data-generating approaches and clinical translational research in terms of PE prediction and prevention, clinical treatment, and long-term CVD management.
Keywords: Pre-eclampsia, hypertensive disorders in pregnancy, cardiovascular risk, precision medicine, cardiac stress test
Introduction
There is an engrained belief that males comprise the major population affected by cardiovascular disease (CVD), which is based on their earlier symptom onset and greater clustering of typical clinical manifestations and traditional risk factors. In contrast, the importance of CVD on women’s health has long been ignored because of the atypical symptoms, non-traditional risk factors and non-specific response to cardiac stress tests [1,2]. Substantial evidence has been documented regarding the under testing and under-treatment of women with CVD [3,4]. In general, It is acknowledged that an age-dependent decline of health status contributes to increased CVD mortality in older women [5], whereas recent studies have demonstrated that young women also have more adverse outcomes with myocardial infarction compared with their male peers [6,7]. CVD is the leading cause of death in women, and contributes to one-third of deaths in women worldwide [8,9]. However, the high prevalence of non-obstructive coronary artery disease (CAD) and the lack of an efficient diagnostic workup make the identification of CVD in women challenging (Figure 1) [10,11].
Figure 1.
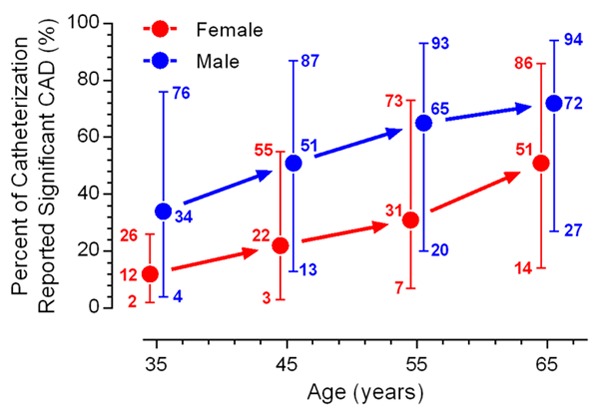
Pretest likelihood of CAD in symptomatic patients based on gender and age. For each point, the lower extended error bar, the solid circle, and the upper extended error bar indicate data from non-anginal chest pain, atypical angina, and typical angina patients, respectively. Data adopted from Reference [11] following modification. Abbreviation: CAD, coronary artery disease.
Hypertensive disorders of pregnancy (HDP) occur in 2% to 10% of pregnant women worldwide [12]. HDP represent a spectrum of disorders that range in severity from gestational hypertension (new hypertension after 20 weeks of gestation) and pre-eclamplsia (PE) to eclampsia, as well as chronic hypertension and chronic hypertension with superimposed PE. Among these disorders, PE and eclampsia are pregnancy-specific, and PE accounts for more than 50% of HDP cases [13]. Accumulating evidence has indicated a consistent association between PE and future cardiovascular risk [14-21]. Moreover, evidence indicates that the US incidence of PE has substantially increased over the previous two decades [22]. Pregnancy may be regarded as a stress test for the female cardiovascular system, which may unmask the symptoms of various chronic diseases that may occur 20 to 30 years later [23,24]. Thus, a confirmative diagnosis of PE could be used as a reliable positive result for a cardiac stress test and thus a prognosis for future CVD risk, which may be used to devise early and personalized interventions to change future well-being in women [25].
PE may precisely unmask the maladaptation to pregnancy-induced hemodynamic and metabolic changes however, it functions more similar a syndrome than a disease. Furthermore, PE is never precise in terms of its clinical features and potential causes, which require a “precision” approach for better reclassification and treatment. In this review, we aimed to summarize the recent evidence to test our hypotheses: (1) a confirmative PE diagnosis is a high risk factor prognosis of future CVD; and (2) management may facilitate future CVD prevention.
We will summarize the recent evidence of PE as a “precision testing result” for future cardiovascular risk, and propose a precision medicine-based approach to address the opportunities and challenges in PE prediction and treatment, as well as future CVD prevention.
Role of cardiac stress tests in ischemic heart disease (IHD) diagnosis in women
The term IHD refers to a multifactorial pathophysiology of coronary atherosclerosis which includes obstructive CAD (at least one main branch of the coronary artery with a fixed stenosis of >70%) and non-obstructive CAD [26]. Cardiac stress tests (including exercise treadmill testing (ETT) with echocardiography, stress echocardiography, stress myocardial perfusion imaging with nuclear medicine, and stress cardiac imaging with magnetic resonance imaging or coronary computed tomographic angiography) are widely used for the diagnostic work up of patients with suspected IHD [27]. The purpose of these tests is to induce an increase in cardiac oxygen consumption, either exertionally or pharmacologically, which leads to an imbalance between the myocardial oxygen supply and demand; this imbalance consequently unmasks pre-existing abnormalities in coronary circulation. The major underlying pathological conditions associated with stress-test identified abnormalities comprise obstructive CAD with fixed stenosis in a coronary artery. Other potential contributors include dysfunctions in the coronary microvasculature and endothelium. However, the fact that myocardial ischemic symptoms in women more are often precipitated by mental or emotional stress, and less frequently by physical exertion compared with men [28], which may be associated with a more aggressive platelet aggregation response in women [29], leads to a challenge in clinical decision-making. Additionally, in symptomatic females with symptoms similar to their male peers, the likelihood of exhibiting obstructive CAD is lower than males [26]. During stress testing, these women are more likely to have “false positive” test results, and as a result of a lack of confidence in accuracy, non-invasive stress tests are often over-used in low-risk individuals with minimal diagnostic benefit [10,30]. Accordingly, a “false positive” result would lead to subsequent invasive tests or even invasive treatment, such as coronary angiography and percutaneous coronary intervention (PCI), which involve increased risks in women [31]. PCI is nearly always appropriately used in patients with acute coronary syndromes, however, the over-use of this invasive and costly procedure is common in patients with mild or stable symptom of CAD [32]. Therefore, the diagnosis of IHD in women using traditional cardiac stress tests is technically challenging and is more likely to render females into a polarizing situation, which includes an under-diagnosis, under-treatment, or over use of invasive approaches.
