Abstract
Background: Qiliqiangxin (QL) capsule is a traditional Chinese medicine which has been approved for the treatment of chronic heart failure. Evidences proved that QL capsules further reduced the NT-proBNP levels and improved left ventricular ejection fraction in CHF patients but the evidence supporting its underlying mechanism is still unclear. Methods and Results: Myocardial infarction (MI) -Heart failure (HF) Sprague-Dawley ratsmodel and neonatal rat cardiac myocytes (NRCMs) were used. Animals were assigned into 4 groups, normal group (n=6), shame-operation group (n=6), MI rats 4 weeks after left anterior descending coronary artery ligation were randomized into vehicle group (n=8), QL group (n=8). QL significantly attenuated cardiac dysfunction and ventricle remodeling as echocardiography and hemodynamic measurements showed improvement in left ventricular ejection fraction, fractional shortening, ±dp/dt and left ventricular end diastolic and systolic diameters in QL treated group compared with the vehicle group. Improvements ininterstitial fibrosisand mitochondrial structures were also exhibited by Sirius Red staining, RT-PCR and electron microscopy. QL treatment improved apoptosis and VEGF expression in rats marginal infract area. Complementary experiments analyzed the improved apoptosis and up-regulate of VEGF in ischemia-hypoxia cultivated NRCMs is in an Akt dependent manner and can be reversed by Akt inhibitor. Conclusion: QL capsule can improve cardiac dysfunction and ventricular remodeling in MI-HF ratsmodel, this cardiac protective efficacy may be concerned with attenuated apoptosis and cardiac fibrosis. Up-regulated VEGF expression and Akt phosphorylation may take part in this availability.
Keywords: Qiliqiangxin, myocardial infarction, heart failure, cardiac dysfunction, cardiac fibrosis, VEGF
Introduction
Chronic heart failure (CHF) is a range of clinical syndromes characterized by decreased cardiac function, it is a public health problem worldwide and an important topic in clinical cardiology associated with high morbidity and mortality. Myocardial infarction (MI) is a dominating pathogenesis of HF and provide an efficient experimental model for research; [1] the dysfunction and remodeling of left ventricular after MI combined with a series of pathologic changes including myocyte apoptosis, myofibroblast proliferation, interstitial fibrosis plays a key role in HF development and take the most common causes of cardiovascular morbidity and mortality [2,3]. CHF is also a heavy burden in China, epidemiological studies showed that the incidence of HF in Chinese adults is 0.9% for the general population [4]. Despite the development and progression in drug treatment strategy for HF under the instructions of guidelines, the mortality continues to rise [5]. Traditional Chinese Medicine (TCM) is an efficient complement for conventional treatment whose clinical effect have gained favorable acceptance both in China and worldwide.
Chinese herbs have demonstrated safety and efficacy in the treatment of heart failure refer to this theory, [6-8] Qiliqiangxin (QL) isa formulation based on several common medicinal herbs in TCM which has been applied for the treatment of CHF patientssince ancient China. QL capsule was approved by the Chinese Food and Drug Administration for heart failure treatment in 2004. J Zhang performed a first multicenter; randomized; double-blind; parallel-group and placebo-controlled study for QL in HF treatment, QL capsule demonstrated a significantly greater reduction in the NT-proBNP level from baseline than control group at the 12-week follow-up (p=0.002) and also showed superior performance with respect to New York Heart Association functional classification; left ventricular ejection fraction (LVEF); 6-min walking distance and quality of life [9]. Recent studies demonstrated that QL produce cardiac protection effect through a variety of pathways involved in anti-inflammation, anti-fibrosis and anti-apoptosis; QL could regulate the balance between pro-inflammatory and anti-inflammatory cytokines like tumor necrosis factor-alpha, interleukin-10 and interleukin-6 in cardiomyocytes [10]. It may also down-regulate the cardiac chymase signaling pathway and chymase-mediated AngII production [11]. Moreover, it can regulate cardiomyocyte apoptosis, proliferation and mitochondrial function which finally improved cardiac remodeling and cardiac function [12]. In MI rats, QL can play an efficient role in attenuate cardiac remodeling by increasing PPARγ level sand regulate the balance between tumor necrosis factor-alpha and interleukin-10 and improves cardiac function [13,14]. Our present study used a rat model of HF induced by permanent left anterior descending coronary artery (LAD) occlusion and neonatal rat cardiac myocytes which are similar to the HF patients caused by MI and ischemia heart disease to evaluate the cardiac protective effect and its underlying mechanisms of QL capsule.
Materials and methods
Animal model and study design
Male Sprague-Dawley (SD) rats weighted 200 g around were used in this study. Myocardial infarction was induced by permanent LAD ligation. Animal study was performed in accordance with the Guide for the Care and Use of Laboratory Animals (US Department of Health, Education, and Welfare, Department of Health and Human Services, NIH Publication 85-23, revised 1996) and approved by the Animal Experiment Center of Fuwai Hospital, State Key Laboratory of Cardiovascular Disease (2013-5-144-HX).
MI procedure was as previous described [15]. Briefly, SD rats were anesthetized by ketamine (50 mg/kg intraperitoneal) and xylazine (10 mg/kg intraperitoneal), tracheal intubation was performed and connected to the respirator before left thoracotomy. The heart was exposed and left coronary was occluded permanently using 6-0 monofilament nylon sutures (Johnson &; Johnson Medical, Waterloo, Belgium). Sham operated rats underwent all the same procedures except coronary ligation. Chronic MI rats 4 weeks followed the procedure were regarded as HF, then echocardiography measurements were taken and randomized into vehicle group (V), QL group (Q). Normal SD rats (N) and sham-operated group (S) were used as control, Q group were given QL 0.6 g/kg•d by intragastric administration; V, N, S groups received the same volume of distilled water which was used for QL dissolution. All animals were feed under the same condition for 5 weeks.
