Abstract
Early stages of melanoma can be treated by surgical resection of tumor, but there is still no effective treatment once it is progressed to metastatic phases. Although growing family of both metastasis promoting and metastasis suppressor genes have been reported, the molecular mechanisms governing melanoma metastatic cascade are still not completely understood. Therefore, defining the molecules that govern melanoma metastasis may aid the development of more effective therapeutic strategies for combating cancer. In the present study, we found that Serpin Peptidase Inhibitor 2, Serpine2 was involved in the metastasis of melanoma cells. The requirement of Serpine2 in the migration of melanoma cells was confirmed by gene silencing and over-expression in vitro. Moreover, down-regulation of Serpine2 expression strikingly inhibited melanoma cellular metastasis in vivo. Finally, we found that Serpine2 promotes melanoma metastasis through the glycogen synthesis kinase 3β, GSK-3β signaling pathway. To conclude, our findings suggested a novel mechanism underlying the metastasis of melanoma cells which might serve as a new intervention target for the treatment of melanoma.
Keywords: Serpine2, melanoma, migration, invasion, GSK-3β
Introduction
Melanoma is a highly aggressive skin malignant cancer and although early stages of melanoma can be successfully treated by surgical resection of the tumor [1], there is still no effective treatment for melanoma once it is progressed to metastatic [2]. Metastasis, considered crucial for the transition of melanoma cells from a primary site to distant tissue, is now established as one of the hallmarks of melanoma and is the most life-threatening factor of cancer. Therefore, many studies have been designed to explore therapy targeting tumor metastasis and agents that will inhibit tumor cell invasion and metastasis [3]. Although few studies have identified few metastasis-promoting and metastasis suppressor genes, the molecular mechanisms governing this process are still not completely understood and the treatment efficiency of metastatic melanoma has not been significantly improved [4]. A limited number of studies testing metastasis-promoting protein and activity using murine derived melanoma cells [5,6], however, these results may not be most representative of human tumor cells. Accordingly, further study to investigate related underlying mechanisms is urgently required to find new potential targets for development novel agents to treatment melanoma.
In the present work, we reported the identification of a novel role of Serpine2 whose disruption impaired the metastasis of melanoma cells. Oncomine, a cancer microarray database and web-based data-mining platform aimed at facilitating discovery from genome-wide expression analyses [7]. Differential expression analyses comparing most major types of cancer with respective normal tissues as well as a variety of cancer subtypes and clinical-based and pathology-based analyses are available for exploration. The expression level of metastasis associates genes in melanoma was analyzed using Oncomine. Based on the large-scale expression profiling analyses, the gene Serpine2 over-represented is strongly associates with melanoma metastasis [8]. Moreover, Serpine2 is strongly overrepresented in the metastatic melanoma cells as compared with the melanocyte cell. We hypothesized that Serpine2 may play an important part in melanoma metastasis process.
Recently, Serpine1 (or plasminogen activator inhibitor-1, PAI-1) has been reported to promote angiogenesis and to induce tumor cell migration [9,10] while Serpine2 (or protease nexin-1, PN-1) appears to enhance the invasive potential of pancreatic, breast and lung cancer cells [11]. Furthermore, serpine1 is over-expressed in highly aggressive human breast tumors while Serpine2 levels are elevated in pancreatic tumors, breast tumors, oral squamous carcinomas, and liposarcomas [12]. Collectively, these results point to a role for Serpine2 in controlling cell mobility and, alternatively, cell metastasis into the tumor microenvironment. In the present work, we reported the identification of a novel role of Serpine2 in the metastasis of melanoma cells. By silencing and over-expression Serpine2, we confirmed the role of Serpine2 in the migration and invasion of melanoma cells in vitro. Moreover, reduction Serpine2 expression in melanoma cells significantly impaired their metastasis in vivo. Further investigation demonstrated that GSK-3β signaling pathway is involved in Serpine2-promoted melanoma migration and metastasis.
Materials and methods
Cell culture
Melanoma A375, B16F10, SK-MEL-2, SK-MEL-5, B16BL6 cells, and adult human epidermal melanocytes NHEM-a were purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences. Cells were cultured in DMEM or 1640 with 10% fetal calf serum (FCS) (Gibco, Invitrogen, USA), and 1% Penicillin/Streptomycin mix (Gibco, Invitrogen, USA) and maintained at 37°C in a humidified atmosphere containing 5% CO2. Specific inhibitor for GSK-3β (SB216763) was obtained from Selleck (USA). Lipofectamine 2000 was purchased from Invitrogen (USA).
Plasmids and DNA constructs
The pDisrup retroviral vector was constructed based upon MMLV retroviral vector pLNCX as backbone. The splicing donor and acceptor were designed according to human adenovirus type 2 major late mRNA intron sequence [13]. For pDisrup clone selections, cells were selected with Blasticidin S. HCl at 25 µg/ml (Invitrogen, USA). The short small interfering RNA (siRNA) was constructed with sequence specifically targeted to Serpine2 gene: (#1: 5’-AGGAACCATGAATTGGCAT-3’) or (#2: 5’-GCAGUG UGCCUGUCACUACUU3-3’). The hemagglutinin (HA)-tagged constitutively active (S9A) GSK3β (Plasmids 14754, Addgene, Cambridge, MA) was deposited by Dr. Jim Woodgett [14]. Serpine2-bio-His (Addgene plasmid # 53416) was deposited by Dr. Gavin Wright [15]. Transient transfection was performed using the Lipofectamine RNAi MAX reagent (Invitrogen) and following the manufacturer’s instructions.
Oncomine analysis
The expression level of Serpine2 genes in the melanoma was analyzed using Oncomine [16]. For this, we compared clinical specimens of cancer vs. normal patient from the Haqq Melanoma database [17]. The keywords were “melanoma” and “metastasis”. In order to reduce our false discovery rate, we selected p < 0.01 as a threshold. We analyzed the results for their p-values and fold change.
Lactate dehydrogenase (LDH) toxicity assay
The LDH released into cell cultures is an index of cytotoxicity and evaluation of the permeability of cell membrane. Cells were seeded in 96-well plate at a density of 3 × 103 cells per well. After incubation with vehicle (0.1% DMSO) or 1% Triton X-100 for 24 h, cell supernatants were collected and analyzed for LDH activity using LDH cyto-toxicity assay kit from Keygen biotech [18]. The absorbance of formed formazan was read at 490 nm on a microplate reader.
