Abstract
Portulaca oleracea L., (POL) is one of commonly used medicine-food herbs and has a cosmopolitan distribution in many countries. Many studies showed that POL exhibited a wide range of pharmacological effects such as anti-inflammatory and liver complaints. In the clinical studies, POL was usually used for the treatment of UC disease and the clinical efficacy was well, but the mechanism and scientific intension was still unknown. In the present study, we studied the protective effects of the ethanol extract from POL on dextran sulphate sodium-induced UC in C57BL/6 mice model through oxidative stress and inflammatory pathway. The results demonstrated that the ethanol extract from POL could exhibit the effective protection for the DSS induced UC by increasing the colon length, decreasing body weight loss and the disease activity index score, inhibiting oxidative stress response through the MDA, NO, SOD activities, reducing the mRNA expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and the protein expressions of TNF-α and NF-kB p65. These results may prove that POL could be considered as a useful and effective botanical compound from the edible plant to be used in UC through the oxidative stress and inflammatory activities.
Keywords: Ulcerative colitis, Portulaca oleracea L, antioxidation, anti-inflammation
Introduction
Ulcerative colitis (UC) is one of the idiopathic of inflammatory bowel disease that causes long-lasting inflammation and ulcers in digestive tract and affects the innermost lining of large intestine and rectum [1]. The main symptom of UC is diarrhea mixed with blood, of gradual onset. UC has an incidence of 1.2 to 20.3 cases per 100,000 persons per year, and a prevalence of 7.6 to 246.0 cases per 100,000 per year [2]. The highest incidence and prevalence of UC are seen in northern Europe and northern areas of individual countries or other regions [3]. The etiology is still no direct known although UC is the most common form of inflammatory bowel disease by far [4]. There are many possible factors such as genetics, environmental and stress, the widely accepted hypothesis was genetic component to individual susceptibility caused by ongoing mucosal immune response [5]. To date, the primary aims of medical therapy for patients with UC are provide an improved quality of life by maintaining remission of symptoms and mucosal inflammation. In recent studies implicated that the oxidative stress and inflammatory pathways disorders may be improved the understanding of the pathogenic process of UC, and these pathways perhaps become the therapeutic targets for finding the new drug [6,7].
Traditional Chinese medicine (TCM) has been used for treating UC for literally thousands of years, and has a wealth of theoretical basis and clinical experience. Although TCM is seem to be not sufficiently available, the TCM is alternative approach to medical care of UC in many western countries. Portulaca oleracea L. (POL) is one of the medicine-food herbs and commonly known as machixian in China and pursley in the USA [8]. POL, as “vegetable for long life” in Chinese folklore, has a cosmopolitan distribution and widely used in many countries [9-12]. Many studies showed that POL exhibited a wide range of pharmacological effects such as anti-inflammatory [13], skeletal muscle relaxant [14], liver complaints [15,16], analgesic [13], wound-healing activities [17], antibacterial [18], stomach and mouth ulcers [19].
Modern studies have shown that POL was a rich source of linolenic acid (LNA) and α-tocopherol (α-TCP) [20], ethanol extract from POL could attenuated acetaminophen-induced liver injury and the carbon tetrachloride induced liver injury in mice [15,16]. The aqueous extract from POL could ameliorates diabetic vascular inflammation and endothelial dysfunction in db/db Mice [21]. In the clinical studies, POL was usually used for the treatment of UC disease and clinical efficacy of the POL was well. Meanwhile, the mechanism and scientific intension was still unknown limits its use in clinical.
In the present study, we studied the protective effects of ethanol extract from POL on dextran sulphate sodium-induced UC in C57BL/6 mice model by histopathological analysis, microscopic score, the antioxidant markers such as SOD, MDA and NO level of colons, as well as by determination of inflammation markers such as the mRNA expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), the protein expressions of TNF-α and NF-kB p65.
Materials and methods
Animals
C57BL/6 male mice (8-10 weeks) were provided by the Experimental Animal Center of Fourth Military Medical University (Xi’an, China). Experiments were carried out in accordance with the published National Institutes of Health guidelines and all procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Shaanxi University of Chinese Medicine. The mice were kept in cages with the room temperature maintained at 25 ± 2°C with a light-dark cycle of 12 h each day.
Ethanol extraction from Portulaca oleracea L
The ethanol extraction from POL was prepared as described according to the literature [11]. The air dried aerial parts of Portulaca were purchased from Shannxi province, China. The POL (10 kg) was extracted with 8 times of 80% ethanol for 2 times (1 h/time), and get ride of the ethanol under vacuum. The 10% NaOH was used to modified the pH to 6.5~7, then get the precipitation after centrifuged at 5000 rpm and oven dried. We get the end extract approximately 54.3 g which was black powder and tasted with lightly odorless.