In 2011, for the first time, the AHA included PE as a risk factor for CVD, and in 2014, it included PE as a risk factor for stroke in women [33,34]. The 2014 AHA consensus statement on the role of non-invasive testing in the clinical evaluation of women with suspected IHD incorporated an updated evaluation of obstructive and non-obstructive CAD and proposed a risk stratification-based diagnostic workup of cardiac stress tests [27]; however, this statement did not include PE in this risk stratification algorithm. Emerging evidence indicates that following the inclusion of parameters such as heart rate recovery, functional capacity, and treadmill scores during ETT, the diagnostic and prognostic accuracies of ETT in symptomatic females with suspected IHD are enhanced. Therefore, future work is warranted to incorporate PE history into the diagnostic criteria for women with suspected IHD.
Pregnancy is an inescapable and long-term cardiac stress “test” for women
Approximately 80% and 90% of women in developed and developing countries, respectively, will have at least one pregnancy in their life time [35]. Pregnancy is characterized by a cascade of physiologic changes that pose a substantial burden on BP regulation, glucose and lipid metabolism, and immune tolerance. During pregnancy, the demand of establishing another circulatory system for the fetus requires a coordinated increase in blood volume. It is estimated that cardiac output increases 35% to 50% during pregnancy, which is initiated during gestational week 5 and reaches a plateau by gestational weeks 16 to 20 through term [36]. As a consequence of left ventricular overload, there is a 10% to 20% increase in the left ventricular mass during pregnancy [36]. As a result of a biphasic change in peripheral arterial resistance, BP exhibits a slightly U-shaped pattern during pregnancy (Figure 2). The BP level will decreases to the nadir at mid-pregnancy and gradually returns to a pre-pregnant level through term. Therefore, the occurrence of gestational hypertension is a paradoxical response to pregnancy-induced physiological BP regulation. Thus, pregnancy unmasks defects in BP regulation in response to hemodynamic alterations. Moreover, pregnancy-induced resetting of glucose and lipid homeostasis, which is manifested by decreased insulin sensitivity (insulin sensitivity of late normal pregnancy is reduced by 50% to 70% compared with normal, non-pregnant women [37]) and increased circulating triglycerides (very low density lipoprotein triglyceride concentrations increase 3-fold from 14 weeks of gestation through term [37]) and low density lipoprotein, mimics the pathological alterations present in metabolic syndrome.
Figure 2.
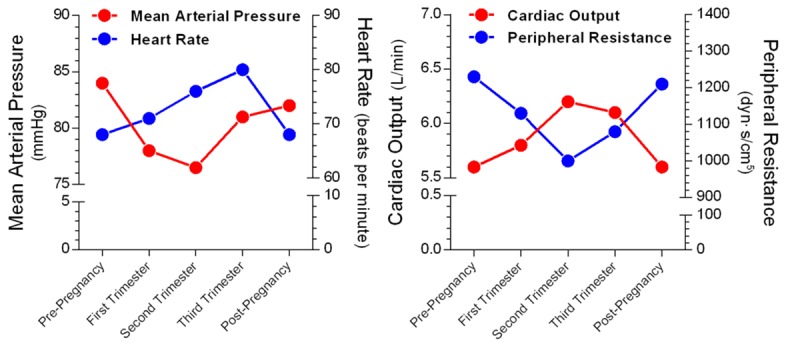
Hemodynamic alterations in normal pregnancy. Each point represents the mean value during a normal pregnancy. Data adopted from Reference [36] following modification.
Recent epidemiological evidence indicates that in metabolic syndrome patients, among the clustering of traditional risk factors, hypertension is the predominant component for risk stratification [38-40]. Notably, the risk for future end-stage renal dysfunction may be increased by 20-fold in patients with a previous history of HDP; thus, PE may also be regarded as a “failed renal stress test” [41]. In this regard, pregnancy is capable of inducing various changes in homeostasis resetting, in which an imbalance comprises are key pathophysiological bases that underlie hypertension, metabolic disorders and renal dysfunction. These inescapable alterations represent chronic stress that lasts for 20 to 30 weeks during pregnancy. Moreover, lipid deposition in the maternal uterine artery walls regularly occurs in PE, which resembles the early stages of atherosclerosis; it is referred to as “acute atherosis” and is thought to regress after delivery [42]. Thus for PE patients, the maladaptation to pregnancy-induced chronic stress also recapitulates the pathological features of atherosclerosis and provides unique human models to investigate the mechanism for atherosclerosis and future cardiovascular health. Accumulating evidence suggests that a previous history of PE is consistently associated with future CVD risk (Figure 3) [18-21]. Moreover, recent evidence also indicates that a previous maternal history of PE is associated with cardiovascular alterations in offspring (Figure 4) [43-47], which may thus provide a tremendous opportunity for potential CVD preventative strategies for both generations.
Figure 3.