Echocardiographic measurements
Echocardiographic data was obtained prior to andafter drug treatment in all animals under general anesthesia using a 12-MHz phased-array transducer (Sonos 7500, Philips). At least 6 consecutive cardiac cycles were measuredfor each animal, left ventricular inner diameter at end of systolic (LVESD) and diastolic (LVEDD) were recorded in the parasternal long-axis view and used to calculate the left ventricular fractional shortening (FS) and left ventricular ejection fraction (LVEF): FS (%) = (LVEDD-LVESD)/LVEDD, LVEF (%) = (LVEDD3-LVESD3)/LVEDD3. Measurement and analysis were performed by the same experienced investigator in a blinded fashion.
Hemodynamic measurements
At the end of the treatment after echocardiographic measurements, animals were still anesthetized and the right common carotid artery was catheterized with a fluid-filled polyethylene catheter (P50) to record the hemodynamic parameters by Powerlab ML750 (AD Instrument) via connecting to a pressure transducer (TRI 21, Letica Scientific Instruments). The catheter was advanced into the left ventricular cavity to record intracavitary pressure. Hemodynamic assessment of LV contractility was achieved by pressure volume analysis including systolic pressure (SBP), diastolic pressure (DBP), left ventricular enddiastolic pressure (LVEDP) and the maximum positive and negative values of dP/dt (+dP/dt max and -dP/dt max). Animals were euthanized with overdose of anesthetic medicationafter hemodynamic recordings. Heartswererapidly removed, weighted and reserved for western blot analysis and histologic analysis.
Cardiac fibrosis assessment
Ventricles samples were transversely divided, fixed in 10% paraformaldehyde-buffered solution for 24 h andembedded in paraffin. 4-μm thick sections were cut and stained with Sirius red stain for the measurement of the area of interstitial fibrosis. The interstitial fibrosis area was calculated as the ratio of the sum of the total area of interstitial fibrosis to the sum of the total connective tissue area plus the cardiomyoctyes area in all the marginal area of the LV [16]. Each filed was quantified using the Image J analysis program (NIH, Bethesda, MD, USA).
Transmission electron microscopy
Fresh samples of myocardial tissues form the marginal infarct area were obtained and fixed with 2.5% glutaraldehyde overnight at 4°C, and then fixed with 1% osmium tetroxide for 2 h after washed three times by phosphate buffered saline. 3 ultra-thin sections were acquired with standard procedure using an ultratome (Leica, Solms, Germany) on uncoated copper grids and stained with 0.2% lead citrate/1% uranyl acetate. Images of different magnifications were recorded by JOEL-1400EX transmission electron microscope (Tokyo, Japan).
Cell culture and treatment
Neonatal rat cardiac myocytes (NRCMs) was isolated from 2-day-old SD rats purchased from Beijing Fang-YY Laboratory Animal Co. [17]. In brief, neonatal rat hearts were taken out and washed, the ventricles were minced after wipe off the atria and other adhering interstitial tissues and dissolved in N-2-hydroxyethylpiperazine-N’-2-ethane sulfonic acid (HEPES)-buffered saline solution. The tissues were dispersed in a series of incubations at 37°C in HEPES-buffered saline solution. Cells were resuspended in Dulbecco modified eagle medium (DMEM)/F-12 medium (GIBCO) after centrifugation and cultured in a humidified incubator with 5% CO2 and 95% air. Ischemia-hypoxia cell culture was achieved by incubation in a sealed, hypoxic GENbox jar fitted with a catalyst (BioMérieux, Marcy l’Etoile, France) using serum-free DMEM for 24 hours, cells diluted to 1×106 per milliliter and plated in at 1 mL per well on six-well plates and were assigned into five different groups on the basis of their treatment: control group (C) was cultured under normal condition, vehicle group (V)suffered ischemia-hypoxia culture only , QL 0.5 mg/mL was added into the DMEM in QL-treated group (Q)under ischemia-hypoxia condition, GSK69069 (Selleck, Shanghai, China) was used as an Akt inhibitor at 0.5 μmol/L, QL+GSK69069 group (Q+G), GSK69069 group (G) were also suffered ischemia-hypoxia.
MTT assay
The MTT (3(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) assay was used to study the NRCMS growth in serum-free DMEM under hypoxia condition with or without QL treatment. Cells in a 96-well plate were treated with different medium containing or absent of QL in hypoxia condition for 24 hours. The MTT stock solution (10 mL of 10 mg/mL, Sigma) was added to the remaining medium, cultures were incubated for an additional 4 h at 37°C. The medium was discarded after incubation, the insoluble dark blue formazan was dissolved in 100 μL DMSO and quantified at 570 nm with a reference wavelength of 630 nm using a microtiter plate reader (infinite M200PRO, TECAN). The survival of the control group was defined as 100%, and that of treated groups was expressed as a percentage of the positive control value.
Flow cytometry
NRCMsapoptosis in C, V, Q, Q+G and G groups were assessed using an Annexin V-FITC/PI apoptosis detection kit (BD Biosciences Clontech, USA) following the manufacturer’s instructions. Apoptotic and necrotic cells were distinguished on the basis of annexin V-FITC reactivity and PI exclusion and testedbythe Accuri C6 flow cytometer (Becton Dickinson, USA).