Induction of colitis
DSS induced colitis was performed as previously described method [22]. Mice were randomly divided into five groups (control group, colitis control group and three experimental groups) with eight in each group. Mice were given drinking water in control group and drinking water containing 5% DSS (MW 36000-50000, MP Biomedicals) in colitis control group and three experimental groups for 7 days and then all of the mice were shifted to normal tap water in day 8. The animals were given free access to water during the experiment. The mice in normal control and colitis control groups were orally administrated with 0.5 ml phosphate-buffered saline. Three experimental groups were orally administrated with different dosage of the ethanol extract of POL (100, 200 mg/kg and 400 mg/kg) in 0.5 ml phosphate-buffered saline for seven days from the first day of induction DSS.
Assessment of colitis
The animals were weighed and monitored for the appearance of the gross rectal bleeding and stool consistency daily throughout the experimental period. The overall disease severity of each animal in this study was assessed through the disease activity index score which was used for colitis evaluation as described earlier by Cooper. The disease activity index score was ranged from 0 to 6 and each score was provided as follows: visible fecal blood (0 = normal, 1 = slightly bloody; 2 = bloody; and 3 = blood in whole colon) and diarrhea (0 = normal; 1 = slightly loose feces; 2 = loose feces; and 3= watery diarrhea) [23].
Sample collection
After induction of colitis for 7 days, animals were sacrificed by 3% chloral hydrate anesthesia in day 8. The colon was excised and rinsed in saline to remove fecal residue, then measured the length. A small section from each colon was placed in 4% paraformaldehyde. The remaining colon was frozen in liquid nitrogen and stored at -80°C.
Histopathological examination
Hematoxylin and eosin (HE) staining was performed to reveal the morphological features of the colon. Samples for histology were obtained from the small section of colon excised which were fixed in 4% paraformaldehyde then dehydrated, and subsequently embedded in paraffin blocks. Slices with 5 μm sections were stained with HE in accordance with the standard procedures for histological evaluation. According to the degree of inflammation and the presence of edema and/or ulcerations, the scoring of microscopic damage of the colonic mucosa in DSS-induced colitis was set as follows: 0 = normal; 1 = slight inflammation; 2 = moderate inflammation and/or edema; and 3 = heavy inflammation and/or ulcerations [23].
Determination of SOD and MDA and NO level activities in colon
The excised colons that stored at -80°C (n = 8 for each group) were homogenized in 0.1 M phosphate buffer (pH 7.4) and then centrifuged at 15,000 g for 10 minutes. The supernatant fraction was used for the measurements of the nitrogen monoxide (NO), superoxide dismutase (SOD), and malondialdehyde (MDA) content using the corresponding kits (Nanjing Jiancheng Biochemistry Co., Nanjing, China).
Determination of MPO activity in colon
The Myeloperoxidase (MPO) activity was determined using the excised colons that stored at -80°C (n = 8 for each group) were homogenized in 0.1 M phosphate buffer (pH 7.4) and then centrifuged at 15,000 g for 10 minutes, the supernatant fraction was used for the measurements of MPO content in colons using the MPO detection kits (Nanjing Jiancheng Biochemistry Co., Nanjing, China). The MPO activity was represented as U/mg protein and defined as the quantity of enzyme degrading 1 μmol of peroxide per minute at 37°C.
Real-time quantitative RT-PCR
RNA was extracted from the excised colons that stored at -80°C using TRI reagent following to the manufacturer’s protocol. The resulting solution was diluted 50-fold with TE buffer, and the cDNA was produced by using the revertaid first strand cDNA synthesis kit for first-standing cDNA synthesis. RT-PCR was performed on cDNA samples using the MyiQ single-color RT-PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). The expression of TNF-α, IL-6 and IL-1β was analyzed by real-time RT-PCR and using β-actin to normalize the expression level of the mRNA genes. The primer sequences (forward 5’-3’, reverse 5’-3’) for gene expression were used as follows: TNF-α, GACAGTGACCTGGACTGTGG, TGAGACA- GAGGCAACCTGAC; IL-6, CCCTGACAGACCCGGACTTA, GCCGAGACTGTTGTTCCATAAT; IL-1β, GTAGCCCA -CGTCGTAGCAAA, CCCTTCTCCAGCTGGGAGAC; The cycling protocol of Real-time RT-PCR was conducted at a DNA denaturation temperature of 95°C for 5 min and followed by 40 cycles of 95°C for 15 s, 60°C for 20 s and an elongation temperature 72°C for 40 s.