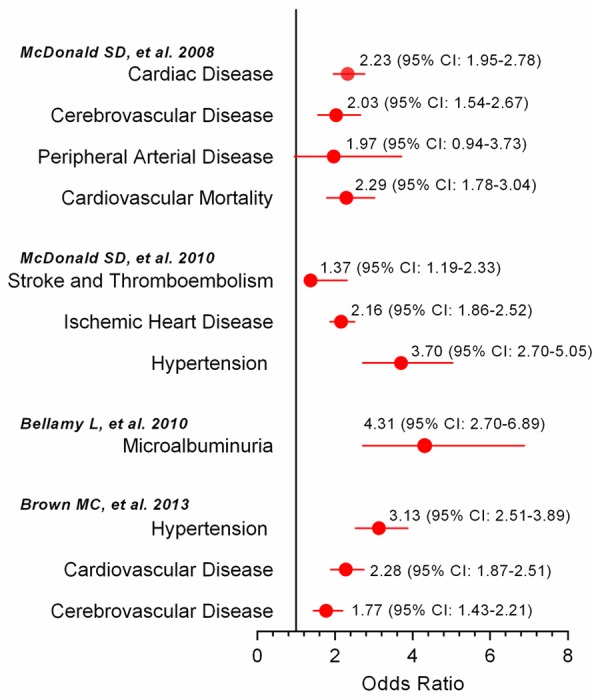
Increased postpartum cardiovascular risk in women with a previous history of pre-eclampsia compared with normal pregnancies. Data adopted following modification from recent meta-analyses on pre-eclampsia and future cardiovascular risk/disease (References [18-21]). Abbreviations: QRISK indicates the global lifetime risk; SBP, systolic blood pressure; DBP, diastolic blood pressure; T2DM, type 2 diabetes mellitus.
Figure 4.
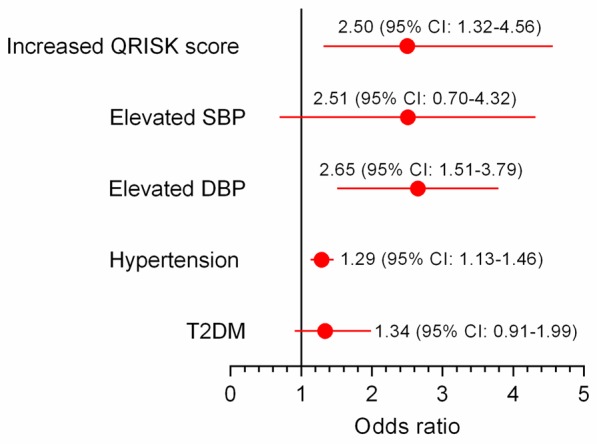
Increased cardiovascular risk in young adulthood in offspring of hypertensive pregnancies compared with normal pregnancies. Data adopted following modification from References [46,47]. Abbreviations: QRISK, indicates the global lifetime risk; SBP, systolic blood pressure; DBP, diastolic blood pressure; T2DM, type 2 diabetes mellitus.
In a mechanistic view, the underlying pathogenesis of PE, which ostensibly presents as an elevated BP level, is more complex than primary hypertension. In addition to the pregnancy-specific factors (including placental ischemia, spiral artery transformation, hypoxia and trophoblast invasion), other common etiologies associated with primary hypertension, i.e., endoplasmic reticulum stress, oxidative stress, antibodies to type-1 angiotens in II receptor, vascular inflammation, endothelial dysfunction, dyslipidemia, and insulin resistance, have been demonstrated to play a role in PE [48,49]. In general, at least three categories of etiologies underlie the pathogenesis of PE: pregnancy-specific (referred to as placental PE [50,51]), non-specific (referred to as maternal PE [50,51]), and the blended form. Thus, PE should be regarded as a syndrome more than a disease with multiple and non-specific clinical parameters [52]. Therefore, despite the fact that PE is associated with a reliable positive testing result for pregnancy-induced hemodynamic and metabolic stress, the “precision test result” needs a “precision” approach for re-classification and management.
Precision medicine-based strategy for PE
As outlined by the US National Academy in 2011, the goal of precision medicine is to establish a more precise definition of and classification of clinical diseases that ultimately lead to better diagnosis and treatment [53]. To achieve this promising goal, the integration of data from genomics, microbiome, exposures, behaviors, and traditional clinical tests, as well as participant contributed data, is warranted. In the following section, we aim to provide an overview of the potential paths with prospective needs to be addressed by precision medicine-based approaches for future PE research.
Genetic background for PE
Among the currently acknowledged risk factors for PE, several components are associated with the hereditary tendency, including history of PE in a previous pregnancy, multi-fetus gestation, obesity, family history of PE (mother or sister) and pre-existing medical conditions (e.g., chronic hypertension, diabetes, antiphospholipid syndrome, thrombophilia, or autoimmune disease). Thus, the attempt to identify candidate genes that predispose women to subsequent PE has long been pursued by clinicians for high-risk pregnancy stratification. The most frequently used method is a candidate gene-based association approach, which is based on statistical correlations between single nucleotide polymorphisms (SNPs) of candidate genes of interest and disease prevalence. However, selection bias is the most prominent limitation of this methodology. As summarized by a recent meta-analysis and systemic review of maternal genotype and severe PE [54], among 57 studies that evaluated 50 genotypes, only 2 genotypes that involved thrombophilia (coagulation factor V gene polymorphism rs6025 and coagulation factor II gene rs1799963) were validated in severe PE. This study questions the majority of candidate gene studies regarding PE susceptibility. Our current understanding of the pathogenesis of PE is limited; thus, it is conceivable that a candidate gene approach is vulnerable to missing important and yet-undiscovered molecular pathways.