Western blot analysis
Tissues in the margin area of infarction heart and lysed NRCMs from each group were collected for protein analysis. Samples were homogenized in RIPA buffer (Roche) and centrifuged at 13000 rpm for 10 minutes, the supernatants were collected. Protein concentration was determined by bicinchoninic acid assay. Samples (30 μg/lane) were mixed with loading buffer in a 12% SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked in 5% skim milk for 1 hour at room temperature and incubated overnight at 4°C with the primary antibodies as follows: anti-Bcl2 (Cell Signaling Technology, #2870), anti-Bax (Cell Signaling Technology, #2772), anti-Caspase3 (Cell Signaling Technology, #9662), anti-Akt (Cell Signaling Technology, #4691), anti-Phospho-Akt (Cell Signaling Technology, #4060) and anti-GAPDH (GSGB-BIO, Beijing, China) diluted 1:1000. Membranes were then washed and incubated in secondary antibodies at 1:5000. Protein expression levels were determined by analyzing the signals captured on the nirtocellulose membranes using a chemi-doc image analyzer (FluorChemo M FM0488, Protein Simple) and analyzed using Image J (NIH, Bethesda, MD, USA).
RT-PCR analysis
mRNA expressions in the LV marginal area were measured by quantitative real-time polymerase chain reaction (RT-PCR). Total RNA was isolated with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions and the concentration was measured by NanoDrop 2000 (Thermo Scientific). RNA samples (2 μg) were further reverse-transcribed with the PrimeScript RT Reagent Kit (Perfect Real Time, TaKaRa Biotechnology, Japan) using the manufacturer’s protocol. The mRNA levels of VEGF (forward 5’-GTG AGC CTT GTT CAG AGC G-3’ and reverse: 5’-GAC GGT GAC GAT GGT GG-3’), Collagen I (forward 5’-ATC AAG GTC TAC TGC AAC AT-3’ and reverse: 5’-CAG GAT CGG AAC CTT CGC TT-3’), Collagen III (forward 5’-CTG GAC CAA AAG GTG ATG CTG-3’ and reverse: 5’-TGC CAG GGA ATC CTC GAT GTC-3’) were quantitatively measured using the Applied Biosystems step-one plus 7500 Real-Time PCR System (Life Technologies, USA) using SYBR Select Master Mix (Life Technologies, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, forward 5’-CCT CAA GAT TGT CAG CAA T-3’ and reverse: 5’-CCA TCC ACA GTC TTC TGA GT-3’) was used as the non-regulated control. The assays were performed three times using triplicate wells, threshold cycle (Ct) was calculated using the second-derivative maximum method. The data were analyzed via the delta-delta method, final values are expressed as the ratio versus the control.
Statistical analysis
The results are expressed as expressed as mean ± standard deviation. The statistical significance was calculated by one-way analysis of variance (ANOVA), followed by the Tukey post-hoc when first two groups were evaluated by the Student t test showed significant differenceusing SPSS17.0 for Windows (student version). P<0.05 was considered statistically significant.
Results
Qiliqiangxin improved LV remodeling and cardiac function in HF rats
All baseline data before treatment including body weight (BW), heart rate (HR), LVEF between Q and V group showed no significant differences among groups (Supplementary Table 1). At the end of the treatment,there was no significant difference inthe HR, BW between QL group and the V group while the ratio of left ventricular weight (LVW) to BW was significantly high in V group indicating the LV remodeling caused by MI was inhibited by QL treatment (Table 1). QL treated group had a significantly lower LVEDD, LVESD and higher LVEF and FS compared with V group at the end of treatment (Figure 1, representative echocardiography of each group were showed in Supplementary Figure 1). Hemodynamic measurement showed decreased LVEDP (Figure 2A) andincreased ±dp/dt (Figure 2B and 2C), systolic and diastolic pressure (Figure 2D and 2E) in QL treated rats, indicating the improved LV function in HF rats.
Table 1.
Metabolic Parameters
| MI | ||||
|---|---|---|---|---|
|
|
||||
| N (n=6) | S (n=6) | V (n=8) | Q (n=8) | |
| BW (g) | 509.2±32.4 | 515.2±38.8 | 519.7±34.0 | 522.8±50.7 |
| HR | 446.7±48.9 | 444.1±50.0 | 453.8±71.3 | 443.2±38.0 |
| LVW/BW (mg/g) | 2.08±0.22 | 2.10±0.18 | 2.47±0.18* | 2.13±0.16 |
Body weight, heart rate and ratio of left ventricular weight to body weight. No significant intergroup differences were noted of BW and HR, the value of LVW/BW in V group was significantly higher than N and S groups and the increase can be suppressed in Q group. V, non-treated heart failure rats with chronic MI; N, normal control group; S, sham-operated group; Q, QL 0.6 g/kg·d treated heart failure rats with chronic MI. Data are analyzed by one-way ANOVA followed by the Tukey post-hoc, values are mean ± SD;
P<0.05 compared with N group.
Figure 1.
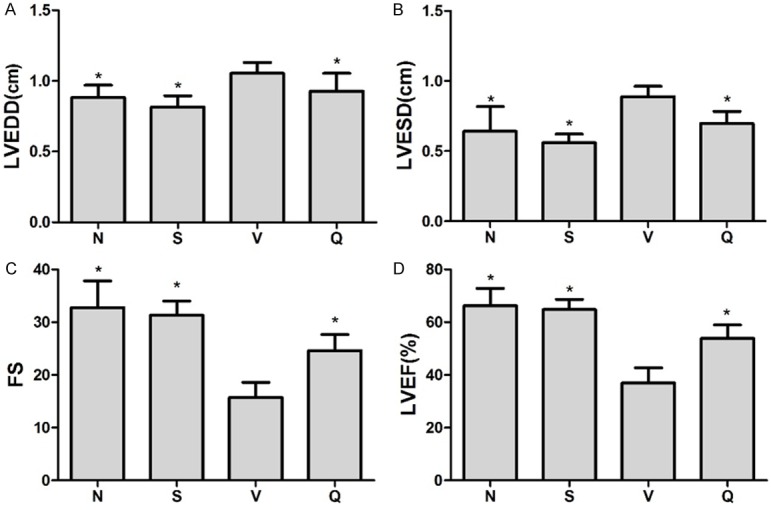
Echocardiographic data at the end of treatment. Graphs show echocardiographic assessments of LVEDD (A), LVESD (B), FS (C) and LVEF (D). LVEDD and LVESD was significantly higher whereas FS and LVEF was significantly decreased in V group. QL treatment had distinctly improved LV function include lower LVEDV, LVESV and significantly higher LVEF and FS compared with V group. Values areanalyzed by one-way ANOVA followed by the Tukey post-hoc and expressed as mean ± SD, *P<0.05 vs. V group.