Western blot analysis
The colons (n = 8 for each group) were homogenized and then the protein was extracted. Colons were washed twice with ice-cold PBS. The protein expression of TNF-α and NF-kB p65 was determined by the BCA method (Beyotime, China) according to the manufacturer’s protocol. Equal amounts of protein extracts were separated using 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis for western blot. The gels were blotted onto a nitrocellulose membrane and incubated with the primary antibodies of TNF-α (Abcam) NF-κB p65 (Abcam), and β-actin were used as a loading control.
Statistical analysis
The statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as Mean ± standard deviation (SD), and differences between groups were compared with one-way ANOVA or t-tests as appropriate. P-value less than 0.05 presented statistical significance. The microscopic sores were expressed as the median and analyzed using Kruskal-Wallis test and differences between groups were compared with Mann-Whitney U test.
Results
Protective effects of POL on DSS induced UC
The UC manifested as a notable loss of body weight accompanied by diarrhea and bloody feces [24]. The body weight change and the disease activity index score were shown in Figure 1. The body weight of mice was increased continuously in the control group. The notable loss of body weight was revealed in DSS induced UC group and showed the significantly decreased compared with the control group (P<0.05). After administrated with different dosage of POL (100, 200 mg/kg and 400 mg/kg), the loss of body weight was reversed and have the significantly increased compared with the DSS induced UC group from day 4 (P<0.05). The disease activity index scores were consistent with the result of body weight, the diarrhea and visible fecal blood were appeared in control group and the disease activity index score was zero from day 1 to 7. The disease activity index scores were elevated markedly in DSS induced UC group. The disease activity index scores were significantly reduced in the different dosage of POL treatment groups and ameliorated the diarrhea and visible fecal blood symptoms compared with the DSS induced UC group (P<0.05).
Figure 1.
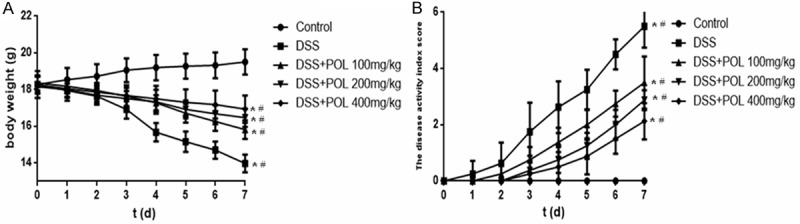
Protective effects of POL against DSS induced UC. The ulcerative Colitis was induced by administration with 5% dextran sulfate sodium (DSS) for 7 d. The different dosage of POL (100, 200 mg/kg and 400 mg/kg) were orally administrated for 7 d, respectively. (A) The body weight change and (B) the disease activity index score were evaluated daily. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, #P<0.05 vs DSS.
Effect of POL on histopathological changes in DSS induced UC
As shown in Figure 2C and 2D, the colon length was 5.1 ± 0.5 cm in DSS induced UC group which was significantly shorter than the control group (7.5 ± 0.8 cm). In the POL treatment groups, the colon length were 6.05 ± 0.6, 6.4 ± 0.4, 6.76 ± 0.8 cm in different dosage (100, 200 mg/kg and 400 mg/kg). According to the HE staining (Figure 2A and 2B), the histological features of the colons were typical of the normal structure in the control group, and the high level of inflammatory cells infiltration, mucosal edema and thickening of the colon wall were shown in DSS induced UC group. After the treatment of POL, the inflammatory cells infiltration and ulcer area were significantly decreased compared with DSS induced UC group, the adhesion and edema were also alleviated with the dosage dependent manner. The scoring of microscopic damage of the colonic mucosa was corresponding with the result of HE staining, the severe inflammation damage was exhibited in DSS induced UC group, and the inflammation damage was ameliorate in the different dosage of the POL treatment group.
Figure 2.