In contrast, a genome-wide association study (GWAS), which uses an unbiased approach to simultaneously investigate thousands of SNPs, may provide a useful tool for genetic screening of high risk pregnancies. To the best of our knowledge, only two PE/HDP related GWAS studies have been published [55,56]: one study of 293 Caucasian women (177 cases and 117 controls) in the US failed to identify associations between SNPs and PE [55]; another study of 1078 Caucasian women (538 cases and 540 controls) in Australia failed to identify significant GWAS SNPs with PE [56]. The major reason that accounts for these negative findings is the inadequately powered sample size. Recently, a large international collaboration project, referred to as the InterPregGen study, was established with funding from the European Union. This study involves research groups from Finland, Iceland, Kazakhstan, Norway, the United Kingdom and Uzbekistan, and aimes to enroll more than 10,000 individuals to identify robust associations between genotypes and PE. This statistically powered study will provide important clues for PE pathogenesis, prevention, and treatment. Attempts to identify potential genetic markers for PE are also highly warranted in non-Caucasian ethnic groups, because novel generalized preventive and treatment strategies should be based on multiethnic GWASs and interethnic comparisons.
Gut microbiome and PE
The gut microbiome has increasingly been recognized as an important contributor to the pathogenesis of metabolic disorders. In 2012, Koren and colleagues first reported that gut microbiota substantially change from first to third trimesters and are coordinated with metabolic changes during pregnancy [57]. Additional evidence indicates that the infant comes into contact with microbes originated from the maternal gut via physiological gut microbial translocation during vaginal delivery and through breast milk [58]. Thus, a mother’s gut microbiota may have a long-term influence on the infant’s future risk of gastrointestinal, allergic, autoimmune and metabolic diseases. Accordingly, the modulation of gut microbiota via maternal probiotic intervention during pregnancy, as well as during breastfeeding, may potentially change future disease trajectories in both mother and infant. For example, a Mediterranean dietary pattern, which is characterized by a high-level consumption of fruits, vegetables, legumes, nuts, red wine, and fish, is associated with beneficial microbiome-related metabolomic profiles [59]. A recent 9 year follow-up study of 3582 women demonstrated that pre-pregnancy adherence to a Mediterranean dietary pattern is associated with a reduced incidence of HDP (highest quartile vs. lowest quintile: RR: 0.58; 95% CI: 0.42 to 0.81) [60]. These promising findings suggest that the use of metagenomic approaches may be helpful in the identification of links between specific changes in gut microbial abundance and diversity and the metabolic alterations associated with the incidence and severity of PE. These links may, in turn, serve as potential therapeutic targets.
Nutritional evaluation and PE impact of dietary salt intake
Nutritional factors, especially high energy consumption, are associated with PE [61]. Moreover, an increase in calorie consumption is associated with an increase in dietary salt intake and metabolic syndrome [62-65]. Dietary salt intake is the most important environmental and modifiable risk factor for hypertension [66]. In general, an increase in dietary salt (NaCl) intake leads to volume expansion, and is positively associated with changes in BP level, the magnitude of which is defined as salt sensitivity [67]. During pregnancy, the need for volume expansion requires a parallel increase in salt intake. As a result of a gradual decrease in peripheral resistance, the volume expansion paradoxically occurs with a BP reduction to the nadir at mid-pregnancy [68]. This phenomenon leads to the speculation that reduced dietary salt intake may represent a potential trigger for PE, and has been referred as inverse salt sensitivity [69]. If this phenomenon could be consistently observed in pregnant women, an increase in dietary salt intake may thus comprise a therapeutic approach. However, the relationship between salt intake and PE is strongly debated [70,71], which is largely attributable to the lack of a reliable method to measure long-term sodium retention/excretion in the body. The most frequently used, but also technically difficult method is 24-h urinary sodium measurement [72]. However, a single 24-h urine collection to monitor sodium excretion has also been demonstrated to be a poor reflection of an individual’s average salt consumption owing to the large day-to-day variations in salt intake [73]. Another issue is that an increase in dietary salt intake is inevitably associated with a concomitant increase in energy intake, which is particularly true in pregnant women, and may thus also confound the relationship between salt intake and PE. To investigate the relationship between dietary salt intake and PE, the extracellular tissue sodium content which is now recognized as the third compartment of sodium storage in the body (the other two compartments are plasma and cellular sodium), may serve as a reservoir that reflects the long-term salt intake status [74,75]. Moreover, it may also provide reliable quantitative information regarding sodium storage using 23Na magnetic resonance imaging [76,77]. It is well established that salt sensitivity is determined by genetic factors [78-81]. Therefore, with the use of unbiased genetic approaches, combined with novel imaging technology to provide reliable measurements of long-term salt consumption status, the existence of inverse salt sensitivity and the genetic background associated with PE, as well as potential therapeutic strategies may be identified.
In addition to the previously discussed modifiable and non-modifiable contributors associated with PE that may be investigated using precision medicine-based approaches, other methodologies, such as serum and placental proteomics, DNA methylation, microRNA, and lncRNA, may also be integrated and applied to PE-focused precision medicine. A combination of these approaches may, in turn, lead to better prediction, treatment and preventative strategies.
Future perspectives and open questions
The benefit of PE as a “precision” positive stress result for future CVD risk as well as its underlying complexity, which should be addressed by precision medicine-based appro-aches, lead to many open questions for future research topics. Here we listed three questions with potential implications for PE as a therapeutic window that may affect future CVD trajectories.
Can PE patients be effectively instructed to follow clinical recommendations during follow-up?