Figure 2.
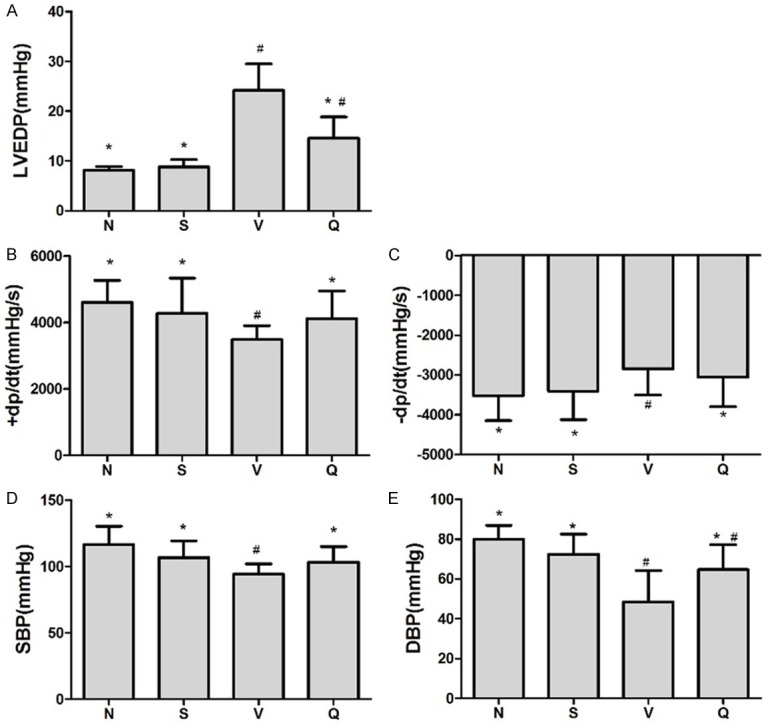
Hemodynamic assessments of left ventricular end-diastolic pressure. Cardiac function assessed by intraventricular pressure measurement, graphs show (A) LVEDP; (B and C) The maximum positive and negative values of dP/dt; (D and E) Systolic pressure (SBP) and diastolic pressure (DBP). QL treatment showed improved cardiac systolic function in HF rats induced by chronic MI compared with V group including lower LVEDP, increased +dp/dt, -dp/dt and SBP; DBP in Q group also increased to some extent although it was still lower than N group (P=0.029) but showed no significant difference comparing with S group. Values are mean ± SD, *P<0.05 vs. V group; # P<0.05 vs. N group.
Qiliqiangxin prevented rats’ myocardial fibrosis after chronic MI
The extent of interstitial fibrosis in the marginal areas of the infarct is shown in Figure 3. The interstitial fibrosis caused by chronic MI was significantly decreased in QL treated group compared with V group (17.39%±2.01% VS 43.22%±5.84%, P<0.001). Furthermore, mRNA expression of Collagen type I and III which are fibrosis-related also showed a distinct decrease in Q group compared with V group (Figure 4B and 4C).
Figure 3.
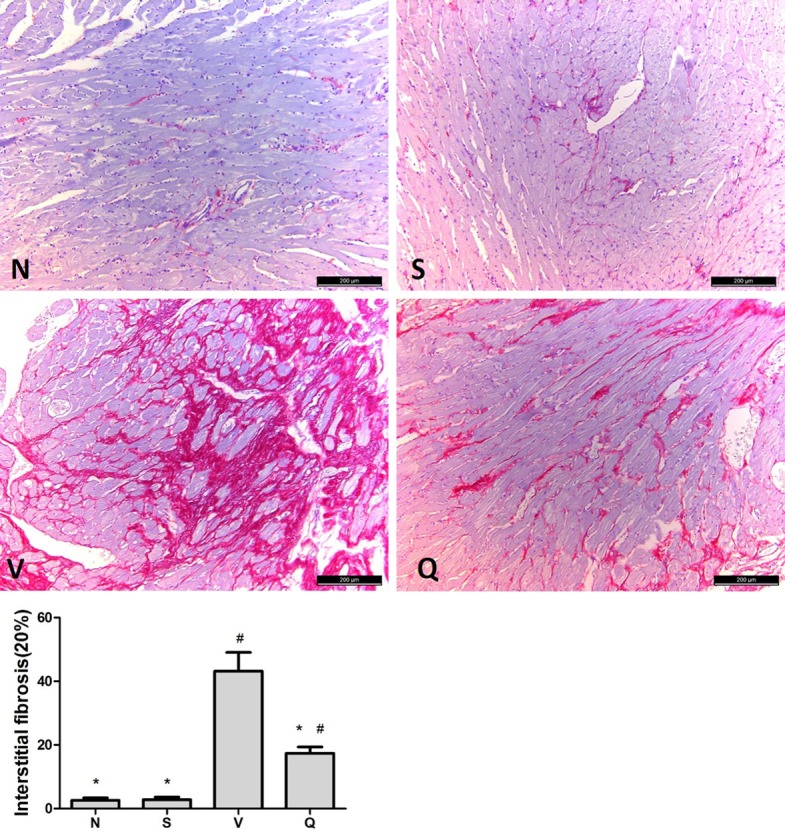
Interstitial fibrosis. Effects of QL on interstitial fibrosis assessed by Sirius red staining, fibrotic area is stained red, scan bar, 200 μm. N: normal group; S: sham-operated group; V: vehicle group; Q: QL treated group. Summary data for interstitial fibrosis is expressed as means ± SD and analyzed by one-way ANOVA followed by the Tukey post-hoc, *P<0.05 vs. V group; #P<0.05 vs. N group.
