Abstract
Purpose: Delayed wound healing is an intractable complex of diabetes and substance P (SP) is proved to benefit wound healing, whose functioning mechanism remains elusive. This study aims at revealing whether the influence of SP on diabetic wound healing is dependent on inflammatory responses, particularly NF-κB. Methods: Skin fibroblasts of genetically diabetic mice were co-cultured with bone marrow-derived macrophages, and treated with SP, SP + L703,606 (a neurokinin-1 receptor antagonist), or SP + MG132 (an inhibitor of NF-κB). For macrophages, their migration ability was assessed by Transwell experiments, and their M2 polarization was analyzed by flow cytometry and markers for M2 phenotype. Pro-inflammatory factors in the supernatant were detected by enzyme-linked immunosorbent assay. In fibroblasts, the transcription levels of the four pro-inflammatory factors and the protein levels of NF-κB regulators like inhibitor of NF-κB alpha (IκBα) and IκB kinases (IKKs) were monitored by real-time quantitative PCR and western blot, respectively. Results: SP could significantly induce migration to fibroblasts (P<0.01), M2 polarization (P<0.001) and pro-inflammatory factor concentration (P<0.01) in the co-culture system. It also promotes the transcription process of pro-inflammatory factors in fibroblasts (P<0.01), and induce activation of IKKα/β and phosphorylation of IκBα, which caused NF-κB activation. All these effects were reversed if NF-κB was inhibited. Conclusion: The promoting effects of SP on diabetic wound healing was dependent on enhanced inflammatory responses, especially the activation of NF-κB. This study provided evidence for the potential usage of SP in accelerating diabetic wound healing.
Keywords: Substance P, diabetic wound healing, inflammatory response, NF-κB
Introduction
Poor wound healing is an important complex of diabetes, leading to worsened non-healing ulcers, especially foot ulcers, and even wound infection or osteomyelitis [1]. In normal persons, various cell types and a series of reaction cascades are involved in the effective wound healing, which includes the aggregation of platelets and inflammatory cells, such as neutrophils and macrophages, and then the functioning of fibroblasts. During the process, macrophages release growth factors and fibroblasts secrete extracellular matrix (ECM) material, both contributing to the rapid healing of wounds [2]. However, this set of wound healing processes is disrupted in diabetic patients, whose inflammatory reactions are possibly to be impaired due to the metabolic abnormalities of diabetes [3].
Macrophages are the primary sources of cytokines during wound healing, when they are activated into two phenotypes, namely the classically activated macrophages (M1) and the alternatively activated macrophages (M2) [4]. The M1 phenotype, triggering the pro-inflammatory response that kills intracellular pathogens, can be induced by interferon gamma (IFN-γ) or lipopolysaccharide, while the M2 phenotype, which is important for the anti-inflammatory response and immune responses to parasites, is induced by interkeukin-4 (IL-4) or IL-13 [5]. Typically, M1 polarized macrophages specifically express cell surface molecules like CD68 and CD80, and the surface markers for M2 macrophages include CD163 and CD206 [6,7]. But the boundary between M1 and M2 macrophages is not absolute, for the existence of M1 or M2-like macrophages, which possess the characteristics of M1 and M2 macrophages at the same time. Researchers have found evidence that as the wound matures, the main activation type of macrophages converts from M1 to M2 [8], which may represent the varying degrees of inflammatory responses.
As the important connective tissue cells, fibroblasts respond to the growth factors and cytokines secreted by macrophages, platelets, amongst others, to grow and proliferate. They produce ECM, providing the structural support for cell growth and nutrient transport [9]. An earlier study has indicated that in response to serum stimulation, skin fibroblasts can adjust their transcriptional activities, which consists of genes and intercellular signaling related to remodeling of the clot and ECM, providing an overview on the significance of fibroblasts in wound healing [10].
Substance P (SP) is a kind of neurokinin, which is found to be suppressed in diabetic wound tissues [11], thus inhibiting acute inflammatory responses and leading to failure of wound healing [12]. Very recent studies have proved the functions of SP in facilitating diabetic wound healing by promoting M2 macrophage polarization, ECM formation, fibroblast proliferation and collagen deposition [12,13]. There are also studies revealing the activation roles of SP on NF-κB signaling in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM)-cultured fibroblasts [14]. Albeit these remarkable efforts on revealing roles of SP, the regulatory mechanisms of SP in diabetic wound healing remain unclear.