To date, a major unresolved concern is the lack of communication among obstetricians, cardiologists, and PE patients, which leads to the ineffectiveness of identification and “tracing” young women with a history of HDP [82]. For the majority of pregnant women, after the initial PE attack, the awareness of their future well-being may be considerably reduced because they may be ostensibly “normal” without laboratory signs or symptoms of CVD. A recent report demonstrated that even among women with continuous medical coverage, only 57% of subjects with a HDP history attended a primary care visit within one year after delivery [83]. However, even in apparently healthy normotensive women with a PE history, 1 in 6 women (17%) developed de novo hypertension within 5 years [84]. Thus, all possible efforts should be made to prevent the reappearance of PE patients in cardiology clinics with classical or non-classical symptoms of CVD without regular evaluation during a 20 to 30 year period. To address this gap, educational programs aimed at obstetricians, cardiologists, and family doctors as well as PE patients should be created to increase physician expertise and public awareness of PE and future CVD risk. In this regard, the Maternal Health Clinic, which specializes in the identification and instruction of high risk postpartum patients in Canada, is a successful example of a primary prevention strategy for women’s future health [85]. Furthermore, because there is an average of 20 to 30 years for women with a PE history to manifest the clinical symptoms of CVD, cohort follow-up and data entry comprise high-cost and labor-intensive work. Given the high penetration of cell phones into groups with different socioeconomic statuses, health-related mobile applications (Apps) may provide an opportunity to overcome many of the barriers of future CVD prevention [86]. A choice of multiple-platform compatible and open-access Apps for PE patient management following a complete evaluation is highly warranted and may provide a communication platform between clinicians and patients, as well as serve as a data-entry access point for patient-reported information for health and research purposes. Thus, government guidance and oversight will help to protect public health, sustain consumer confidence in mobile health products, and encourage high-value innovations [87]. Finally, efforts should be made at the government level to expand medical insurance coverage for preventative health care in women with a history of PE [15].
Do all PE patients have worse future cardiovascular outcomes?
The answer may be no. Accumulating evidence indicates that while the development of typical PE at term is associated with a small but significant increase in risk of future CVD, the development of severe PE is associated with a greater risk of future premature CVD [25,88,89]. This finding suggests a dose-dependent relationship between PE severity and future CVD risk. Furthermore, using a precision medicine-based approach, PE may be reclassified using predominately pregnancy-specific factors and predominately non-specific factors, as well as a blended form. Distinguishing these components is important because for the pregnancy-specific etiology, novel treatment options that target the pregnancy-specific pathology are warranted, and the future cardiovascular risk may be diminished, providing that the target organ injury is not severe and self-limiting once pregnancy is terminated. Moreover, for pregnancy non-specific reasons, these patients should be regarded as a “true” positive stress test and their future risk may be increased because the presence of common ideologies for cardiovascular disease may have a life-long impact on their well-being. Regarding the blended form, a combined strategy may be suitable. The answer to this question may also be helpful to address the association between the pathophysiological mechanism of HDP and future CVD risk.
In which manner, may PE patients benefit from a precision medicine-based strategy before, during, and after pregnancy?
In general, we proposed five critical stages for precision medicine-based PE prediction, reclassification, and clinical management, as well as long-term CVD prevention (Figure 5). From pre-pregnancy through the first and second trimesters, Big-Data generating approaches should focus on PE prediction and preventative strategies. For PE prediction, routine clinical characteristic-based methods may predict only 30% of women who develop PE [13]. Efforts have also been made to improve the predictive potential of these characteristics via the incorporation of uterine artery Doppler scan [90], and other pregnancy-specific markers [91], as well as novel metabolic biomarkers [92]. For PE prevention, aspirin is the only drug with proven efficacy based on robust evidence; however, its effect is not substantial (RR 0.90, 95% CI: 0.84-0.97) [13,93]. For PE treatment, especially during the second and third trimesters, several treatment strategies, such as antioxidation with vitamins C and E [94], anti-coagulation [95], and vitamin D supplementation [96], did not exhibit consistent efficacy in clinical trials despite their promising potential. To date, the only cure for PE is delivery of the placenta [13]. In recent guidelines, anti-hypertensive drugs for HDP are strongly recommended in cases with severe hypertension [97,98]. The findings from the recent CHIPS trial indicated that compared with less-tight BP control (target diastolic BP 100 mmHg), tight BP control (target diastolic BP 85 mmHg) was associated with maternal protection in terms of less severe maternal hypertension or serious maternal complications, without an increase in adverse perinatal outcomes [99]. Therefore, during these two trimesters, the three sub-classifications/patterns of PE based on precision-medicine may provide a valuable platform for designing and testing new treatment options: for the pregnancy-specific pattern, novel treatment options that target pregnancy-specific abnormalities would be tested; for the non-specific pattern, which may, in general, be regarded as hemodynamicand metabolism-induced, traditionally available options that target BP regulation, as well as glucose and lipid modifying treatments with proven safety may be suggested; and a combined strategy may be tested in the blended pattern. Furthermore, efforts should also be made from the second trimester to post-pregnancy period to reduce target organ injuries and cardiovascular mobility. Thus, from the third trimester to the post-pregnancy period, specialized clinics with joint efforts by cardiologists, obstetricians and pediatricians would benefit PE patients and their offspring in terms of education and communication, risk factor monitoring and assessment, and effective pharmacological and lifestyle interventions, as well as offer a platform to provide a mechanistic link between PE and future CVD. Overall, this simplified, 5-stage PE-targeted algorithm would enhance the exploitation of Big-Data generated by precision medicine-based approaches. Moreover, it may also provide novel hypotheses that bridge the gap between Big Data-generating approaches and clinical translational research.