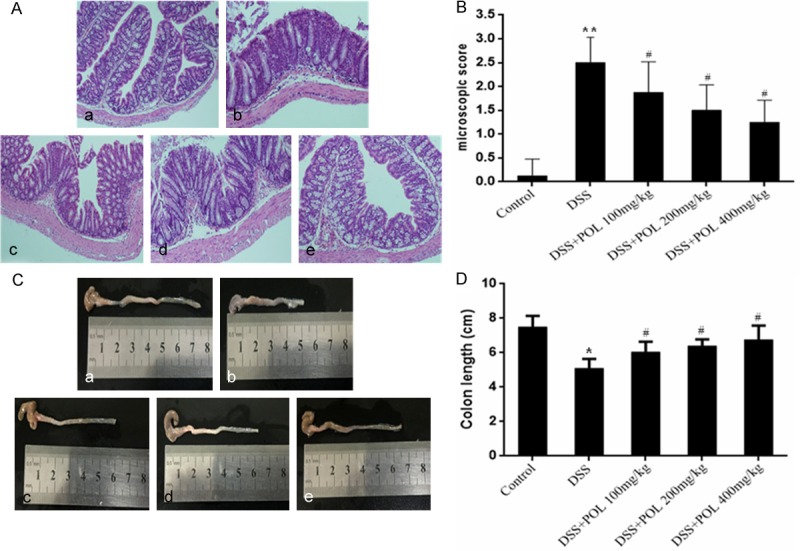
Effect of POL on histopathological changes and colon length in DSS induced UC. (A) The representative images of HE staining (a) control group (b) DSS (c) DSS+POL 100 mg/kg (d) DSS+POL 200 mg/kg (e) DSS+POL 400 mg/kg and (B) the microscopic score were studied for histological evaluation. Data are presented as the median. (n = 8 for each group). (C) The colon of different groups (a) control group (b) DSS (c) DSS+POL 100 mg/kg (d) DSS+POL 200 mg/kg (e) DSS+POL 400 mg/kg and (D) the colon length were compared on the 7th day. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, **P<0.01 vs sham, #P<0.05 vs DSS.
Effects of the POL on SOD and MDA and NO level activities in colon
The results of SOD and MDA and NO Level activities in colon were all shown in Figure 3. The SOD level of the colons was significantly decreased in DSS induced UC group compared with control group. The POL significantly increased the SOD levels to 6.7 ± 0.7, 8.0 ± 0.6, 9.0 ± 0.5 U/mg protein in different dosage (100, 200 mg/kg and 400 mg/kg), respectively. The MDA and NO level activities of the colons were significantly increased in DSS induced UC group compared with control group. In the POL treatment groups, the MDA levels were 0.77 ± 0.1, 0.62 ± 0.08, 0.48 ± 0.15 U/mg protein and the NO levels were 3.1 ± 0.3, 2.8 ± 0.2, 2.5 ± 0.2 U/mg protein in different dosage (100, 200 mg/kg and 400 mg/kg), respectively.
Figure 3.
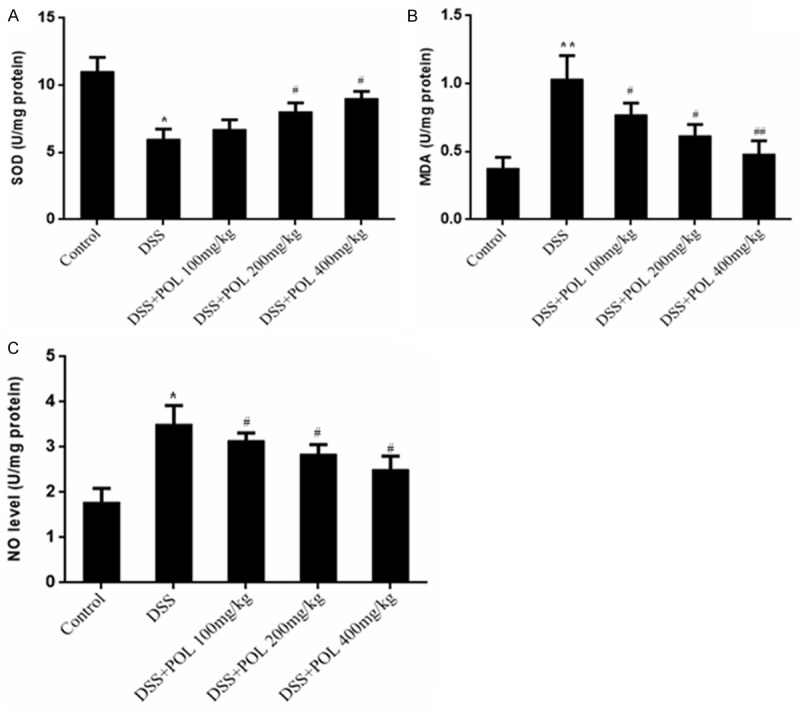
Effects of POL on levels of the SOD and MDA and NO Level activities of colon in DSS induced UC. (A) SOD, (B) MDA and (C) NO Level were measured. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, **P<0.01 vs sham, #P<0.05 vs DSS, ##P<0.01 vs DSS.