Inspired by former studies that NF-κB, a pivotal regulator in inflammation, is involved in wound healing [15,16], we aimed to determine whether the promotive function of SP on diabetic wound healing was dependent on inflammatory responses involving NF-κB. To achieve this aim, we isolated and co-cultured mouse skin fibroblasts and bone marrow-derived macrophages, and treated them with SP, neurokinin-1 receptor antagonist L703,606 or NF-κB antagonist MG132, to detect changes in macrophage migration, polarization, factor amounts in the medium, and factor expression in the fibroblasts. Our data proved that the roles of SP on influencing macrophages and fibroblasts, and promoting diabetic wound healing, are dependent on the activation of NF-κB-modulated inflammatory responses, offering explanations for the functional mechanism of SP.
Materials and methods
Animal
Four female individuals of C57BL/KsJ-db mice (20-30 g) were purchased form Vital River Laboratories (Beijing, China) and raised in caged laboratory conditions for 3 d before anesthetization and sampling. All the animal experiments were approved by the local animal ethics committee and performed according to the instruction of our institute.
Skin fibroblast cell culture
The back skin was harvested from mice and the hair was removed. The skin was cut into small pieces of 5 mm3, and put into dishes for collecting the fibroblasts. Fibroblasts were primarily cultured in DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Gibco). After four passages, the fibroblasts were seeded in a Petri dish and DMEM with 10% FBS was added. Then the cells were cultured in humid atmosphere containing 5% CO2 at 37°C.
Macrophage cell culture
Bone marrow was collected from the left femur of mice and cultured in DMEM with 10% FBS, 2% penicillin-streptomycin (Thermo Scientific, Carlsbad, CA) and 50 ng/mL M-CSF or GM-CSF (PeproTeck, Rocky Hill, NJ) to induce macrophages of different phenotypes. Non-adherent cells were washed out and the adherent macrophages were cultured for use. Macrophages derived from peritoneal cavity were collected from the peritoneal exudate cells by washing out non-adherent cells. Then they were cultured in DMEM with 10% FBS and 2% penicillin-streptomycin.
Co-culture of fibroblasts and macrophages
Fibroblasts (1.5×104) were seeded and adhered to the lower chamber of a Transwell (Corning, New York, USA), and the mix of bone marrow-derived and peritoneal cavity-derived macrophages (1.5×104) was added to the upper chamber, which allowed the interaction of soluble factors between the two cell types. Cells were treated with SP (10 nM, Merck, Darmstadt, Germany), SP + MG132 (2 μM, Merck), or SP + L703,606 (1 μM, Sigma-Aldrich, Shanghai, China), and cells with no treatment were used as the control group. Then the cells were cultured in DMEM supplemented with 10% FBS in humid atmosphere with 5% CO2 at 37°C. Macrophages on the upper side of the membrane were cleared with a cotton swab after 24 h. The cells that migrated to the lower side of the membrane were fixed with 10% methanol for 30 min and stained with crystal violet (Solarbio, Beijing, China) for 20 min. After washed with phosphate buffer saline for two times, the crystal violet was eluted with 33% acetic acid, then the optical density (OD) of the elution was compared to the reference standard by detecting the absorbance at 570 nm. The fibroblasts in the lower chamber were collected for the later experiments by centrifugation, and the supernatants in the lower chamber were collected for enzyme-linked immunosorbent assay (ELISA) to detect the amount of monocyte chemoattractant protein-1 (MCP-1), IL-1β, tumor necrosis factor alpha (TNF-α) and INF-γ in supernatants of the co-culture system using the corresponding ELISA kit (R&D Systems, Minneapolis, MN).
Macrophage polarization assay
The macrophages co-cultured with fibroblasts treated with SP, SP + MG132, SP + L703,606 or not were harvested. Macrophages of 5×105 were incubated with Alexa Fluor 488 anti-F4/80 (1:100, #123119), PE anti-CD206 (1:100, #141705), and PerCP/cy5.5 anti-CD80 (1:100, #104721, BioLegend, San Diego, CA) for 30 min at 4°C. After washed and resuspended with phosphate buffer saline, the cells were detected by FACSCanto II (BD Biosciences, San Jose, CA) to monitor the M2 macrophage number.
Real-time quantitative PCR (qPCR)
Fibroblasts and macrophages in the co-culture system treated with SP, SP + MG132, SP + L703,606 or not were collected separately and lysed in TRIzol (Invitrogen, Carlsbad, CA) for total RNA extraction. The extracted RNAs were purified using DNase I (Invitrogen) to eliminate DNA contamination and quantified by NanoDrop 2000 (Thermo Scientific). The complementary DNAs (cDNAs) were synthesized with PrimeScipt 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China) from 1 μg RNAs. qPCR was performed to detect the arginase 1 (Arg1), c-type lectin domain family 10, member A (Clec10a), and resistin-like molecule alpha (Retnla) expression inmacrophages, and MCP-1, IL-1β, TNF-α and IFN-γ expression in fibroblasts. β-actin was used as an internal reference. 20 ng cDNAs and gene-specific primers (Table 1) were used in each reaction, which was performed on LightCycler 480 (Roche, Basel, Switzerland). Experiments were conducted in triplicate for each sample, and data were analyzed with the 2-ΔΔCt method.