Figure 4.
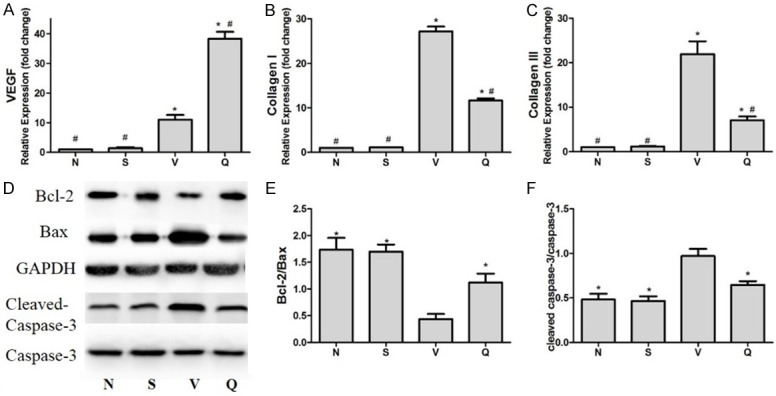
mRNA and protein expressionof MI-HF SD rats. A-C: Effect of QL in mRNA expression in CHF rats. The mRNA level were determined by RT-PCR and normalized to GAPDH housekeeping gene. Values are mean ± SD, *P<0.05 vs. N group; #P<0.05 vs. V group. D, E: QL inhibits mitochondrial-mediated apoptosis in HF rats caused by chronic MI. D: Representative western blot analysis demonstrating the expression of Bcl-2 and Bax in different groups. Densitometric analysis: E: In HF rats caused by MI, the ratio of Bcl-2/Bax was reduced and QL treatment increased the Bcl-2/Bax ratio conversely. F: QL reduced the expression levels of cleaved caspase-3 caused by HF. Data are analyzed by one-way ANOVA followed by the Tukey post-hoc and expressed as the mean ± SD, *P<0.05 vs. V group.
Qiliqiangxin improved myocardial apoptosis and VEGF expression in HF rats
Myocardial apoptosis evaluated by western blot analysis showed Bcl-2 expression in the margin area of infarction was declined in V group compared with N and S groups whereas QL treatment can increase their expression; in contrast, Bax expression increased inversely, therefore, upregulating the Bcl-2/Bax ratio (Figure 4D, 4E). Besides, QL significantly reduced caspase-3 activity compared with the vehicle group (Figure 4D, 4F). Thus, the attenuated MI-induced myocardial apoptosis may play a part in cardiac remodeling protection effect of QL in HF rats after chronic MI.
VEGF is closely associated with angiogenesis and cardiac function after MI [18]. RT-PCR showed VEGF expression was increased by 38.25 and 11.04 fold (P<0.05) in LV marginal area respectively in Q and V group compared with N group which means MI-induced upregulation of VEGF expression was significantly enhanced by QL treatment (Figure 4A).
Qiliqiangxin improved myocardial ultrastructure in HF rats
The ultrastructure of MI and treated myocardial was observed by transmission electron microscopy (TCM). Images of ultra-thin sections from V and Q group showed cardiac muscle fibers granulovascular degeneration accompaniedmitochondria swollen and dissolved remarkably in the LV marginal area after chronic MI (Figure 5A and 5B). This pathologic change can be reversed by QL treatment, in Q group, cardiac muscle fibers granulovascular degeneration was obviously attenuated and mitochondria were relatively proliferated and accumulated in these areas which was a compensatory change corresponding to the chronic myocardial ischemia (Figure 5C and 5D).
Figure 5.
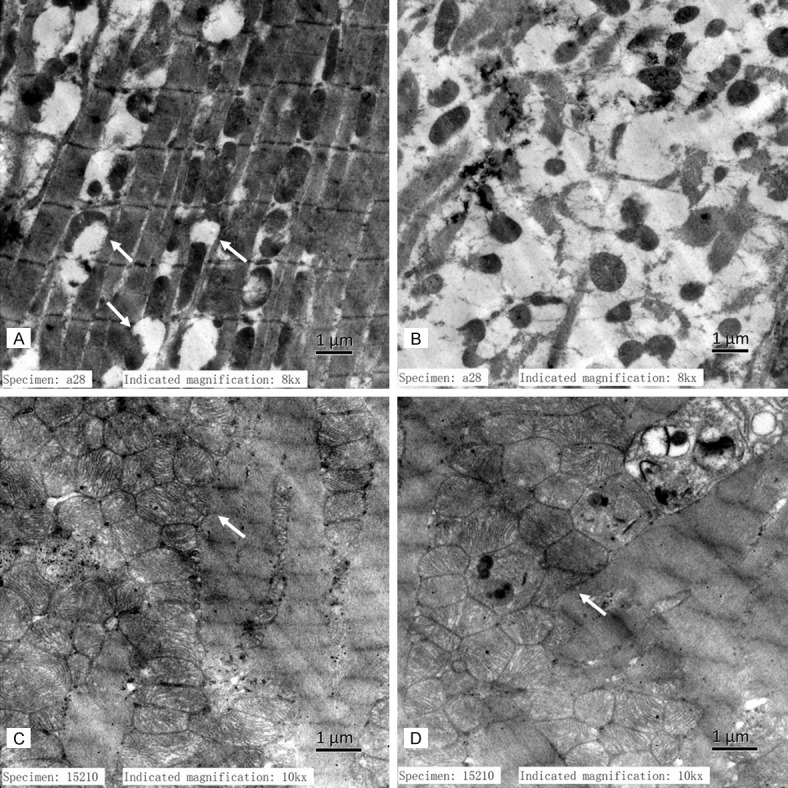
Ultrastructure in HF rats. A and B: Cardiac muscle fibers granulovascular degeneration and mitochondria swollen and dissolved were observed in the infarcted LV marginal area of V group rats; C and D: Cardiac muscle fibers granulovascular degeneration was obviously attenuated and mitochondria were relatively proliferated and accumulated in Q group.