Effects of the POL on MPO activity in colon
The MPO activity was shown in Figure 4. The MPO activity of DSS induced UC group was 1.21 ± 0.21 U/mg protein, almost 4-fold over that of control group. After administrated with different dosage of POL (100, 200 mg/kg and 400 mg/kg), the MPO activity was significantly decreased to 0.86 ± 0.12, 0.64 ± 0.11, 0.46 ± 0.08 U/mg protein, respectively.
Figure 4.
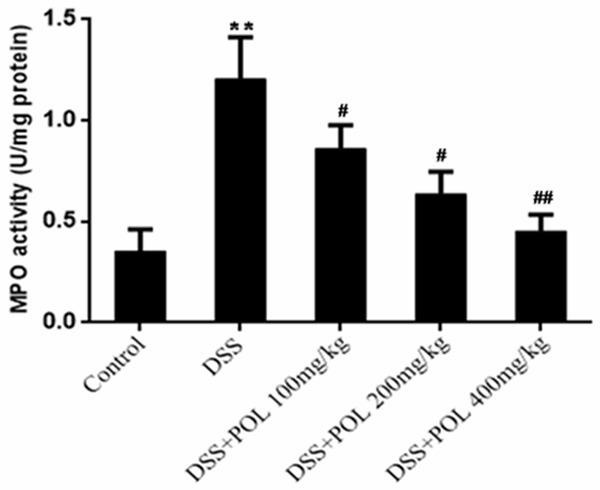
Effects of POL on MPO activities of colon in DSS induced UC. The MPO activities of colon in DSS induced UC Level activities were measured. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, **P<0.01 vs sham, #P<0.05 vs DSS, ##P<0.01 vs DSS.
Effects of the POL on the mRNA expression of TNF-α, IL-6 and IL-1β
The inflammatory cytokines were investigated to show the anti-inflammatory effect of POL, and the mRNA expression of IL-6, IL-1β and TNF-α was analyzed by RT-PCR. As shown in Figure 5, for DSS caused the colonic inflammation, the mRNA expression of IL-6, IL-1β and TNF-α was increased markedly in DSS induced UC group compared with control group (P<0.05). Conversely, all of the proinflammatory cytokines in colon tissues were effectively reduced in the POL treatment groups, and different dosage of POL reduced the mRNA expression in a dose-dependent manner.
Figure 5.
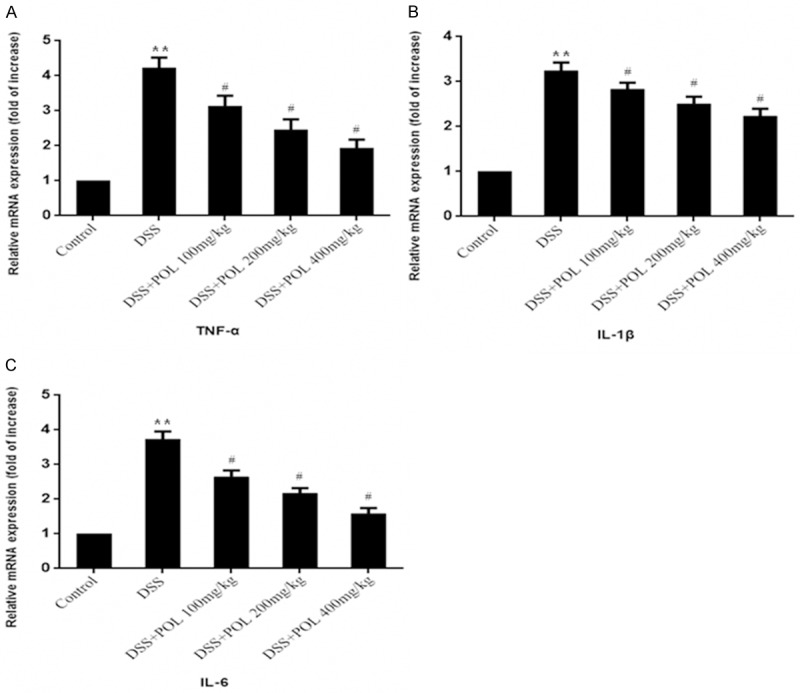
Effects of the POL on the relative mRNA expression of TNF-α, IL-6 and IL-1β of colon in DSS induced UC. The relative mRNA expression of (A) TNF-α, (B) IL-1β and (C) IL-6 activities was measured. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, **P<0.01 vs sham, #P<0.05 vs DSS.