Table 1.
Gene-specific primers used in qPCR
| Gene (Accession number) | Primer sequence (5’ to 3’) | Product size (bp) |
|---|---|---|
| Arg1 (NM_007482) | Fw: CTCCAAGCCAAAGTCCTTAGAG | 184 |
| Rv: AGGAGCTGTCATTAGGGACATC | ||
| Clec10a (NM_010796) | Fw: GGAATCCTCCTACCCGGTCT | 107 |
| Rv: TGCAAACACCACACTACCCA | ||
| Retnla (NM_020509) | Fw: CTCCACTGTAACGAAGACTC | 177 |
| Rv: GCAGTGGTCCAGTCAACGA | ||
| MCP-1 (NM_011333) | Fw: TCAGCCAGATGCAGTTAACGC | 94 |
| Rv: TGATCCTCTTGTAGCTCTCCAGC | ||
| IL-1β (NM_008361) | Fw: ACGGACCCCAAAAGATGAAG | 138 |
| Rv: TTCTTCACAGCCACAATGAG | ||
| TNF-α (NM_013693) | Fw: CCACATCTCCCTCCAGAAAA | 259 |
| Rv: AGGGTCTGGGCCATAGAACT | ||
| IFN-γ (NM_008337) | Fw: TGAGACAATGAACGCTACAC | 143 |
| Rv: CTTCCACATCTATGCCACT | ||
| β-actin (NM_007393) | Fw: TGACAGGATGCAGAAGGAGA | 131 |
| Rv: GCTGGAAGGTGGACAGTGAG |
Western blot
Protein was extracted from the fibroblasts in the co-culture system with lysis buffer (Beyotime, Shanghai, China). Protein samples of the same amount were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked in 5% skim milk for 2 h at room temperature, and incubated in specific primary antibodies (ab7609, ab32135, ab55341, ab32518, ab12135, abcam, Cambridge, UK) overnight at 4°C. β-actin (ab6276, abcam) was used as an internal reference. After washed with Tris-buffered saline containing 0.1% Tween 20, the membrane was incubated in horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. Signals were developed using Enhanced Chemiluminescence Detection Kit for HRP (Biological Industries, Beit-Haemek, Israel), and the band intensity was analyzed by ImageJ 1.49 (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were repeated at least in triplicate with results indicated as the mean ± standard deviation. Data were analyzed using one-way analysis of variance followed by t test with Statistical Product and Service Solutions 19.0 (IBM, New York, USA). Differences were considered significant if P<0.05.
Results
SP promotes migration, M2 polarization and pro-inflammatory factor concentration in the medium
We first examined the migration ability change of macrophages co-cultured with skin fibroblasts after they were treated with SP, neurokinin-1 receptor antagonist L703,606, or NF-κB antagonist MG132. Observation with a microscope revealed that macrophages treated with SP possessed higher migration ability than the other treated groups or the control group (Figure 1A). OD detection further confirmed that the migrated macrophages in the SP-treated group were significantly increased than the other groups (P<0.01, Figure 1B). These results indicated that SP helped to recruit macrophages to skin fibroblasts. But its function was inhibited if NF-κB was suppressed by its corresponding antagonist, which inferred the vital roles of NF-κB in this process.
Figure 1.