Migration assay
Migration of cells was determined by BD Transwell Migration Chamber (BD Biosciences, USA) assay in vitro according to the manufacturer’s instructions. In brief, 1 × 105 cells with 500 µl in serum-free medium were added into the upper chamber and 750 µl of cells conditioned medium was added into the lower chamber. After incubation in humidified tissue culture incubator, 37°C, 5% CO2 atmosphere for 24 h, the non-migration cells in the upper surface of the membrane were removed by “scrubbing” with cotton tipped swab and the cells migrating to the lower surface of the membrane were fixed and stained with 0.5% crystal violet for 30 minutes. Cell counting was then carried out by photographing the membrane through the microscope. Five random fields under microscope were taken and migration cells were quantified [19]. The data were presented as mean ± SE. Differences in the results of different groups were evaluated using either two-tailed Student’s t test or one-way ANOVA followed by post hoc Dunnett’s test.
Invasion assay
Assay was performed with Matrigel-coated chambers from a BioCoat Matrigel Invasion Chamber Kit (BD Biosciences). Cells with 500 µl in serum-free medium were added into the upper chamber and complete medium was added into the lower chamber. After incubation for 24 h, non-invasive cells in the upper surface of the membrane were removed and the cells invasion to the lower surface of the membrane was fixed. Cell counting was then carried out by photographing the membrane through the microscope [20] and five random fields were taken.
Matrix metalloproteins (MMPs) activity assay
The activity of MMP-9 and MMP-2 were determined by QuickZyme MMPs activity assay (QucikZyme BioSciences) according to the manufacturer’s instructions [21]. Briefly, after transfection, cells were washed with fresh medium and replaced with serum-free medium. After additional 24 h, the medium was collected and centrifuged at 10000 g for 10 min. Respective supernatant was added to the 96-well strip coated with MMP-9 antibody or MMP-2 antibody and incubated at 4°C overnight. After washing with wash buffer for 3 times, 50 µl assay buffer was added into the well, followed by adding 50 µl detection reagent. After incubation at 37°C for 1 h, OD405 was measured with Microplate Reader (Bio-Tek).
Western blot
After washing with PBS (3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl and 140 mM NaCl, pH 7.4) twice, cells were extracted with cold lysis buffer (20 mM Tris, 100 mM NaCl, 5 mM EDTA, 1 mM EGTA, 5 mM MgCl2, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM b-glycerolphosphate, 1 mM Na3VO4, 1 mM PMSF, and Roche complete protease inhibitors) and centrifuged at 15,000 g for 15 min at 4°C. Protein concentration of the supernatants was determined with Bradford assay (Biorad, USA). 10-40 µg of samples was separated by electrophoresis on 10% SDS-PAGE and transferred to Polyvinylidene fluoride membrane (Millipore, USA). After blocking with 5% skimmed milk for 1 h, membranes were incubated with different specific primary antibodies in either 5% skimmed milk or 5% bovine serum albumin (BSA). After washing with TBST for 30 min, the membranes were further incubated with corresponding HRP-conjugated secondary antibodies and developed with Pierce’s West Pico chemiluminescence substrate (Millipore, USA). All results were obtained from 3 independent experiments.
Real-time PCR
Total RNA was isolated using TRIzol according to the manufacturer’s instructions (Invitrogen, USA) and the concentration of total RNA was detected by spectrophotometry at OD260. Reverse transcription (RT) was carried out using superscript III reverse transcriptase (Invitrogen, USA) as described in the manufacturer’s manual. The real-time PCR was performed on ABI Prism 7500 Sequence detection system (Applied Biosystems, CA) with the KAPA SYBR® qPCR Kit (KAPA Biosystems, USA) according to the manufacturer’s instructions. The primers used was as follow: Serpine2 (Forward: 5’- CCCTACCATGGTGTGAGAGCAT-3’, Reverse: 5’-GCCTTTGACGGTTCAAACAT-3’), β-actin (Forward: 5’-GCTCTTTTCCAGCCTTCCTT-3’, Reverse: 5’-TGATCCACATCTGCTGGAAG-3’). The target mRNA level of control cells normalized to the level of β-actin mRNA, was defined as 1. Results were obtained from three independent experiments. For gene expression assay, the total RNA was extracted from cell cultures by using the phenol-chloroform method (Trizma; Sigma-Aldrich), treated with DNase-free kit (Ambion; Applied Biosystems), and quantified with an RNA 6000 Nano Assay kit in an Agilent 2100 Bioanalyzer (Agilent Technologies). Single-strand cDNA synthesis from total RNA was prepared by TaqMan reverse transcription reagents (Applied Biosystems), as recommended by the manufacturer. All quantitative real-time PCR reactions were performed with ABI PRISM 7900 HT Fast Real-Time PCR system (Applied Bio-systems). The expression of the housekeeping gene, TATA Binding Protein (TBP), was used to normalize for variances in input cDNA. All measurements were performed in technical duplicates. Raw data were analyzed using SDS2.2 software (Applied Biosystems) to define relative quantity (RQ) and the gene expression data analysis program GEDAS (http://sourceforge.net/projects/gedas) for hierarchical clustering. The following TaqMan Gene Expression Assay (Applied Bio-systems) has been used for the analysis: CXCL-3-Hs00171061_m1, CD44-Hs01075862_m1, MMP-2-Hs01548727_m1, cMET-Dm01843535_g1, CD31-Hs00169777_m1, CCL-2-Hs00234140_m1, IL11-Hs01055413_g1, VEGFA-Hs00900055_m1, bRAF-Hs00269944_m1, cRAF-Hs00234119_m1, MMP-14-Hs01037003_g1, IL-6-Hs00985639_m1, VEGF-C-Hs00153458_m1, CXCL-12-Hs03676656_mH, IL8-Hs00174103_m1, DLL4-Hs01117333_m1, ERK1/2-Hs00946872_m1, PI3K-Hs01046353_m1, AKT-Hs01086102_m1, FGFR1-Hs00915142_m1, Src-Hs01082246_m1, PTEN-Hs02621230_s1, E-cadherin-Hs01023894_m1, BCAR1-Hs01547079_m1, Rap1-Sc04159738_s1, Paxillin-Hs01104424_m1, TP53-Hs01034249_m1, Hif1a-Hs00153153_m1, STAT3-Hs00374280_m1, FAK-Hs00164611_m1, GSK-3β-Ce02424746_m1, mTOR-Hs00234508_m1, CXCL2-Hs00601975_m1, EGFR- Hs01076078_m1.