Effects of the POL on expression of TNF-α and NF-κB
It has been reported that NF-κB p65 was an important inflammation mediating molecular, so the western blot was performed to analysis the expression of TNF-α and NF-κB p65 in colon tissues (Figure 6). Compared with control group, the expression of TNF-α and NF-κB p65 was significantly decreased in DSS induced UC group. POL treatment groups were significantly reduced the expression of TNF-α and NF-κB p65 compared with DSS induced UC group (P<0.05).
Figure 6.
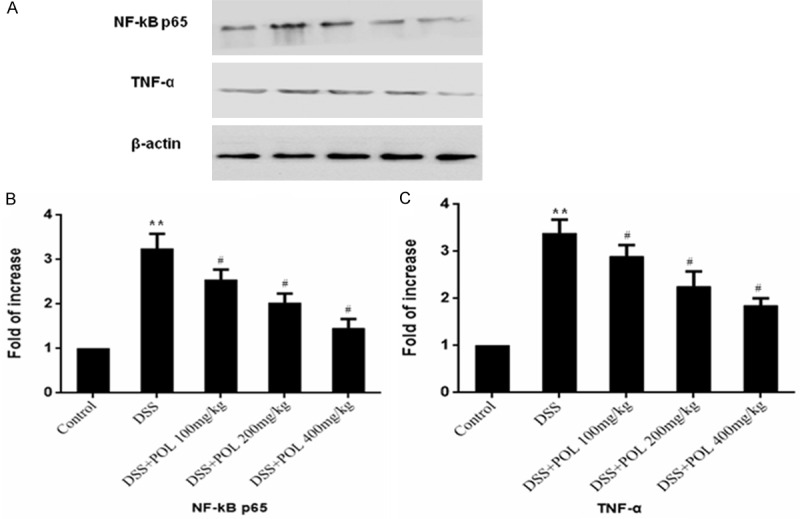
Effects of the POL on expression of TNF-α and NF-κB in DSS induced UC. The expression of (A) TNF-α and NF-κB p65 was evaluated by western blot and the fold of increase of (B) TNF-α and (C) NF-κB p65 was presented compared with the control group. Data are presented as mean ± SD. (n = 8 for each group). *P<0.05 vs sham, **P<0.01 vs sham, #P<0.05 vs DSS.
Discussion
Ulcerative colitis is associated with cellular immunity, oxidative stress and autoimmunity, it was recognized that the immune and oxidative stress dysfunction plays an important role in the pathogenesis of UC [25]. POL has been used for thousands of years in China to treat various ailments in humans, and has the nutritional benefits due to the rich omega-3 fatty acids and antioxidant properties [26,27]. In this study, we want to preliminary clarify the mechanism and scientific intension of the protective effects of POL extract on DSS-induced UC.
The UC model was successfully established by treating mice with 5% DSS for 7 days. The DSS induced UC in the C57BL/6 male mice was widely used model for the studies on UC disease. For the large molecular weight and negative charge of the DSS, the DSS usually administration by oral which could easily absorbed and reaches the colon. The mechanism of DSS induces UC is likely due to the properties of high salt, caused the imbalance of the internal and external osmotic pressure, damage to the epithelial monolayer lining in the colon [28]. And DSS modeling was more safe compared other methods to induce UC. The DSS induced UC group showed that body weight loss, diarrhea, and shorter colon length in our study. After administration of POL, the treatment group exhibited that weight loss, diarrhea, and colonic shortening were significantly reversed and improved. The result of HE staining was consistent with the assessment of colitis, the high level of inflammatory cells infiltration, mucosal edema and thickening of the colon wall were shown in DSS induced UC group. After the treatment of POL, the inflammatory cells infiltration and ulcer area were significantly decreased compared with DSS induced UC group, the adhesion and edema were also alleviated.
MDA is a marker for oxidative stress, results from lipid peroxidation of polyunsaturated fatty acids [29], SOD is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide radical into either ordinary molecular oxygen or hydrogen peroxide and NO could lead to tissue damage in the middle and distal colon through the infiltration of neutrophils [30]. The previous research has shown that oxidative stress induced by excessively produced reactive oxygen metabolites played an important role in the intestinal tissue damage of UC models. In DSS induced UC model, the increased activities of MDA, NO and decreased SOD induced that enhanced free radical activity and reduced endogenous antioxidants level imply that severe oxidative stress occurred during UC. Our study found that with the POL treatment could significantly decrease the level of MDA, NO and increase SOD activity in DSS induced UC model. These results demonstrated that POL may protect against DSS-induced UC via regulation of oxidative stress biomarkers.