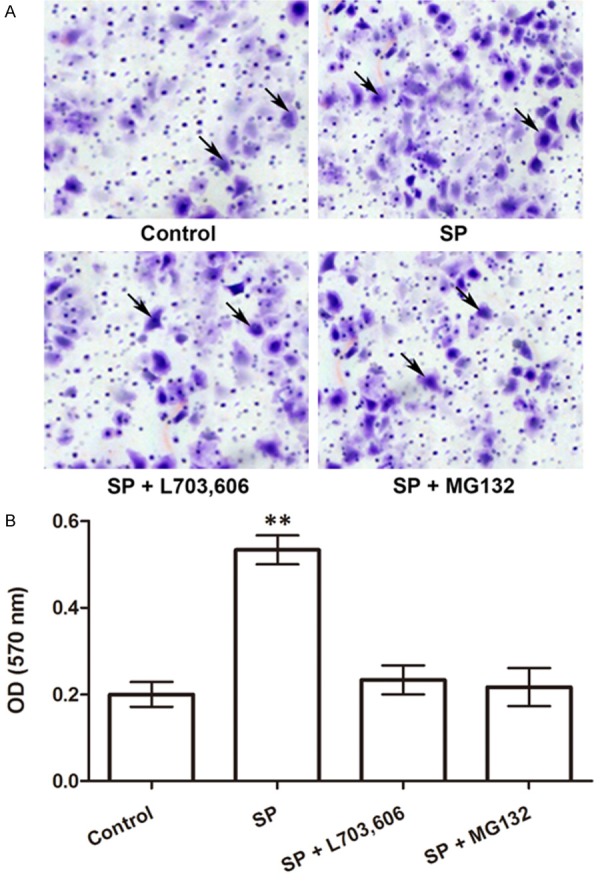
Number of migrated macrophages in the co-culture system is increased by SP. A. Photographs of migrated macrophages (pointed by arrows) observed with a microscope. B. Optical density (OD) under 570 nm of the migrated macrophages (N=5). **P<0.01. SP, substance P. L703,606, neurokinin-1 receptor antagonist. MG132, NF-κB antagonist.
Then we tested whether M2 macrophage polarization happened during this process. The macrophages were collected to detect the expression level of M2 macrophage markers, including Arg1, Clec10a and Retnla [17,18]. Results showed that the mRNA expression of all the three factors was significantly promoted inthe macrophages by SP treatment (P<0.001, Figure 2A-C), while their expression levels remained unchanged when SP or NF-êB was inhibited. More detailed analysis using flow cytometry indicated that the number of M2 macrophages were indeed significantly increased in the SP-treated group (P<0.001, Figure 2D and 2E), in agreement with the mRNA expression results. These results suggested the roles of SP in promoting M2 macrophage polarization, and NF-κB was involved in the execution of SP function.
Figure 2.
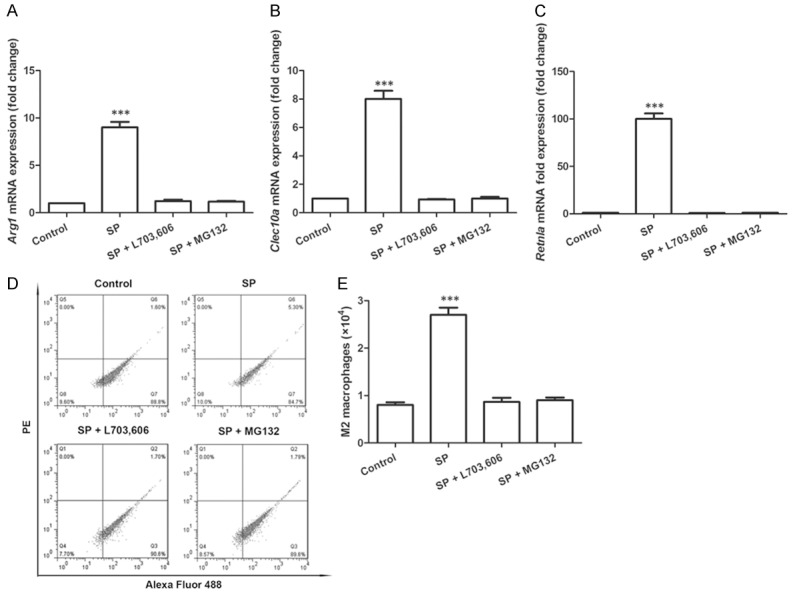
M2 macrophage polarization in the co-culture system is promoted by SP. A. mRNA expression of Arg1 in macrophages (N=3). B. mRNA expression of Clec10a in macrophages (N=3). C. mRNA expression of Retnla in macrophages (N=3). D. Flow cytometry results show the M2 macrophage number (PE-positive and Alexa Fluor 488-positive) is increased by SP. E. Number of M2 macrophages calculated by flow cytometry (N=3). ***P<0.001. SP, substance P. L703,606, neurokinin-1 receptor antagonist. MG132, NF-κB antagonist. Arg1, arginase 1. Clec10a, c-type lectin domain family 10, member A. Retnla, resistin-like molecule alpha.