Animals and experimental metastasis assay
Female BALB/c-nu mice at 6-8 weeks old (15-20 g) were purchased from Shanghai Slack laboratory animal co., LTD. Mice were maintained at dark/light cycles of 12 h duration with food and water available. 12 animals were randomly divided into two experiment groups. For experimental metastasis analysis, the mice were injected at the lateral tail vein with (5 × 105) cells carrying control and Serpine2 siRNA plasmids. Mice were sacrificed 2 weeks after inoculation and all organs were examined for the presence of macroscopic metastases. Consecutive sections were made for every tissue block of the lungs and stained with H&E [22]. Animal handling and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai TongRen Hospital.
Statistical analysis
For quantitative PCR analyses, results were obtained from triplicate experiments on all the samples and data from all trials were averaged. Numerical results were analyzed using independent mean T-test and expressed in mean ± standard error (SE). Statistical analysis was performed using post hoc testing using Bonferroni’s method. Differences were considered statistically significant at p < 0.05.
Results
Serpine2 is a novel melanoma metastasis regulator
The SK-MEL-2 melanoma cell line is a widely used as model system to study the metastasis of melanoma for its high metastatic potential [23]. To identify the key genes involved in migration and metastasis of melanoma, pDisrup vector was transfected into SK-MEL-2 cells to randomly produced insertions into the genomic DNA, and followed by selection with blasticidin to obtain SK-MEL-2 cell clones with genes mutated. The mobility and migration ability of the selected mutant SK-MEL-2 cell clones was determined by Transwell migration assay. Finally, cell clones with increased or decreased migration potential were further analyzed by the RT-PCR and 3’RACE to identify the genes that disrupted by pDisrup vector. Several candidate genes were identified, including a gene named Serpine2 and this candidate was designated as Serpine2mut which exhibited decreased migration potential. To verify whether the Serpine2 gene identified was indeed disrupted in SK-MEL-2 cells, both real-time PCR and western blot assay was carried out to determine Serpine2 gene expression. As shown in Figure 1A and 1B, the expression of Serpine2 was greatly reduced in Serpine2mut cell clone compared to control cells. To determine if loss function of Serpine2 affected SK-MEL-2 metastasis, we performed migration and invasion assay to evaluate the cell motility. As shown in Figure 1C, the migration of Serpine2mut cells was significant decreased compared to control ones. Consistently, there were less Serpine2mut cells that invasive across the membrane of the Transwell chamber compared to the wide type cells (Figure 1D). In summary, disruption of Serpine2 led to reduced SK-MEL-2 cells mobility and significantly impaired the metastasis of melanoma cell SK-MEL-2.
Figure 1.
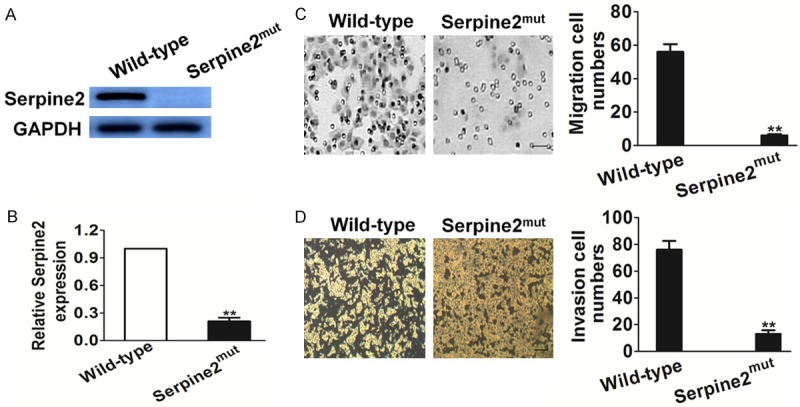
Identification of a novel role of Serpine2 in the metastasis of melanoma. A and B. Serpine2 expression in Serpine2mut cells was analyzed by and Western blotting real-time PCR. C. Migration of wild-type cells and Serpine2mut cells was performed. The number of cell migration was counted. Scale bars: 50 µm. Data were collected from three independent experiments and were average ± S.E. values. **P < 0.01, compared to wild type cells. D. The cell motility potency was evaluated by invasion assay. Representative pictures were taken after staining with crystal violet. Scale bars: 50 µm. Data were collected from three independent experiments and were average ± S.E. values. **P < 0.01, compared to wild type cells.
Serpine2 in melanoma
To investigate the mRNA expression of Serpine2, we performed quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis in normal human melanocyte cell line NHEM-a and a panel of melanoma cell lines. Serpine2 mRNA was up-regulated in all melanoma cell lines compared with that in the NHEM-a (Figure 2A). We also performed western blot analysis to investigate the Serpine2 expression status in the melanoma cells. A significant increase in Serpine2 expression was seen in melanoma cell lines compared with NHEM-a (Figure 2B). The expression level of Serpine2 genes in melanoma was also analyzed using Oncomine (https://www.oncomine.org/). For this, we compared clinical specimens of cancer vs. normal patient from the Haqq Melanoma database to identify the key genes involved in metastasis [17]. We analyzed the results for their p-values and fold change. Oncomine analysis of neoplastic vs. normal tissue showed that Serpine2 was significantly over-expressed in melanoma and associated with cancer metastasis (Figure 2C and 2D).
Figure 2.
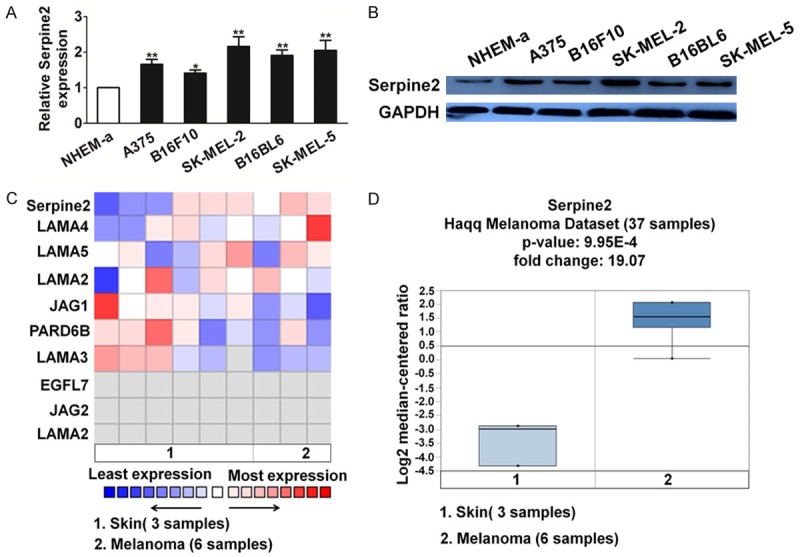
Serpine2 is over-expression in melanoma and be associates with metastasis. A. Quantification of Serpine2 mRNA levels in melanoma-derived cell lines by qRT-PCR analysis. All melanoma cells had significant up-regulation of Serpine2 mRNA compared with that in the NHEM-a (Bars were represented as the mean ± S.E, n=3, *P < 0.05 and **P < 0.01 versus NHEM-a). B. Immunoblotting analysis of Serpine2 protein in the melanoma cell lines and melanocyte cell. Serpine2 protein expressions were up-regulated in all melanoma cell lines examined compared with that in NHEM-a. C. This graphic shown genes relation to metastasis expressed at high levels in melanoma. D. This graphic compared the dataset that the specified protein Serpine2 had significant mRNA over-expression of in melanoma versus normal tissue. The datasets were obtained with the following parameters: p-value threshold of 0.01.