Figure 5.
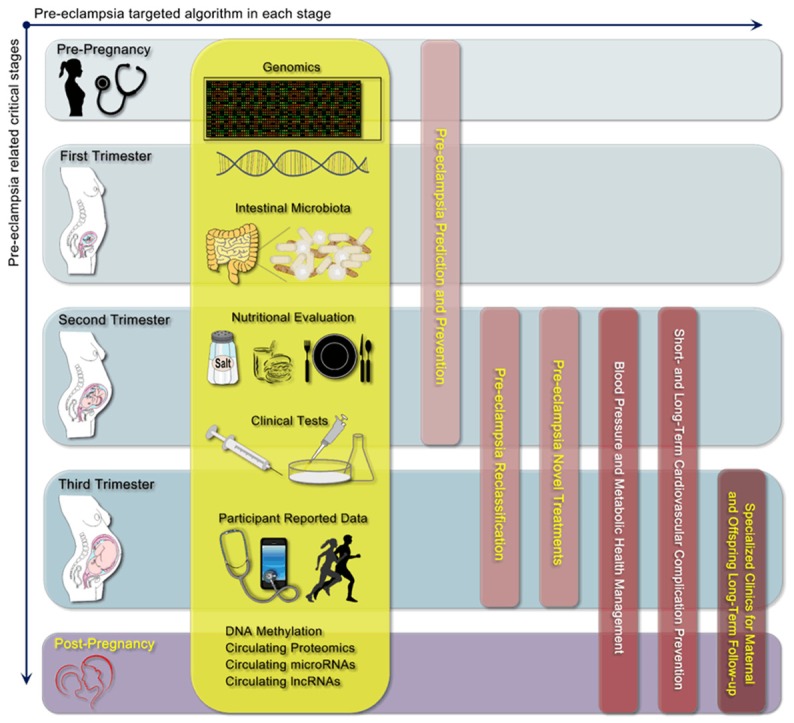
Proposal of a PE-targeted, 5-stage algorithm using precision medicine-based approaches for PE prediction, prevention and clinical treatment, as well as long-term CVD management.
Conclusions
In summary, consistent evidence has demonstrated an association between prior PE and future CVD risk. PE is a reliable positive testing that unmasks multiple pathological features; thus, the development of PE provides a window of valuable opportunity for prediction, screening, prevention and treatment of future CVD. Specifically, the need for precision medicine-based approaches are warranted to define PE-related disease patterns, which serve as a platform to fill the gap between the explosion of a vast amount of information and focused efforts in translational medicine. Moreover, these approaches may ultimately contribute to better diagnosis, treatment, and preventative strategies for PE and related long-term well-being for women.
Acknowledgements
This work was supported by research grants from National Natural Science Foundation of China to Dr. Z.X and Dr. Y-M.L (81170238 and 81570335) and China Scholarship Council and Tianjin Municipal Science and Technology Commission Key Funding to Dr. Y-M.L (15ZXJZSY00010).
Disclosure of conflict of interest
None.
References
- 1.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 2.Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case of depression. Prog Cardiovasc Dis. 2013;55:557–562. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juliard JM, Golmard JL, Himbert D, Feldman LJ, Delorme L, Ducrocq G, Descoutures F, Sorbets E, Garbarz E, Boudvillain O, Aubry P, Vahanian A, Steg PG. Comparison of hospital mortality during ST-segment elevation myocardial infarction in the era of reperfusion therapy in women versus men and in older versus younger patients. Am J Cardiol. 2013;111:1708–1713. doi: 10.1016/j.amjcard.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. doi: 10.1161/CIRCULATIONAHA.111.086892. [DOI] [PubMed] [Google Scholar]
- 7.D’Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1324–1332. doi: 10.1161/CIRCULATIONAHA.114.012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer LC, Svatikova A, Mulvagh SL. The Challenges of Prevention, Diagnosis and Treatment of Ischemic Heart Disease in Women. Cardiovasc Drugs Ther. 2015;29:355–368. doi: 10.1007/s10557-015-6607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 13.Mol BW, Roberts CT, Thangaratinam S, Magee LA, de Groot CJ, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63:1815–1822. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 15.Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin Perinatol. 2015;39:276–283. doi: 10.1053/j.semperi.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Nerenberg K, Daskalopoulou SS, Dasgupta K. Gestational diabetes and hypertensive disorders of pregnancy as vascular risk signals: an overview and grading of the evidence. Can J Cardiol. 2014;30:765–773. doi: 10.1016/j.cjca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovasc Res. 2014;101:579–586. doi: 10.1093/cvr/cvu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 19.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 20.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih T, Peneva D, Xu X, Sutton A, Triche E, Ehrenkranz RA, Paidas M, Stevens W. The Rising Burden of Preeclampsia in the United States Impacts Both Maternal and Child Health. Am J Perinatol. 2016;33:329–38. doi: 10.1055/s-0035-1564881. [DOI] [PubMed] [Google Scholar]
- 23.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 25.Simth GN. Development of preeclampsia provides a window of opportunity for early cardiovascular risk screening and intervention. Expert Rev Obstet Gynecol. 2009;4:355–357. [Google Scholar]
- 26.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, Kramer CM, Min JK, Newby LK, Nixon JV, Srichai MB, Pellikka PA, Redberg RF, Wenger NK, Shaw LJ. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130:350–379. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 28.Al Mheid I, Quyyumi AA. Sex differences in mental stress-induced myocardial ischemia: are women from venus? J Am Coll Cardiol. 2014;64:1679–1680. doi: 10.1016/j.jacc.2014.06.1206. [DOI] [PubMed] [Google Scholar]
- 29.Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O’Connor CM, Velazquez EJ, Jiang W. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669–1678. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huffman MD, van Geertruyden PH. Does low pre-test probability of coronary artery disease reflect overuse of stress testing? JACC Cardiovasc Imaging. 2011;4:1143–1144. doi: 10.1016/j.jcmg.2011.07.007. author reply 1144. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34:1262–1269. doi: 10.1093/eurheartj/ehs481. [DOI] [PubMed] [Google Scholar]
- 32.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Pina IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15-44 years in the United States: National Survey of Family Growth, 2006-2010. Natl Health Stat Report. 2012:1–28. [PubMed] [Google Scholar]
- 36.Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD, Chesley LC. Chesley’s hypertensive disorders in pregnancy. Amsterdam; Boston: Academic Press/Elsevier; 2015. [Google Scholar]