The amount of four pro-inflammatory factors, MCP-1, IL-1β, TNF-α and IFN-γ, in the medium of the co-culture system was monitored by quantitative ELISA. Results indicated the increased amounts of these factors in the medium when cells were treated with SP (P<0.01, Figure 3), inferring the roles of SP in promoting the release of pro-inflammatory factors from macrophages or fibroblasts, which might further influence inflammatory responses. But when NF-κB was inhibited, the amount of these four factors was almost unchanged, which again underscored the roles of NF-κB in the regulatory process of SP. Taken together, SP made great influences on the co-cultured macrophages by inducing their migration, M2 polarization, as well as the pro-inflammatory factor secretion, all of which depended on NF-κB, inferring the functions of SP might rely on mechanisms involving NF-κB.
Figure 3.

Concentration of MCP-1, IL-1β, TNF-α and IFN-γ in the co-culture medium detected by ELISA (N=3). **P<0.01. SP, substance P. L703,606, neurokinin-1 receptor antagonist. MG132, NF-κB antagonist. MCP-1, monocyte chemoattractant protein-1. IL-1β, interleukin-1 beta. TNF-α, tumor necrosis factor alpha. IFN-γ, interferon gamma.
SP activates NF-κB and pro-inflammatory production in fibroblasts
We also examined changes in the co-cultured fibroblasts after SP treatment. First we analyzed the mRNA expression of MCP-1, IL-1β, TNF-α and IFN-γ in fibroblasts. Results showed that the mRNA expression levels of the four factors in fibroblasts were all significantly up-regulated by SP treatment (P<0.05, Figure 4), while they were inhibited at their original levels when SP was inhibited, inferring that SP might induce the production of pro-inflammatory factors in fibroblasts, which was associated with NF-κB, since the transcription levels of these four factors were reduced to the original levels when inhibiting NF-κB. This result implied the promoting functions of SP on inflammatory responses in fibroblasts, possibly due to the response of fibroblasts to macrophages and the increased pro-inflammatory factors in the medium.
Figure 4.
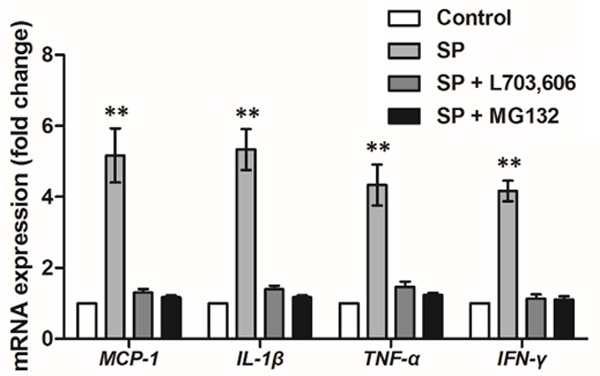
mRNA expression of MCP-1, IL-1β, TNF-α and IFN-γ in the fibroblasts of the co-culture system (N=3). **P<0.01. SP, substance P. L703,606, neurokinin-1 receptor antagonist. MG132, NF-κB antagonist. MCP-1, monocyte chemoattractant protein-1. IL-1β, interleukin-1 beta. TNF-α, tumor necrosis factor alpha. IFN-γ, interferon gamma.
Was NF-κB actually activated in the SP-induced inflammatory responses? To verify this speculation, we next examined the proteins regulating NF-κB in fibroblasts using western blot (Figure 5A and 5B). SP treatment did not influence the expression of inhibitor of NF-κB alpha (IκBα), IκB kinase alpha (IKKα) or IKKβ, while it significantly promoted the phosphorylated forms of these factors (p-IKKα/β and p-IκBα) (P<0.05), suggesting SP, as predicted, could induce the activation of p-IKKα/β and p-IκBα, which was likely to further activate NF-κB and its signaling.Conforming to the former results, the activated NF-κB might be translocated to the nucleus and promote the transcription of MCP-1, IL-1β, TNF-α and IFN-γ in fibroblasts.
Figure 5.
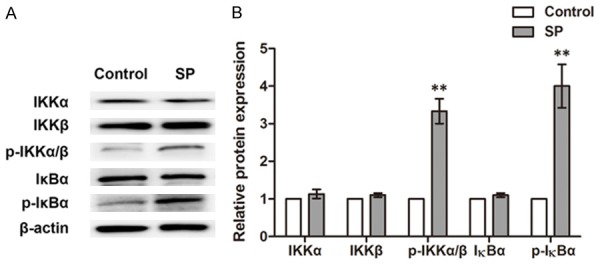
Protein expression of IKKα, IKKβ, p-IKKα/β, IκBα and p-IκBα in the fibroblasts of the co-culture system. A. Western blot results showing the expression changes of these proteins by SP. B. Histogram showing the relative intensity of bands to Control in western blot (N=3). **P<0.01. SP, substance P. IκBα, inhibitor of NF-κB alpha. p-IκBα, phosphorylated IκBα. IKKα, IκB kinase alpha. IKKβ, IκB kinase beta.