Cell proliferation is not inhibited after Serpine2 knockdown
To ascertain Serpine2 was responsible for reduced metastasis in melanoma cells, we transfected SK-MEL-2 cells with Serpine2 siRNA to stably establish SK-MEL-2-siSerpine2 cells with two independent siRNAs specific for Serpine2. We used PCR and Western blot analyses to verify Serpine2 down-regulation at the RNA and protein levels, respectively. Our results shown that Serpine2 RNA and protein expression were down-regulated in the SK-MEL-2 siSerpine2 cells compared with siCTL cells (Figure 3A and 3B).
Figure 3.
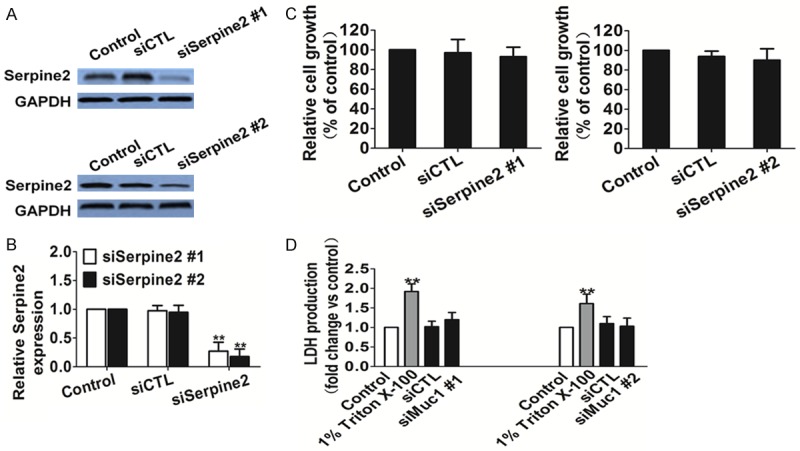
The effect of siSerpine2 on tumor cells growth. A. Western blot shown that the Serpine2 was elevated in control cells and siCTL transfected cells than siSerpine2 cells. GAPDH was used as a loading control. B. Quantification of Serpine2 mRNA levels in transfected cells. siSerpine2 melanoma cells had significant down-regulation of Serpine2 mRNA compared with that in the control cells (Bars were represented as the mean ± S.E, n=3, **P < 0.01 versus control). C. One solution cell proliferation assay analyzed cell proliferation in SK-MEL-2-siSerpine2 and control cells. Data were from three independent experiments and were average ± S.E. values. **P < 0.01, compared to control cells. D. Serpine2 siRNA did not result in LDH release, indicating Serpine2 siRNA brought little toxic effects on melanoma cells. Data were from three independent experiments and were average ± S.E. values. **P < 0.01, compared to control cells.
Because the role of Serpine2 in the melanoma has rarely been examined, we investigated the effect of Serpine2 on the proliferation of SK-MEL-2 cells. For proliferation, no significant changes were observed between the siSerpine2 cells and the control cells (Figure 3C). To validate the toxicity effects of Serpine2 siRNA on melanoma cells, lactic dehydrogenase, LDH assay was carried out. As shown in Figure 3D, Triton X-100 significantly increased LDH release, and siCTL treated as well as Serpine2 knock-down brought little toxic effects on cells compared to vehicle control. Because the down-regulation was stronger in the siSerpine2 #1 cells (Figure 3A), we employed SK-MEL-2-siSerpine2 #1 cells to perform the following studies. These cells are labeled as “siSerpine2” cells in the figures.
The knockdown of endogenous Serpine2 attenuates cell migration and invasion in melanoma cells
We investigated whether reduced melanoma SK-MEL-2 cells migration could be reproduced by gene silencing with siRNA specific for Serpine2. A migration assay was used to investigate the effect of Serpine2 on cell migration. SK-MEL-2-siSerpine2 cells displayed significantly decreased migration compared with control cells (Figure 4A). In addition, we analyzed the cell invasion profile of SK-MEL-2-siSerpine2 cells using invasion analysis. As expected, Serpine2 silencing inhibited this process (Figure 4B).
Figure 4.
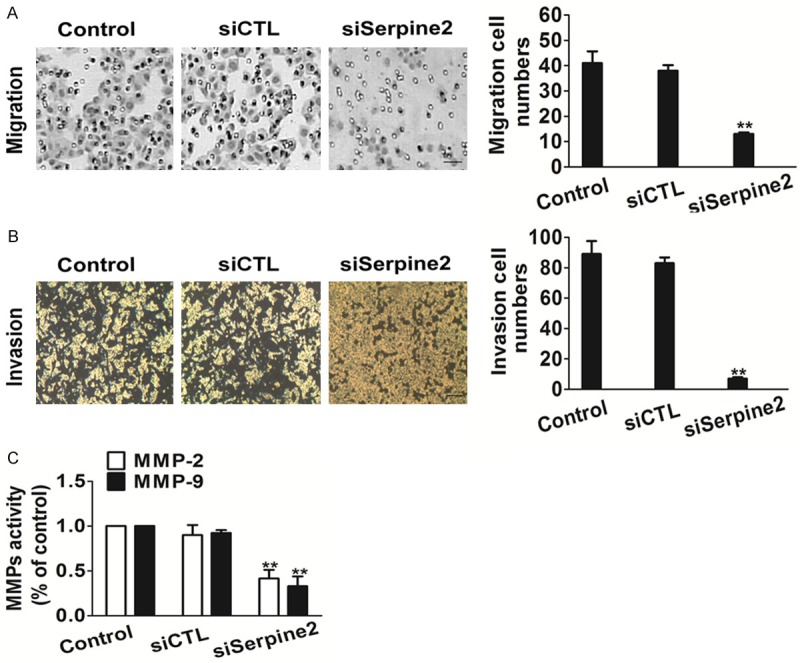
Serpine2 silencing decreases the migration of melanoma SK-MEL-2 cells. A. Migration assay was performed to determine the metastatic potential of cells and representative pictures of the wound distance were taken as indicated. The number of migration cells was quantified. Data were collected from three independent experiments and were average ± S.E. values. **P < 0.01, compared to control cells. (Scale bar represents 50 μm). B. The cell motility was evaluated by Transwell assay and the number of invasion cells was quantified. Scale bars: 50 µm. Data were from three independent experiments and are average ± S.E. values. *P < 0.01, compared to control cells. C. Quantification of MMP-2/9 activity in SK-MEL-2 cells transfected with siSerpine2. Bars are represented as the mean ± S.E. n=3, **P < 0.01 versus siCTL cells.