- 37.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16:255–275. doi: 10.1093/humupd/dmp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas F, Pannier B, Benetos A, Vischer UM. The impact of the metabolic syndrome--but not of hypertension--on all-cause mortality disappears in the elderly. J Hypertens. 2011;29:663–668. doi: 10.1097/HJH.0b013e32834320dc. [DOI] [PubMed] [Google Scholar]
- 39.Garg PK, Biggs ML, Carnethon M, Ix JH, Criqui MH, Britton KA, Djoussé L, Sutton-Tyrrell K, Newman AB, Cushman M, Mukamal KJ. Metabolic syndrome and risk of incident peripheral artery disease: the cardiovascular health study. Hypertension. 2014;63:413–419. doi: 10.1161/HYPERTENSIONAHA.113.01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Hypertension is the most common component of metabolic syndrome and the greatest contributor to carotid arteriosclerosis in apparently healthy Japanese individuals. Hypertens Res. 2005;28:27–34. doi: 10.1291/hypres.28.27. [DOI] [PubMed] [Google Scholar]
- 41.Wagner S, Craici I. Hypertensive pregnancy disorders and future renal disease. Curr Hypertens Rep. 2014;16:484. doi: 10.1007/s11906-014-0484-2. [DOI] [PubMed] [Google Scholar]
- 42.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56:1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 43.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 44.Lawlor DA, Macdonald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, Sattar N, Deanfield J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33:335–345. doi: 10.1093/eurheartj/ehr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. doi: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoulass JC, Robertson L, Denadai L, Black C, Crilly M, Iversen L, Scott NW, Hannaford PC. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J Epidemiol Community Health. 2016;70:414–22. doi: 10.1136/jech-2015-205483. [DOI] [PubMed] [Google Scholar]
- 47.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spracklen CN, Saftlas AF, Triche EW, Bjonnes A, Keating B, Saxena R, Breheny PJ, Dewan AT, Robinson JG, Hoh J, Ryckman KK. Genetic Predisposition to Dyslipidemia and Risk of Preeclampsia. Am J Hypertens. 2015;28:915–923. doi: 10.1093/ajh/hpu242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 51.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 52.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 53.National Research Council (U.S.) Toward precision medicine : building a knowledge network for biomedical research and a new taxonomy of disease. Washington, D.C.: National Academies Press; 2011. Committee on A Framework for Developing a New Taxonomy of Disease. [PubMed] [Google Scholar]
- 54.Fong FM, Sahemey MK, Hamedi G, Eyitayo R, Yates D, Kuan V, Thangaratinam S, Walton RT. Maternal genotype and severe preeclampsia: a HuGE review. Am J Epidemiol. 2014;180:335–345. doi: 10.1093/aje/kwu151. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Triche EW, Walsh KM, Bracken MB, Saftlas AF, Hoh J, Dewan AT. Genome-wide association study identifies a maternal copy-number deletion in PSG11 enriched among preeclampsia patients. BMC Pregnancy Childbirth. 2012;12:61. doi: 10.1186/1471-2393-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson MP, Brennecke SP, East CE, Goring HH, Kent JW Jr, Dyer TD, Said JM, Roten LT, Iversen AC, Abraham LJ, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Laivuori H, Austgulen R, Blangero J, Moses EK. Genome-wide association scan identifies a risk locus for preeclampsia on 2q14, near the inhibin, beta B gene. PLoS One. 2012;7:e33666. doi: 10.1371/journal.pone.0033666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Backhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–576. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 59.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2015 doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 60.Schoenaker DA, Soedamah-Muthu SS, Callaway LK, Mishra GD. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women’s Health. Am J Clin Nutr. 2015;102:94–101. doi: 10.3945/ajcn.114.102475. [DOI] [PubMed] [Google Scholar]
- 61.Schoenaker DA, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: a systematic review and meta-analysis of observational studies. BMC Med. 2014;12:157. doi: 10.1186/s12916-014-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363–384. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 63.Grimes CA, Wright JD, Liu K, Nowson CA, Loria CM. Dietary sodium intake is associated with total fluid and sugar-sweetened beverage consumption in US children and adolescents aged 2-18 y: NHANES 2005-2008. Am J Clin Nutr. 2013;98:189–196. doi: 10.3945/ajcn.112.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension. 2008;51:629–634. doi: 10.1161/HYPERTENSIONAHA.107.100990. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 67.Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65–76. doi: 10.1097/MNH.0b013e32835b3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grindheim G, Estensen ME, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30:342–350. doi: 10.1097/HJH.0b013e32834f0b1c. [DOI] [PubMed] [Google Scholar]
- 69.Gennari-Moser C, Escher G, Kramer S, Dick B, Eisele N, Baumann M, Raio L, Frey FJ, Surbek D, Mohaupt MG. Normotensive blood pressure in pregnancy: the role of salt and aldosterone. Hypertension. 2014;63:362–368. doi: 10.1161/HYPERTENSIONAHA.113.02320. [DOI] [PubMed] [Google Scholar]