MPO was one of cytotoxic enzymes in the neutrophils, as a relevant factor in tissue injury in many inflammatory processes [31]. Its activity can positively reflect the number of neutrophil granulocytes and trigger a variety of inflammatory diseases. As we know, DSS would significantly increased MPO accumulation in the colon tissue, and our study also demonstrated that the MPO activity was markedly increased in DSS induced UC model. Our study found that with the POL treatment could significantly decrease the elevation of MPO activity. This result may demonstrate that POL has a beneficial effect of anti-inflammatory on UC.
Inflammatory as well as oxidative stress was played an important role in the pathogenesis of UC. IL-6 was secreted by T cells and macrophages to stimulate the inflammatory and auto-immune processes in UC. IL-6 acts as a pro-inflammatory cytokine caused the colon damage leading to inflammation. IL-1β was a cytokine protein produced by activated macrophages acts as an important mediator of the inflammatory response, and with the increased production of IL-1β would cause auto-immune processes that damage the colon tissue [32]. TNF-α was a cytokine that has a wide variety of functions, and would cause apoptotic cell death, proliferation, differentiation and inflammation. TNF-α was involved in systemic inflammation and the over-expression of TNF-α was lead to the intestinal mucosal impairment [33]. In the DSS induced UC model group, for DSS induced UC was an inflammatory process, the proinflammatory cytokines of IL-6, IL-1β and TNF-α was significantly increased and result in intestinal tissue damage. Our results showed that POL reduced the mRNA levels of IL-6, IL-1β and TNF-α in DSS induced UC mice, suggesting that protective effect of POL in the colon tissue from damage induced by DSS was related to the down regulation of inflammatory cytokines, such as IL-6, IL-1β and TNF-α.
NF-κB was a protein which consists of the p50 and p65/RelA polypeptides controls transcription of DNA and cytokine production, played a key role in the regulation of transcription and the expression of many cytokines involved in inflammatory responses, such as TNF-α. Incorrect regulation of NF-κB could cause the viral infection, improper immune development, inflammatory and autoimmune diseases. Activated NF-κB accelerates the expression of TNF-α and as extracellular stimulating factor, the increasing expression of TNF-α could activate NF-κB, further cascade of the inflammatory reaction [34]. In addition, reactive oxygen species was also able to activate NF-κB pathway, and the antioxidant activities of drugs may inhibit NF-κB pathway in recent studies [35,36]. In this study, the western blot assay exhibited that the expression of TNF-α and NF-κB was significantly increased in the DSS induced UC model group compared with the control group. Our results showed that POL significantly reduced the expression of both TNF-α and NF-κB on DSS induced UC mice compared with DSS induced UC group. It was may showed that POL has its anti-inflammatory effect through inhibition of NF-κB activation and reduction of TNF-α expression.
Overall, in this study we firstly demonstrate that POL could exhibited effective protection for the DSS induced UC by increasing the colon length, decreasing body weight loss and the disease activity index score, inhibiting oxidative stress response through the MDA, NO, SOD activities, reducing the mRNA expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and the protein expressions of TNF-α and NF-kB p65. These results may prove that POL could be considered as a useful and effective botanical compound from the edible plant to be used in UC.
Acknowledgements
We thank the grant from the National Natural Science Foundation of China (No.81173156) and Education Department of Shaanxi Provincial Government, China (No.2011JG19) and Administration of Traditional Chinese Medicine of Shaanxi Province, China (No.jc09) for financial support.
Disclosure of conflict of interest
None.