Matrix metalloproteinases (MMPs) consist of a multigene family of zinc-dependent extracellular matrix (ECM) remodeling endopeptidases implicated in pathological processes, such as carcinogenesis [24]. We then tested the effect of Serpine2 knock-down on the activity of MMP-2/9. Consistent with cells assay result, the activity of MMP-2/9 was also markedly decreased with the down-expression of Serpine2 (Figure 4C). Collectively, these results suggest that Serpine2 promotes melanoma cells metastasis and MMPs activity.
GSK-3β pathway is involved in Serpine2-mediated melanoma cells metastasis
We next compared the transcriptional profile of genes involved in metastasis between SK-MEL-2-siSerpine2 cells and control cells. This comprehensive approach revealed that siSerpine2 decreased the expression of several pro-metastasis molecules, including mammalian target of rapamycin, mTOR, GSK-3β, and extracellular regulated protein kinases, ERK1/2 (Figure 5A). To future determine the potential signaling pathways which are involved in Serpine2-mediated melanoma cell migration and invasion, several potential signaling pathways related to migration and invasion of cancer cells were assayed by western blot assay. As shown in Figure 5B, the basal level of GSK-3β activation was found to be significantly down-regulated in Serpine2 siRNA cells. In combination, these results strongly suggest that Serpine2 facilitates the activation of GSK-3β signaling pathway in melanoma cells.
Figure 5.
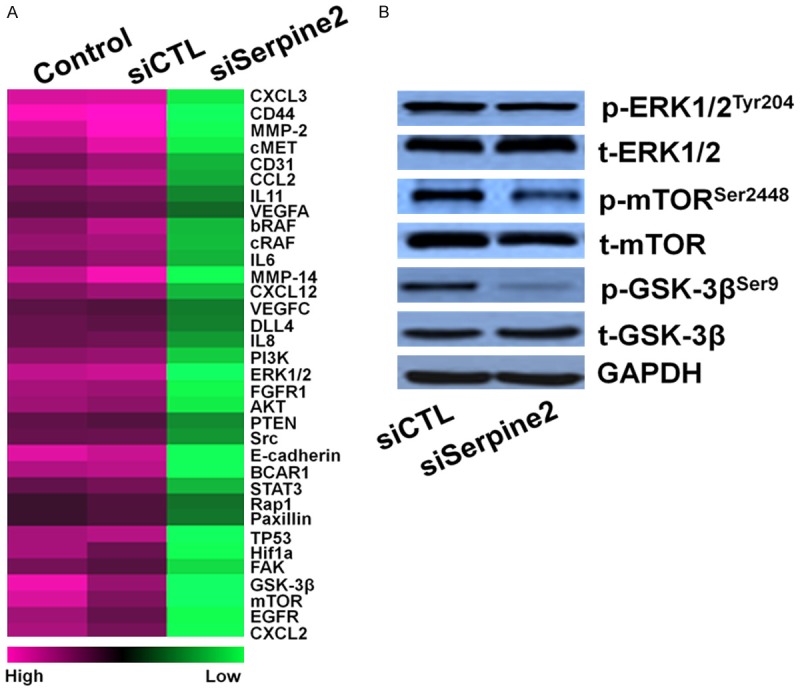
Serpine2 regulates GSK3β pathway in melanoma cells. A. Serpine2 down-regulated the expression of pro-metastasis factors in siSerpine2 cells. Gene expression analysis was performed by real-time PCR comparing control SK-MEL-2 cells. Differentially modulated genes were selected and analyzed by hierarchical clustering. B. Western blot shown that the phosphorylation of GSK-3β was inhibited in cells transfected with siSerpine2.
Confirmed GSK-3β pathway is involved in Serpine2-mediated melanoma cells metastasis
Among the metastasis factors that were pinpointed by the transcriptional profile, we focused on GSK-3β because this molecule is a well known master switch of the metastasis program. To confirm the role of GSK-3β in Serpine2-mediated cell metastasis, constitutively active form of GSK-3β (S9A-GSK3β) was introduced into Serpine2-silenced cells and the expression of S9A-GSK3β was confirmed by western blot with anti-HA-tag and anti-GSK-3β antibody (Figure 6A). As expected, active GSK-3β largely restored the impaired migration (Figure 6B) and invasion (Figure 6C) in Serpine2-silenced SK-MEL-2 cells. To conclude, these data indicated that GSK-3β signaling is involved in Serpine2 promoted metastasis of melanoma cells.
Figure 6.