Qiliqiangxin improvedcell vitality and apoptosis inischemia-hypoxic cultivated NRCMs in an Akt dependent manner
To get a further understanding of cardiac protection effectof QL in HF patients caused by chronic ischemic heart disease, NRCMs cultivated under ischemia-hypoxic condition were used. MTT assay showed QL treatment improved cell vitality as the OD value of Q group increased significantly compared with V group (Figure 6A) and flow cytometry confirmed that QL treatment improved cell apoptosis caused by ischemia-hypoxic cultivation (Figure 6B). RT-PCR and western blot data showed the protection for NRCMs of QL was associated with VEGF expression upregulate in an Akt dependent manner. VEGF expression raised 18.7-folds more compared with V group (Figures 6 and 7) whereas the Akt inhibitor GSK69069 blocked Akt phosphorylation notably and VEGF up-regulation disappeared at the same time.Besides, GSK69069 can block the cell vitality and apoptosis improvement caused by QL (Figures 6 and 7), Bcl-2/Bax expression also showed the same tendency, In V group, Bcl-2 declined in comparison with N group whereas QL treatment increased their expression; in contrast, Bax were inversely to Bcl-2 expression and GSK69069 partly blocked this effect (Figure 7C, 7D).
Figure 6.
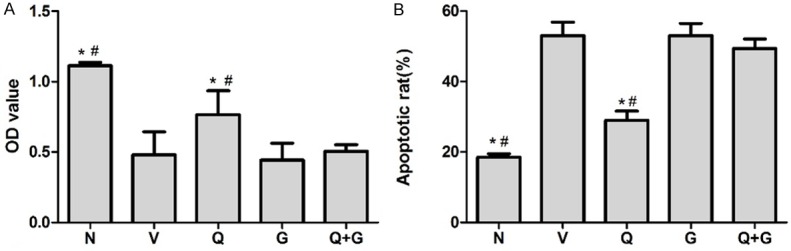
Effect of QL on cell vitality and apoptosis of ischemia hypoxic cultivated NRCMs. A: MTT assay showed the OD value of Q group increased significantly compared with V group; B: Flow cytometry confirmed that QL treatment improved cell apoptosis, preincubation of Akt inhibitor GSK69069 can block this effect as the OD value and apoptotic rate in Q+G group showed no significant difference with V group. C: Control group, V: Vehicle group, Q: QL-treated group, G: GSK69069 group, Q+G: QL + GSK69069 group. Values are expressed as the mean ± SD, one-way ANOVA followed by the Tukey post-hoc were applied,*P<0.05 vs. V group, #P<0.05 vs. Q+G group.
Figure 7.
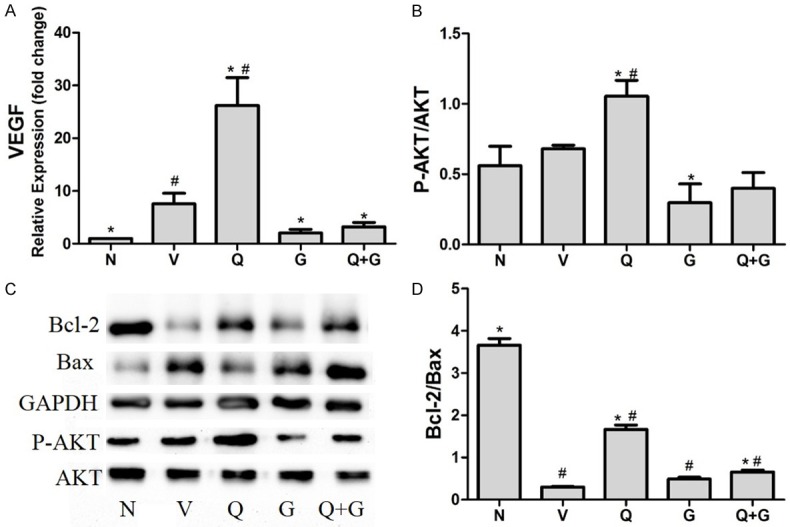
mRNA and protein expression of ischemia-hypoxic cultivated NRCMs. (A) Effect of QL in VEGF mRNA expression in NRCMs under ischemia hypoxic condition. The mRNA level were normalized to GAPDH housekeeping gene, VEGF expression increased in V group due to ischemia hypoxic stimulate, but QL treatment increased VEGF expression to 26.2 folds compared with only 7.5 folds in V group, Akt inhibitor reduced this effect both alone and together with QL. (B-D) Western blot analysis showed the phosphorylation Akt and expression of Bcl-2 and Bax in different ischemia hypoxic cultivated NRCM groups (C). Densitometric analysis: (B) QL increased Akt phosphorylation and this effect can be reduced in present of Akt inhibitor; (D) QL treatment increased the Bcl-2/Bax ratio compared with V group, while this effect was blocked by Akt inhibitor GKS69069. C: Control group, V: Vehicle group, Q: QL-treated group, G: GSK69069 group, Q+G: QL + GSK69069 group. Data are expressed as mean ± SD and analyzed by one-way ANOVA followed by the Tukey post-hoc, *P<0.05 vs. V group, #P<0.05 vs. N group.