References
- 1.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 5.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77. doi: 10.3748/wjg.v20.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkhayat ES, Ibrahim SR, Aziz MA. Portulene, a new diterpene from Portulaca oleracea L. J Asian Nat Prod Res. 2008;10:1039–1043. doi: 10.1080/10286020802320590. [DOI] [PubMed] [Google Scholar]
- 9.Jin R, Lin ZJ, Xue CM, Zhang B. An improved association-mining research for exploring Chinese herbal property theory: based on data of the Shennong’s Classic of Materia Medica. J Integr Med. 2013;11:352–365. doi: 10.3736/jintegrmed2013051. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Xu X, Huang Y, Xu L, Chen G. Field enhancement sample stacking for analysis of organic acids in traditional Chinese medicine by capillary electrophoresis. J Chromatogr A. 2012;1246:35–39. doi: 10.1016/j.chroma.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Wang WY, Wang XL, Dong LW, Yue YT, Xin HL, Ling CQ, Li M. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. J Ethnopharmacol. 2009;124:246–250. doi: 10.1016/j.jep.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–2601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Chan K, Islam MW, Kamil M, Radhakrishnan R, Zakaria MN, Habibullah M, Attas A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. Sativa (Haw.) Celak. J Ethnopharmacol. 2000;73:445–451. doi: 10.1016/s0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 14.Parry O, Marks JA, Okwuasaba FK. The skeletal muscle relaxant action of Portulaca oleracea: role of potassium ions. J Ethnopharmacol. 1993;40:187–194. doi: 10.1016/0378-8741(93)90067-f. [DOI] [PubMed] [Google Scholar]
- 15.Liu XF, Zheng CG, Shi HG, Tang GS, Wang WY, Zhou J, Dong LW. Ethanol extract from portulaca oleracea L. attenuated acetaminophen-induced mice liver injury. Am J Transl Res. 2015;7:309–318. [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H, Liu X, Tang G, Liu H, Zhang Y, Zhang B, Zhao X, Wang W. Ethanol extract of Portulaca Oleracea L. reduced the carbon tetrachloride induced liver injury in mice involving enhancement of NF-kappaB activity. Am J Transl Res. 2014;6:746–755. [PMC free article] [PubMed] [Google Scholar]
- 17.Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XJ JY, Qu Z, Xia J, Wang L. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro. Chin J Microecol. 2002;14:277–280. [Google Scholar]
- 19.Karimi G, Hosseinzadeh H, Ettehad N. Evaluation of the gastric antiulcerogenic effects of Portulaca oleracea L. extracts in mice. Phytother Res. 2004;18:484–487. doi: 10.1002/ptr.1463. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira MC, Carvalho IS, Brodelius M. Omega-3 fatty acid desaturase genes isolated from purslane (Portulaca oleracea L.): expression in different tissues and response to cold and wound stress. J Agric Food Chem. 2010;58:1870–1877. doi: 10.1021/jf902684v. [DOI] [PubMed] [Google Scholar]
- 21.Lee AS, Lee YJ, Lee SM, Yoon JJ, Kim JS, Kang DG, Lee HS. Portulaca oleracea Ameliorates Diabetic Vascular Inflammation and Endothelial Dysfunction in db/db Mice. Evid Based Complement Alternat Med. 2012;2012:741824. doi: 10.1155/2012/741824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Xu Y, Zhang C, Deng L, Chang M, Yu Z, Liu D. Protective Effect of Calculus Bovis Sativus on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Evid Based Complement Alternat Med. 2015;2015:469506. doi: 10.1155/2015/469506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54:175–189. doi: 10.1080/10408398.2011.579361. [DOI] [PubMed] [Google Scholar]
- 26.Petropoulos S, Karkanis A, Fernandes A, Barros L, Ferreira IC, Ntatsi G, Petrotos K, Lykas C, Khah E. Chemical Composition and Yield of Six Genotypes of Common Purslane (Portulaca oleracea L.): An Alternative Source of Omega-3 Fatty Acids. Plant Foods Hum Nutr. 2015;70:420–426. doi: 10.1007/s11130-015-0511-8. [DOI] [PubMed] [Google Scholar]
- 27.Uddin MK, Juraimi AS, Hossain MS, Nahar MA, Ali ME, Rahman MM. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. ScientificWorldJournal. 2014;2014:951019. doi: 10.1155/2014/951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 29.Gueraud F, Tache S, Steghens JP, Milkovic L, Borovic-Sunjic S, Zarkovic N, Gaultier E, Naud N, Helies-Toussaint C, Pierre F, Priymenko N. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic Biol Med. 2015;83:192–200. doi: 10.1016/j.freeradbiomed.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179–189. doi: 10.1097/00054725-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Hong T, Dong M, Meng Y, Mu J. Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol Med Rep. 2013;7:565–570. doi: 10.3892/mmr.2012.1225. [DOI] [PubMed] [Google Scholar]
- 32.Yap DY, Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol. 2010;2010:365083. doi: 10.1155/2010/365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711–1747. doi: 10.1089/ars.2012.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marupanthorn K, Tantrawatpan C, Tantikanlayaporn D, Kheolamai P, Manochantr S. The Effects of TNF-alpha on Osteogenic Differentiation of Umbilical Cord Derived Mesenchymal Stem Cells. J Med Assoc Thai. 2015;98(Suppl 3):S34–40. [PubMed] [Google Scholar]
- 35.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]