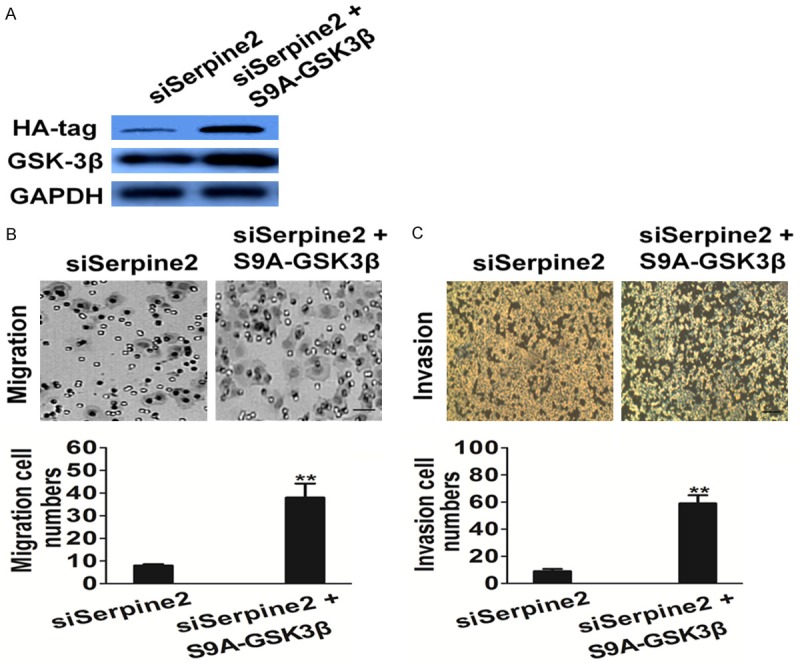
Serpine2 facilities the migration of melanoma cells via GSK3β pathway. A. Expression of S9A-GSK3β was confirmed by western blot with antibody against HA-tag and GSK3β, and GAPDH was used as loading control. B. Transwell assay was performed to determine the motility of cells co-transfected with Serpine2 silencing plasmid and S9A-GSK3β plasmid. Columns were data collected from three independent experiments. Representative pictures were taken after staining with crystal violet. Scale bar represents 50 μm. Data were from three independent experiments and are average ± S.E. values. **P < 0.01, compared to siSerpine2 cells. C. Invasion assay was performed to determine the invasion of cells co-transfected with Serpine2 silencing plasmid and S9A-GSK3β plasmid. Columns were data collected from three independent experiments. Scale bar represents 50 μm. Data were from three independent experiments and are average ± S.E. values. **P < 0.01, compared to siSerpine2 cells.
Serpine2 knockdown reduces the metastasis of melanoma cells in vivo
To further investigate the role of Serpine2 in the metastasis of tumor cells in vivo, an experimental metastasis assay was performed. Control and Serpine2 knock-down cells were injected into the lateral tail vein of BALB/c-nu mice. 2 weeks post inoculation, animals were sacrificed and all the major organs were checked for the generation of lung metastasis. The tumor metastasis was mainly observed by hematoxylin and eosin (HE) staining in the lungs (Figure 7A). The numbers of lung metastatic lesions in the mice injecting with siSerpine2 cells were greatly decreased compared with the respective controls group (Figure 7B). To further examine whether Serpine2 knock-down suppress melanoma cells growth via GSK-3β, GSK-3β activity in lung tissues were assayed by western blot analysis with specific antibody. Mice implantation with Serpine2 knockdown cells showed a significant reduction of p-GSK-3βSer9 in lung tissue (Figure 7C). All these results implied that Serpine2 silencing indeed perturbed the metastasis of melanoma cells not only in vitro but also in vivo via suppressed GSK-3β.
Figure 7.
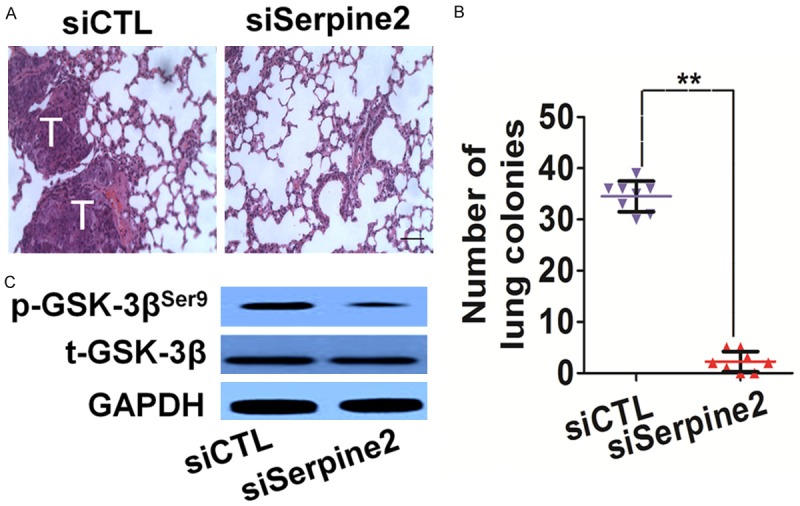
The metastasis of melanoma SK-MEL-2 is impaired by Serpine2 silencing in vivo. A. siCTL and siSerpine2 cells were injected into the lateral vein of mice respectively. Representative pictures of lungs from mice were taken and HE staining after 2 weeks of injection with control cells or with Serpine2 knock-down cells. “T” in white color represented metastases foci. Scale bar represents 100 μm. B. Numbers of lung metastasis were quantified and showed by each data point. Data were from three independent experiments and were average ± S.E. values. n=8, **P < 0.01, compared to mice injected with siTCL cells. C. Western blot showed that the phosphorylation of GSK-3β was inhibited in lung tissue from mice injected with Serpine2 siRNA cells.
Confirm Serpine2 as a novel melanoma metastasis regulator
To further confirm the role of Serpine2 in melanoma cells metastasis, we transfected Serpine2-bio-His into SK-MEL-2 cells. The transfection efficiency was confirmed by western blot with Serpine2 antibody (Figure 8A). The metastasis of Serpine2 over-expressed SK-MEL-2 cells was then examined by migration and invasion assay. As shown in Figure 8B, there were more Serpine2 over-expressed cells migrated across the membrane compared with cells transfected vector. Using invasion analysis, we investigated the effects of Serpine2 over-expression on invasion in tumor cells. In accordance with migration result, we found that SK-MEL-2 transfected with Serpine2 resulted in significantly increasing invasion (Figure 8B).
Figure 8.