- 70.Duley L, Henderson-Smart D, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst Rev. 2005:CD005548. doi: 10.1002/14651858.CD005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan AE, Scheelbeek PF, Shilpi AB, Chan Q, Mojumder SK, Rahman A, Haines A, Vineis P. Salinity in drinking water and the risk of (pre)eclampsia and gestational hypertension in coastal Bangladesh: a case-control study. PLoS One. 2014;9:e108715. doi: 10.1371/journal.pone.0108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 73.He FJ, MacGregor GA. Cardiovascular disease: salt and cardiovascular risk. Nat Rev Nephrol. 2012;8:134–136. doi: 10.1038/nrneph.2011.220. [DOI] [PubMed] [Google Scholar]
- 74.Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A, Luft FC. Spooky sodium balance. Kidney Int. 2014;85:759–767. doi: 10.1038/ki.2013.367. [DOI] [PubMed] [Google Scholar]
- 75.Titze J. A different view on sodium balance. Curr Opin Nephrol Hypertens. 2015;24:14–20. doi: 10.1097/MNH.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 76.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 77.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J. (23)Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]
- 78.Gu D, Kelly TN, Hixson JE, Chen J, Liu D, Chen JC, Rao DC, Mu J, Ma J, Jaquish CE, Rice TK, Gu C, Hamm LL, Whelton PK, He J. Genetic variants in the renin-angiotensin-aldosterone system and salt sensitivity of blood pressure. J Hypertens. 2010;28:1210–1220. [PMC free article] [PubMed] [Google Scholar]
- 79.Li C, Yang X, He J, Hixson JE, Gu D, Rao DC, Shimmin LC, Huang J, Gu CC, Chen J, Li J, Kelly TN. A gene-based analysis of variants in the serum/glucocorticoid regulated kinase (SGK) genes with blood pressure responses to sodium intake: the GenSalt Study. PLoS One. 2014;9:e98432. doi: 10.1371/journal.pone.0098432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Defago MD, Gu D, Hixson JE, Shimmin LC, Rice TK, Gu CC, Jaquish CE, Liu DP, He J, Kelly TN. Common genetic variants in the endothelial system predict blood pressure response to sodium intake: the GenSalt study. Am J Hypertens. 2013;26:643–656. doi: 10.1093/ajh/hps099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Q, Hixson JE, Rao DC, Gu D, Jaquish CE, Rice T, Shimmin LC, Chen J, Cao J, Kelly TN, Hamm LL, He J. Genetic variants in the apelin system and blood pressure responses to dietary sodium interventions: a family-based association study. J Hypertens. 2010;28:756–763. doi: 10.1097/HJH.0b013e3283370d32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, Margolis KL, O’Connor P, Magid DJ, Bibbins-Domingo K. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57:717–722. doi: 10.1161/HYPERTENSIONAHA.110.168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bennett WL, Chang HY, Levine DM, Wang L, Neale D, Werner EF, Clark JM. Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data. J Gen Intern Med. 2014;29:636–645. doi: 10.1007/s11606-013-2744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scholten RR, Lotgering FK, Hopman MT, Van Dijk A, Van de Vlugt M, Janssen MC, Spaanderman ME. Low Plasma Volume in Normotensive Formerly Preeclamptic Women Predisposes to Hypertension. Hypertension. 2015;66:1066–1072. doi: 10.1161/HYPERTENSIONAHA.115.05934. [DOI] [PubMed] [Google Scholar]
- 85.Cusimano MC, Pudwell J, Roddy M, Cho CK, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. 2014;210:438, e431–439. doi: 10.1016/j.ajog.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Neubeck L, Lowres N, Benjamin EJ, Freedman SB, Coorey G, Redfern J. The mobile revolution--using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol. 2015;12:350–360. doi: 10.1038/nrcardio.2015.34. [DOI] [PubMed] [Google Scholar]
- 87.Cortez NG, Cohen IG, Kesselheim AS. FDA regulation of mobile health technologies. N Engl J Med. 2014;371:372–379. doi: 10.1056/NEJMhle1403384. [DOI] [PubMed] [Google Scholar]
- 88.Manten GT, Sikkema MJ, Voorbij HA, Visser GH, Bruinse HW, Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. 2007;26:39–50. doi: 10.1080/10641950601146574. [DOI] [PubMed] [Google Scholar]
- 89.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, Khan KS, Aquilina J, Thangaratinam S. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014;43:500–507. doi: 10.1002/uog.13275. [DOI] [PubMed] [Google Scholar]
- 91.Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Mol BW, Pajkrt E. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119:778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 92.Kenny LC, Broadhurst DI, Dunn W, Brown M, North RA, McCowan L, Roberts C, Cooper GJ, Kell DB, Baker PN. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010;56:741–749. doi: 10.1161/HYPERTENSIONAHA.110.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 94.Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:503, e501–512. doi: 10.1016/j.ajog.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greer IA, Brenner B, Gris JC. Antithrombotic treatment for pregnancy complications: which path for the journey to precision medicine? Br J Haematol. 2014;165:585–599. doi: 10.1111/bjh.12813. [DOI] [PubMed] [Google Scholar]
- 96.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 99.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A, Helewa M, Hutton E, Lee SK, Lee T, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin JM. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]