Discussion
Our study revealed that QL could improve LV function, cardiac remodeling and reserve mitochondrial morphology to a certain extentin HF rats induced by chronic MI, the possible mechanism may be associated withattenuated apoptosis and increased VEGF expression in an Akt dependent manner. This is the first attempt to evaluate the cardiac protection effects of QL in HF in a rat model, beyondthe limitations of previous clinic trials, the effects of QL in LV dysfunction were surveyed by means of research techniques such as pathological observation and histological examination which could give us a intuitionistic evidence and help us to reveal the possible mechanisms. The increased LVEF, FS and ±dp/dt suggest an ameliorated cardiac function, especially systolic function as the intraventricular hemodynamic showed an increased SBP of QL treated rats compared with V group; SBP of Q group showed no significant difference with normal rats; DBP also increased in Q group compared with Vgroup but still lower than N group (P=0.029). In addition, QL also reduced the increased LVEDP caused by MI considerably (V VS Q group, P<0.001), suggesting QL may also improve LV diastolic function. Meantime, decreased LV weight to body weight, interstitial fibrosis and Col I and Col III expression elucidated improved cardiac remodeling. TCM observation showed cardiac muscle fibers granulovascular degeneration was obviously attenuated and mitochondria were relatively proliferated and accumulated due to QL treatment.
QL was said to attenuate apoptosisin AMI mice [14] and we also found QL reduced the Bcl-2/Bax ratio and activated caspase-3 in our experiment. Bcl-2 and Bax together can impact apoptosis through mitochondrial dependent pathway, Bcl-2 blocks mitochondrial cytochrome Crelease and prevents subsequent activation of caspase-3 while translocation of Bax causes release of cytochrome C and result in an activation of caspase-3 and cell apoptosis [19]. This data suggested the effect of QL in improving LV dysfunction may not be entirely attributable to its cardiac inotropic effect, which is QL’s main effect on HF patients theoretically based on traditional Chinese medicine pharmacology.VEGF is an endothelial cell mitogen that can regulate both angiogenesis and nonangiogenesis process in cardiovascular system. Researchers found VEGF could facilitate cardiomyocytes regeneration andprotect it from apoptosis with the activationof phosphatidylinositol-3 kinase (PI-3K) and theupregulation of Bcl-2 expression [20]. Our data showed QL could up-regulate VEGF expression in the infarction border area of HF rats. To gain a better understanding of the mechanisms, we cultured NRCMs in ischemia hypoxic environment and found that QL improved cell vitality and apoptosis with up-regulated VEGF expression, Akt blocker can block these effects. These findings remind us VEGF may play an important role in QL therapeutic mechanism in an Akt dependent manner.
VEGF is important for cardiac remodeling and repair within the damaged and diseased myocardium [21,22]. It plays an important part in angiogenesis and stromal deposition associated with myocardial infarction. Studies showed increase VEGF expression of myocytes adjacent to the infarct zone and new vessels infiltrating the infarct also overexpressed in MI rats [23,24]. VEGF can also prevent apoptosis and regulate cell survival under certain conditions such as hypoxia, glucose deprivation by activating antiapoptotic proteins and inhibiting proapoptotic signaling like Bad, caspase-9, caspase-3 [25]. Recent studies also found a linkage between VEGF and tissue metabolism, which could lead to the development of new therapies for the treatment of HF [26]. Akt serves as a central regulator in VEGF expression. Akt acts on TSC1-TSC2/Rheb/mTORC1 pathway and increases the protein level of hypoxia inducible factor 1α which stimulates VEGF release [27]. Activation of PI3K/Akt-dependent signaling has been shown to prevent cardiomyocytes apoptosis and protect themyocardium [28,29]. Our results found that the level of phosphor-Akt was elevated and the cell apoptosis was reduced in QL group compared with that in vehicle group, so the cardiac protect effectof QL was mediated partially through the VEGF expression in PI3K/Akt dependent manner.
In conclusion, QL could significantly improve the cardiac function and remodeling in HF rats caused by chronic MI, it also attenuated cell apoptosis and increased cell vitality in ischemia hypoxic cultured NRCMs. The underlying mechanism may attribute to attenuation of cardiomyocyte apoptosis mediated by VEGF expression in PI3K/Akt dependent manner. For a Traditional Chinese Medicine of great antiquity, our results indicated a guarantee deffect in HF rats model and a new perspective in the molecular mechanisms of QL which makes it even evidence-based both fundamentally and clinically.