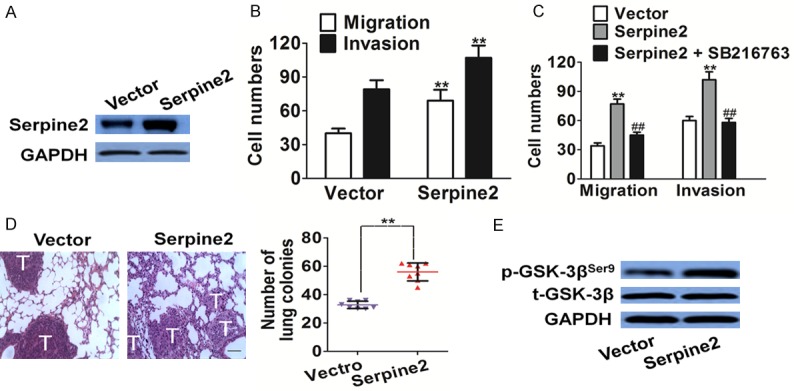
Serpine2 over-expression promotes melanoma cells metastasis. A. The expression of Serpine2 was determined western blot with Serpine2 antibody. GAPDH was used as a loading control. B. The cell motility was evaluated by Transwell assay and the number of migration and invasion cells was quantified after Serpine2 transfection. Data were from three independent experiments and are average ± S.E. values. **P < 0.01, compared to vector cells. C. In the presence of SB216763 (2 nM), transwell migration and invasion assay was conducted to evaluate the cell metastasis after Serpine2 transfection. Data were from three independent experiments and were average ± S.E. values. **P < 0.01, compared to transfected vector cells; ##P < 0.01, compared to transfected Serpine2 cells. D. Control vector transfected and Serpine2 over-expression cells were injected into the lateral vein of mice respectively. Representative pictures of lungs from mice were taken after 2 weeks of injection with control cells or with Serpine2 over-expression cells. Numbers of lung metastasis were quantified and showed by each data point. Data were from three independent experiments and were average ± S.E. values. **P < 0.01, compared to mice injected with vector transfected cells. Scale bars: 100 μm. E. Western blot shows that the phosphorylation of GSK-3β was increased in lung tissue from mice injected with Serpine2 over-expression cells.
To confirm the role of GSK-3β in Serpine2 mediated cell metastasis, SB216763, a GSK-3β specific inhibitor was also employed to dissect the role of GSK-3β signaling in cell migration [25]. As shown in Figure 8C, Serpine2 promoted metastasis in SK-MEL-2 cells were also abolished by SB216763, as shown by the Transwell migration and invasion assay. To conclude, these data indicate that GSK-3β signaling is involved in Serpine2 promoted metastasis of melanoma cells.
To further investigate the role of Serpine2 in the metastasis of melanoma cells in vivo, Serpine2 over-expression cells were injected into the lateral tail vein of BALB/c-nu mice. 2 weeks post inoculation, animals were sacrificed and we found that injection of Serpine2 over-expression cells resulted in the formation of more lung colonies than vector cells (Figure 8D). These results implied that Serpine2 indeed perturbed the metastasis of melanoma cells not only in vitro but also in vivo. Serpine2 has been identified as a key regulator in the GSK-3β and influences cell migration and invasion in vitro. To investigate Serpine2-mediated metastasis in vivo was through regulation of GSK-3β, GSK-3β activity in lung tissues was assayed by western blot. Mice injected with Serpine2 over-expression cells showed a significant increasing of phospho-GSK-3β expression in lung (Figure 8E).
Discussion
Currently, the dismal prognosis of malignant melanoma is due to the late stage at which it is usually diagnosed and the highly invasive and metastatic potential of melanoma, resulting in a poor overall survival of patients with malignant melanoma [26]. To develop effective new strategies for the treatment of malignant melanoma metastasis, it is of prime importance to elucidate the molecular events involved in the metastasis of this disease and to identify efficient therapeutic targets [2]. In the present study, we identified a novel role for Serpine2 in the metastasis of melanoma cells. We found that Serpine2 promotes the metastasis of melanoma cells in vitro and in vivo through the GSK-3β signaling pathway. Our results may provide a new target for intervention in the melanoma treatment and may improve the future treatment of melanoma.
Cancer metastasis is a multistep process involving complex and highly coordinated interactions between tumor cells and a constantly changing host microenvironment. DNA microarray technology has led to an explosion of oncogenomic analyses, generating a wealth of data and uncovering the complex gene expression patterns of cancer. Here, we present Oncomine, a cancer microarray database and web-based data-mining platform aimed at facilitating discovery from melanoma metastasis genes expression analyses. Oncomine analysis showed significant levels of Serpine2 in melanoma metastasis. We also performed qRT-PCR and immunoblotting using melanoma-derived cell lines. Based on the observation that the Serpine2 is strongly over-represented in the highly metastatic melanoma cells, we hypothesized that Serpine2 may play an important part in this complex process.
The extra-cellular Serpine2, also called Protease Nexin-1 (PN-1) belongs to the Serpin gene superfamily. Serpins are expressed in tissues throughout the body and function in many physiological processes including inflammation, tumor growth and metastasis [12]. In particular, Serpine2 is up-regulated in a large number of invasive/metastatic tumors including breast, prostate, pancreatic, colorectal, oral-squamous, and testicular cancers and is required for tumor growth and malignant progression. Serpine2 is up-regulated by ERK signal transduction and forms covalent complexes with its protease substrates in the extra-cellular matrix (ECM) following secretion. Taken together, these studies reveal the complexity of Serpine2 functions during tumorigenesis. So far, no investigation has been carried out on the involvement of Serpine2 in melanoma metastasis. In this work, we firstly reported a novel role for Serpine2 in the metastasis of melanoma cells. Investigation with gene silencing of Serpine2 showed that the metastasis of melanoma cells was significantly decreased as revealed by the wound healing assay and invasion assay.
The underlying molecular mechanism for Serpine2-regulated melanoma metastasis is identified to be related to GSK-3β signaling pathway. Our results showed that down-expression Serpine2 in melanoma cells induces down-regulation of GSK-3β phosphorylation. Furthermore, restored GSK-3β activity by an active form GSK-3β plasmid could rescue the impaired migration and invasion of melanoma cells induced by Serpine2 silencing. GSK-3β has been implicated to play roles in cancers which are resistant to chemo-, radio-, and targeted therapy. Targeting GSK-3β may be a means to overcome the resistance of these cancers to certain chemotherapeutic drugs, radiation and small molecule inhibitors [27]. More convincingly, Serpine2 silencing markedly impaired the lung metastasis of melanoma cells in vivo. We found that tumors in nude mice derived from transplanted siSerpine2 cells displayed little lung metastasis than did tumors derived from control cells. In the presence of SB216763, a specific inhibitor of GSK-3β signaling pathway, Serpine2 over-expression promoted migration was also significantly inhibited.
All these data presented that Serpine2 is involved in the metastasis of melanoma cells. Due to the important roles of Serpine2 in the metastasis of melanoma cells, it may serve as an attractive target for molecular targeting cancer therapy. In the further work, it is worthwhile to elucidate the precise roles of Serpine2 in regulating the GSK-3 beta signaling thus mediating the metastasis in melanoma cells.
Disclosure of conflict of interest
None.