Discussion
In this study, we investigated the influences of SP on inflammatory responses in co-cultured mouse macrophages and skin fibroblasts. SP induces migration to fibroblasts, M2 polarization of macrophages, and pro-inflammatory factors secreted to the co-culture system. SP also promotes the inflammatory responses in fibroblasts by activating NF-κB, indicated by the up-regulated transcription of factors mediated by NF-κB, and the promoted p-IKKα/β and p-IκBα levels in fibroblasts.
As mentioned above, SP is a neuropeptide with a high affinity for neurokinin-1 receptor [19]. So L703,606, a neurokinin-1 receptor antagonist, was used in this study to achieve the inhibition of SP. When the neurokinin-1 receptor is inhibited by L703,606, the consumption of SP is blocked, which in turn suppresses SP production SP, thus leading to a significantly reduced SP level [20]. We evaluated M2 macrophage polarization by calculating the number of M2 polarized macrophages marked with M2-specific cell surface molecules using flow cytometry, as well as the detection of M2-specific cell markers, Arg1, Clec10a and Retnla [18] in macrophages. Results reflected significant changes in macrophage activities under SP treatment, including the promoted migration to fibroblasts, the induced M2 polarization degree. On the contrary, L703,606 treatment could completely reverse the promoting functions of SP, which further proved the changes in macrophages and cytokine release were indeed brought about by SP.
MCP-1 has been found to be released by human skin fibroblasts under SP treatment [21]. In this study, we detected the concentration of pro-inflammatory cytokines, MCP-1, IL-1β, TNF-α and IFN-γ [22-24], in the medium of the co-culture system, and found their concentration was increased. Together with the results of their up-regulated mRNA levels in the fibroblasts, it could be deduced that the increase of their concentration in the medium could be caused by the release by the fibroblast. However, we could not eliminate the possibility that these cytokines were partly released by the co-cultured macrophages. The M2 polarization of macrophages in wound healing is usually ac-companied by the induction of pro-inflammatory release. The increased release of MCP-1 has been reported in macrophages and keloid CD14+ cells, which helps accelerating the migration of human THP-1 monocytes [25] or proliferation of fibroblasts [26]. IL-1β is proved to be associated with the macrophage polarization towards M2 phenotype [27], and its expression is not found in M2 polarized macrophages [28]. So the four pro-inflammatory factors detected in this study not only proved the promoted release of pro-inflammatory factors by fibroblasts, but also reflected the M2 macrophage polarization from another perspective, implying the enhanced inflammatory responses in the co-culture system.
Cell activation by cytokines like IL-1β and TNF-α causes the activation of IKKs, and further the phosphorylation of IκBs, an inhibitory molecule of NF-κB. Phosphorylated IκBs can be recognized by the ubiquitin ligase machinery, which leads to the polyubiquitination and degradation of IκBs. Then NF-κB is free from IκBs and translocated into the nucleus, where it executes the functions as a transcription factor to further regulate the transcription process of target genes [29-32]. Hence in this study the proteasome inhibitor MG132 was used block NF-κB activation by suppressing the phosphorylation or degradation of IκBα. Results showed that SP significantly increased the mRNA levels of MCP-1, IL-1β, TNF-α and IFN-γ in fibroblasts, inferring their promoted transcription process. However, when NF-κB was blocked by MG132, these factors were inhibited at their original levels, underscoring the pivotal position of activated NF-κB in SP regulatory mechanisms (Figure 6), which were also indicated by the results discussed earlier. It is noteworthy that the four factors examined in this study can also regulate NF-κB [33-36], implying the NF-κB-centered signaling cascade. More evidence to support the activated NF-κB by SP treatment was that levels of p-IKKα/β and p-IκBα were promoted by SP, suggesting the activation of IKKs and degradation of IκBα, which induced NF-κB activation. Taken together, after the addition of SP to the co-culture system of macrophages and fibroblasts, IKKs were activated and IκBα were degraded, inducing the activation of NF-κB, which might be translocated into the nucleus, promoting the transcription of its target genes, such as the pro-inflammatory MCP-1, IL-1β, TNF-α and IFN-γ in fibroblasts. These activated factors might again mediate NF-κB, composing a signaling cascade to induce macrophage aggregation and inflammatory responses at the site of injury, which contributes to quick wound healing.
Figure 6.