Acknowledgements
We thank Shijiazhuang Yiling Pharmaceutic (Hebei, China) for providing the QL capsular. Specially, I want to present my most sincere and honest thankfulness to my doctoral supervisor Professor Jian Zhang, this paper is on the guidance and mentor of his support. I would also like to thank Professor Yuhui Zhang and all the researchers, from whose work I quoted in my paper.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YF, Weltman NY, Xiang L, Youmans S, Krause D, Gerdes AM. Improvement of left ventricular remodeling after myocardial infarction with eight weeks L-thyroxine treatment in rats. J Transl Med. 2013;11:40. doi: 10.1186/1479-5876-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lautamäki R, Knuuti J, Saraste A. Recent Developments in Imaging of Myocardial Angiotensin Receptors. Current Cardiovascular Imaging Reports. 2014;7:1–6. [Google Scholar]
- 4.Yang YN, Ma YT, Liu F, Huang D, Li XM, Huang Y, Tang Q, Chen BD, Ma X, Xie X, Du L, Gao X, Wang YH, Gulinaer B, Yu ZX. [Incidence and distributing feature of chronic heart failure in adult population of Xinjiang] . Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:460–464. [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 6.Song YH, Li BS, Chen XM, Cai H. Ethanol extract from Epimedium brevicornum attenuates left ventricular dysfunction and cardiac remodeling through down-regulating matrix metalloproteinase-2 and -9 activity and myocardial apoptosis in rats with congestive heart failure. Int J Mol Med. 2008;21:117–124. [PubMed] [Google Scholar]
- 7.Wen-Ting S, Fa-Feng C, Li X, Cheng-Ren L, Jian-Xun L. Chinese medicine shenfu injection for heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:713149. doi: 10.1155/2012/713149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao MJ, Wang SR, Zhao MJ, Lv XL, Xu H, Li L, Gu H, Zhang JL, Li G, Cui XN, Huang L. The Effects of Velvet Antler of Deer on Cardiac Functions of Rats with Heart Failure following Myocardial Infarction. Evid Based Complement Alternat Med. 2012;2012:825056. doi: 10.1155/2012/825056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Zhang J, Huang J, Ma A, Yang J, Li W, Wu Z, Yao C, Zhang Y, Yao W, Zhang B, Gao R. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065–1072. doi: 10.1016/j.jacc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Jiang K, Ding X, Fu M, Wang S, Zhu L, He T, Wang J, Sun A, Hu K, Chen L, Zou Y, Ge J. Qiliqiangxin inhibits angiotensin II-induced transdifferentiation of rat cardiac fibroblasts through suppressing interleukin-6. J Cell Mol Med. 2015;19:1114–1121. doi: 10.1111/jcmm.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Yang P, Li F, Tao L, Ding H, Rui Y, Cao Z, Zhang W. Therapeutic effects of astragaloside IV on myocardial injuries: multi-target identification and network analysis. PLoS One. 2012;7:e44938. doi: 10.1371/journal.pone.0044938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y, Lin L, Ye Y, Wei J, Zhou N, Liang Y, Gong H, Li L, Wu J, Li Y, Jia Z, Wu Y, Zhou J, Ge J. Qiliqiangxin inhibits the development of cardiac hypertrophy, remodeling, and dysfunction during 4 weeks of pressure overload in mice. J Cardiovasc Pharmacol. 2012;59:268–280. doi: 10.1097/FJC.0b013e31823f888f. [DOI] [PubMed] [Google Scholar]
- 13.Xiao H, Song Y, Li Y, Liao YH, Chen J. Qiliqiangxin regulates the balance between tumor necrosis factor-alpha and interleukin-10 and improves cardiac function in rats with myocardial infarction. Cell Immunol. 2009;260:51–55. doi: 10.1016/j.cellimm.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Tao L, Shen S, Fu S, Fang H, Wang X, Das S, Sluijter JP, Rosenzweig A, Zhou Y, Kong X, Xiao J, Li X. Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci Rep. 2015;5:8374. doi: 10.1038/srep08374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki T, Izumi Y, Nakamura Y, Yamashita N, Fujiki H, Osada-Oka M, Shiota M, Hanatani A, Shimada K, Iwao H. Tolvaptan Improves Left Ventricular Dysfunction after Myocardial Infarction in Rats. Circulation Heart Failure. 2012;5:794–802. doi: 10.1161/CIRCHEARTFAILURE.112.968750. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki T, Yamashita N, Izumi Y, Nakamura Y, Shiota M, Hanatani A, Shimada K, Muro T, Iwao H, Yoshiyama M. The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res. 2012;35:34–40. doi: 10.1038/hr.2011.139. [DOI] [PubMed] [Google Scholar]
- 17.Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008;283:29730–29739. doi: 10.1074/jbc.M805514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Shi C, Hou X, Zhao Y, Chen B, Tan B, Deng Z, Li Q, Liu J, Xiao Z, Miao Q, Dai J. Modified VEGF targets the ischemic myocardium and promotes functional recovery after myocardial infarction. J Control Release. 2015;213:27–35. doi: 10.1016/j.jconrel.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Henning RJ, Dennis S, Sawmiller D, Hunter L, Sanberg P, Miller L. Human umbilical cord blood mononuclear cells activate the survival protein Akt in cardiac myocytes and endothelial cells that limits apoptosis and necrosis during hypoxia. Transl Res. 2012;159:497–506. doi: 10.1016/j.trsl.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, Ding B, Ha T, Gao X, Li C. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther. 2005;12:196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 22.Hori R, Nakagawa T, Yamamoto N, Hamaguchi K, Ito J. Role of prostaglandin E receptor subtypes EP2 and EP4 in autocrine and paracrine functions of vascular endothelial growth factor in the inner ear. Bmc Neuroscience. 2010;11:1–9. doi: 10.1186/1471-2202-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CT, Hung MC. Beyond anti-VEGF: dual-targeting antiangiogenic and antiproliferative therapy. Am J Transl Res. 2013;5:393–403. [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Chuang DM. HDAC inhibitors mitigate ischemia-induced oligodendrocyte damage: potential roles of oligodendrogenesis, VEGF, and anti-inflammation. Am J Transl Res. 2014;6:206–223. [PMC free article] [PubMed] [Google Scholar]
- 25.Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–39. [PubMed] [Google Scholar]
- 26.Kivela R, Bry M, Robciuc MR, Rasanen M, Taavitsainen M, Silvola JM, Saraste A, Hulmi JJ, Anisimov A, Mayranpaa MI, Lindeman JH, Eklund L, Hellberg S, Hlushchuk R, Zhuang ZW, Simons M, Djonov V, Knuuti J, Mervaala E, Alitalo K. VEGF-B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol Med. 2014;6:307–321. doi: 10.1002/emmm.201303147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Petrilli AM, Fuse MA, Donnan MS, Bott M, Sparrow NA, Tondera D, Huffziger J, Frenzel C, Malany CS, Echeverri CJ, Smith L, Fernández-Valle C. A chemical biology approach identified PI3K as a potential therapeutic target for neurofibromatosis type 2. Am J Transl Res. 2014;6:471–93. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