References
- 1.Mort RL, Jackson IJ, Patton EE. The melanocyte lineage in development and disease. Development. 2015;142:620–632. doi: 10.1242/dev.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonsalves CF, Eschelman DJ, Thornburg B, Frangos A, Sato T. Uveal Melanoma Metastatic to the Liver: Chemoembolization With 1,3-Bis-(2-Chloroethyl)-1-Nitrosourea. AJR Am J Roentgenol. 2015;23:1–5. doi: 10.2214/AJR.14.14001. [DOI] [PubMed] [Google Scholar]
- 3.Aris M, Barrio MM. Combining immunotherapy with oncogene-targeted therapy: a new road for melanoma treatment. Front Immunol. 2015;9:46. doi: 10.3389/fimmu.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olszanski AJ. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J Manag Care Spec Pharm. 2014;20:346–356. doi: 10.18553/jmcp.2014.20.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azijli K, Stelloo E, Peters GJ, VAN DEN Eertwegh AJ. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014;34:1493–1505. [PubMed] [Google Scholar]
- 6.Spagnolo F, Queirolo P. Upcoming strategies for the treatment of metastatic melanoma. Arch Dermatol Res. 2012;304:177–84. doi: 10.1007/s00403-012-1223-7. [DOI] [PubMed] [Google Scholar]
- 7.Ortega CE, Seidner Y, Dominguez I. Mining CK2 in Cancer. PLoS One. 2014;9:e115609. doi: 10.1371/journal.pone.0115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Shuman MA, Cohen RL. Co-expression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung cancer cells. Int J Cancer. 1995;60:501–6. doi: 10.1002/ijc.2910600413. [DOI] [PubMed] [Google Scholar]
- 9.Vaillant C, Valdivieso P, Nuciforo S, Kool M, Schwarzentruber-Schauerte A, Méreau H, Cabuy E, Lobrinus JA, Pfister S, Zuniga A, Frank S, Zeller R. Serpine2/PN-1 Is Required for Proliferative Expansion of Pre-Neoplastic Lesions and Malignant Progression to Medulloblastoma. PLoS One. 2015;10:e0124870. doi: 10.1371/journal.pone.0124870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergeron S, Lemieux E, Durand V, Cagnol S, Carrier JC, Lussier JG, Boucher MJ, Rivard N. The serine protease inhibitor serpinE2 is a novel target of ERK signaling involved in human colorectal tumorigenesis. Mol Cancer. 2010;9:271. doi: 10.1186/1476-4598-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh YS, Yu J, Kim BC, Choi B, Han TS, Ahn HS, Kong SH, Lee HJ, Kim WH, Yang HK. Overexpression of Plasminogen Activator Inhibitor-1 in Advanced Gastric Cancer with Aggressive Lymph Node Metastasis. Cancer Res Treat. 2015;47:718–26. doi: 10.4143/crt.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Wang B, Xing AY, Xu KS, Li GX, Yu ZH. Prognostic significance of SERPINE2 in gastric cancer and its biological function in SGC7901 cells. J Cancer Res Clin Oncol. 2015;141:805–12. doi: 10.1007/s00432-014-1858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303:701–4. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Vandenbriele C, Kauskot A, Verhamme P, Hoylaerts MF, Wright GJ. A human platelet receptor protein microarray identifies FcepsilonR1alpha as an activating PEAR1 ligand. Mol Cell Proteomics. 2015;14:1265–74. doi: 10.1074/mcp.M114.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Chong CC, Chen GG, Lai PB. A Seven-microRNA Expression Signature Predicts Survival in Hepatocellular Carcinoma. PLoS One. 2015;10:e0128628. doi: 10.1371/journal.pone.0128628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR, Allen RE, Singer MI, Leong SP, Ljung BM, Sagebiel RW, Kashani-Sabet M. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005;102:6092–7. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adaramoye O, Erguen B, Oyebode O, Nitzsche B, Höpfner M, Jung K, Rabien A. Antioxidant, antiangiogenic and antiproliferative activities of root methanol extract of Calliandra portoricensis in human prostate cancer cells. J Integr Med. 2015;13:185–93. doi: 10.1016/S2095-4964(15)60175-3. [DOI] [PubMed] [Google Scholar]
- 19.Choedon T, Mathan G, Kumar V. The traditional Tibetan medicine Yukyung Karne exhibits a potent anti-metastatic activity by inhibiting the epithelial to mesenchymal transition and cell migration. BMC Complement Altern Med. 2015;15:182. doi: 10.1186/s12906-015-0707-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Seong I, Min HJ, Lee JH, Yeo CY, Kang DM, Oh ES, Hwang ES, Kim J. Sox10 controls migration of B16F10 melanoma cells through multiple regulatory target genes. PLoS One. 2012;7:e31477. doi: 10.1371/journal.pone.0031477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto-Guzman A, Villegas-Comonfort S, Cortes-Reynosa P, Perez-Salazar E. Role of arachidonic acid metabolism in Stat5 activation induced by oleic acid in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2013;88:243–9. doi: 10.1016/j.plefa.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Liu XY, Tang QS, Chen HC, Jiang XL, Fang H. Lentiviral miR30-based RNA interference against heparanase suppresses melanoma metastasis with lower liver and lung toxicity. Int J Biol Sci. 2013;9:564–77. doi: 10.7150/ijbs.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schayowitz A, Bertenshaw G, Jeffries E, Schatz T, Cotton J, Villanueva J, Herlyn M, Krepler C, Vultur A, Xu W, Yu GH, Schuchter L, Clark DP. Functional profiling of live melanoma samples using a novel automated platform. PLoS One. 2012;7:e52760. doi: 10.1371/journal.pone.0052760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cathcart J, Pulkoski-Gross A, Cao J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee-Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 26.Nagahara A, Nakayama M, Oka D, Tsuchiya M, Kawashima A, Mukai M, Nakai Y, Takayama H, Nishimura K, Jo Y, Nagai A, Okuyama A, Nonomura N. SERPINE2 is a possible candidate promotor for lymph node metastasis in testicular cancer. Biochem Biophys Res Commun. 2010;391:1641–6. doi: 10.1016/j.bbrc.2009.12.105. [DOI] [PubMed] [Google Scholar]
- 27.Darrington RS, Campa VM, Walker MM, Bengoa-Vergniory N, Gorrono-Etxebarria I, Uysal-Onganer P, Kawano Y, Waxman J, Kypta RM. Distinct expression and activity of GSK-3α and GSK-3β in prostate cancer. Int J Cancer. 2012;131:E872–83. doi: 10.1002/ijc.27620. [DOI] [PubMed] [Google Scholar]