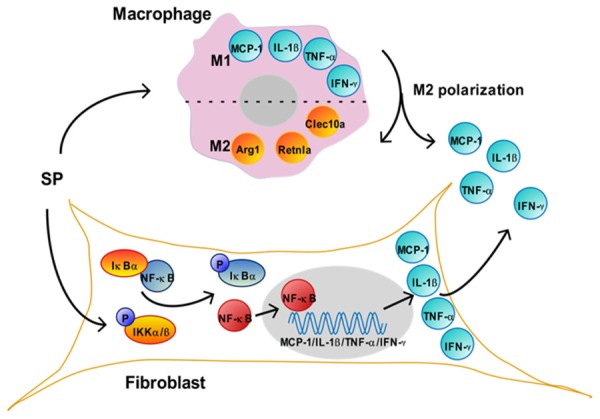
Mechanism of SP in the co-culture system. SP treatment induces the migration of macrophages to fibroblasts and M2 polarization. The markers for M1 macrophages, including MCP-1, IL-1β, TNF-α and IFN-γ, are released by macrophages during the polarization, thus helping to increase the concentration of these factors in the medium. Accordingly, the markers for M2 macrophages, including Arg1, Retnla and Clec10a are up-regulated.In fibroblasts, SP induces the phosphorylation of IKKα/β, which degrades IκBα, thus releasing and activating NF-κB. The activated NF-κB is translocated to the nucleus (grey), where it binds to MCP-1, IL-1β, TNF-α or IFN-γ, and activates the transcription of these genes. These up-regulated cytokines in fibroblast are released to the co-culture medium, which also help to increase their concentration in the medium. SP, substance P. MCP-1, monocyte chemoattractant protein-1. IL-1β, interleukin-1 beta. TNF-α, tumor necrosis factor alpha. IFN-γ, interferon gamma. Arg1, arginase 1. Clec10a, c-type lectin domain family 10, member A. Retnla, resistin-like molecule alpha. IκBα, inhibitor of NF-κB alpha. IKKα/β, IκB kinase alpha/beta. NF-κB, nuclear factor kappa B.
In summary, this study uncovers roles of SP in co-cultured macrophages and skin fibroblasts of genetically diabetic mice. SP promotes migration to fibroblasts and M2 polarization of macrophages, and pro-inflammatory release by fibroblasts and macrophages, leading to the activation of NF-κB and its signaling in fibroblasts, which contributes to enhanced inflammatory responses and quick wound healing. These findings reveal the regulatory mechanisms of SP in inflammatory responses and underscore the potential usage of SP in accelerating diabetic wound healing.
Disclosure of conflict of interest
None.
References
- 1.Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725–1735. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 2.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 3.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 6.Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281:51–61. doi: 10.1016/j.cellimm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Linden MA, Fletcher JA, Morris EM, Meers GM, Laughlin MH, Booth FW, Sowers JR, Ibdah JA, Thyfault JP, Rector RS. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc. 2015;47:556–567. doi: 10.1249/MSS.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2009;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15:1385–1400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 10.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson JJ, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 11.Tao N, Yong F, Zhi-gang M, Peng-gao Y, Xiao-hui H. Expression of neuropeptide substance P during wound healing of deep partial thickness scalding in diabeitc rats. Journal of Shanghai Jiaotong University (Medical Science) 2009;29:673–676. (in Chinese) [Google Scholar]
- 12.Leal EC, Carvalho E, Tellechea A, Kafanas A, Tecilazich F, Kearney C, Kuchibhotla S, Auster ME, Kokkotou E, Mooney DJ, LoGerfo FW, Pradhan-Nabzdyk L, Veves A. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185:1638–1648. doi: 10.1016/j.ajpath.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kant V, Kumar D, Kumar D, Prasad R, Gopal A, Pathak NN, Kumar P, Tandan SK. Topical application of substance P promotes wound healing in streptozotocin-induced diabetic rats. Cytokine. 2015;73:144–155. doi: 10.1016/j.cyto.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Matayoshi T, Goto T, Fukuhara E, Takano H, Kobayashi S, Takahashi T. Neuropeptide substance P stimulates the formation of osteoclasts via synovial fibroblastic cells. Biochem Biophys Res Commun. 2005;327:756–764. doi: 10.1016/j.bbrc.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Ming T. Effects of advanced glycation end-products (AGEs) on skin keratinocytes by nuclear factor-kappa B (NF-κB) activation. Afr J Biotechnol. 2012:11132–11142. [Google Scholar]
- 16.O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews JA, Kasahara DI, Ribeiro L, Wurmbrand AP, Ninin FM, Shore SA. gammadelta T Cells Are Required for M2 Macrophage Polarization and Resolution of Ozone-Induced Pulmonary Inflammation in Mice. PLoS One. 2015;10:e0131236. doi: 10.1371/journal.pone.0131236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastrup H, Schwartz TW. Septide and neurokinin A are high-affinity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS lett. 1996;399:264–266. doi: 10.1016/s0014-5793(96)01337-3. [DOI] [PubMed] [Google Scholar]
- 20.Sio SW, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol. 2008;180:8333–8341. doi: 10.4049/jimmunol.180.12.8333. [DOI] [PubMed] [Google Scholar]
- 21.Zhi-Gang J, Yong F, Min Y, Wei-rong Y, Peng X, Yi-Tao J, Tao N. Molecular mechanisms of substance P-induced monocyte chemoattractant protein-1 secretion in fibroblasts under high glucose culture condition. Journal of Shanghai Jiaotong University (Medical Science) 2012;32:1302–1307. (in Chinese) [Google Scholar]
- 22.Giunti S, Pinach S, Arnaldi L, Viberti G, Perin PC, Camussi G, Gruden G. The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 2006;69:856–863. doi: 10.1038/sj.ki.5000197. [DOI] [PubMed] [Google Scholar]
- 23.Grellner W, Georg T, Wilske J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. 2000;113:251–264. doi: 10.1016/s0379-0738(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 24.Sakallioglu AE, Basaran O, Karakayali H, Ozdemir BH, Yucel M, Arat Z, Haberal M. Interactions of systemic immune response and local wound healing in different burn depths: an experimental study on rats. J Burn Care Res. 2006;27:357–366. doi: 10.1097/01.BCR.0000216330.93056.06. [DOI] [PubMed] [Google Scholar]
- 25.Arana L, Ordonez M, Ouro A, Rivera IG, Gangoiti P, Trueba M, Gomez-Munoz A. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: involvement in ceramide 1-phosphate-stimulated cell migration. Am J Physiol Endocrinol Metab. 2013;304:E1213–1226. doi: 10.1152/ajpendo.00480.2012. [DOI] [PubMed] [Google Scholar]
- 26.Liao WT, Yu HS, Arbiser JL, Hong CH, Govindarajan B, Chai CY, Shan WJ, Lin YF, Chen GS, Lee CH. Enhanced MCP-1 release by keloid CD14+ cells augments fibroblast proliferation: role of MCP-1 and Akt pathway in keloids. Exp Dermatol. 2010;19:e142–150. doi: 10.1111/j.1600-0625.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 27.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–2127. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scotton CJ, Martinez FO, Smelt MJ, Sironi M, Locati M, Mantovani A, Sozzani S. Transcriptional Profiling Reveals Complex Regulation of the Monocyte IL-1 beta System by IL-13. J Immunol. 2005;174:834–845. doi: 10.4049/jimmunol.174.2.834. [DOI] [PubMed] [Google Scholar]
- 29.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 30.Phelps CB, Sengchanthalangsy LL, Huxford T, Ghosh G. Mechanism of I kappa B alpha binding to NF-kappa B dimers. J Biol Chem. 2000;275:29840–29846. doi: 10.1074/jbc.M004899200. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genome Res. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 33.Hwang SY, Woo CW, Au-Yeung KK, Siow YL, Zhu TY, O K. Homocysteine stimulates monocyte chemoattractant protein-1 expression in the kidney via nuclear factor-kappaB activation. Am J Physiol Renal Physiol. 2008;294:F236–244. doi: 10.1152/ajprenal.00331.2007. [DOI] [PubMed] [Google Scholar]
- 34.Lee YR, Lee JH, Noh EM, Kim EK, Song MY, Jung WS, Park SJ, Kim JS, Park JW, Kwon KB, Park BH. Guggulsterone blocks IL-1beta-mediated inflammatory responses by suppressing NF-kappaB activation in fibroblast-like synoviocytes. Life Sci. 2008;82:1203–1209. doi: 10.1016/j.lfs.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Jang EJ, Nahm DH, Jang YJ. Mouse monoclonal autoantibodies penetrate mouse macrophage cells and stimulate NF-kappaB activation and TNF-alpha release. Immunol Lett. 2009;124:70–76. doi: 10.1016/j.imlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Ishida Y, Kimura A, Iwakura Y, Mukaida N, Kondo T. IFN- Protects Cerulein-Induced Acute Pancreatitis by Repressing NF- B Activation. J Immunol. 2007;178:7385–7394. doi: 10.4049/jimmunol.178.11.7385. [DOI] [PubMed] [Google Scholar]


