Abstract
Previous studies reported remarkable high incidence of depression in cancer patients compared with the general population. Colorectal carcinoma (CRC) is one of the most frequent malignancies worldwide and has been found to be one of the malignancies with the highest incidence of patient depression. Thus, strategies that may alleviate CRC-associated depression may significantly improve the patients’ life quality and outcome of the therapy. Ginsenoside Rh2 (GRh2) has been reported to have therapeutic effects on various diseases. However, whether it may also play a potential role in alleviating tumor-associated depression in CRC patients is unknown. Here, we studied the role of GRh2 in the control of depression in CRC using a mouse model. CRC was induced in mice through orthotopic implantation. GRh2 or control vehicle was then given to the mice twice per week for 4 weeks, after which the mice were subjected to a forced swim test (FST), a tail suspension test (TST) and a sucrose intake test (SIT). We found that the mice that received GRh2 treatment significantly improved their behaviors in all FST, TST and SIT tests, seemingly through decreases in the depression-associated cytokines, interleukin 6 (IL-6), IL-18 and tumor necrosis factor-alpha. Moreover, GRh2 significantly increased survival time of the CRC-mice. Together, our data suggest that GRh2 may alleviate tumor-associated depression in mice carrying CRC and highlight GRh2 treatment as a potential beneficial therapy for CRC-associated depression in patients.
Keywords: Ginsenoside Rh2 (GRh2), colorectal carcinoma (CRC), cancer-associated depression
Introduction
Previous studies have demonstrated the immense impact of psychological distress on life quality and illness trajectory in cancer patients. Moreover, this emotional distress has been entitled as “the sixth vital sign in cancer care” [1], which requests health care providers to attach no less importance to the monitoring of emotional distress than to the monitoring of “traditional” vital signs such as blood pressure or heart rate [2]. Patients are believed to benefit from early recognition and adequate treatment of emotional burden and tumor-associated depression [3]. However, whether any medicine could be together with individual psychological coping strategies could have significant impact on treatment outcome for patients’ depression as well as the overall survival in cancer patients is largely unknown.
Colorectal cancer (CRC) is a common malignant tumor that develops from the epithelial cells in the colon or rectum of the gastrointestinal tract [4-6]. The prognosis of CRC largely results from the presence of distal metastases or not, since in situ cancer and cancer with invasion of lymph nodes are both highly treatable [4-6]. However, distal metastases of CRC to the liver, lungs or other organs cause difficulties for treatments, leading to poor therapeutic outcome [7-10]. Many CRC patients have been shown to suffer from depression [11-13]. Hence, strategies to reduce the impact of depression on the outcome of the therapy appear to be extremely important.
Ginsenoside Rh2 (GRh2) is a well-characterized component in red ginseng with potential bioactivity. GRh2 and its derivatives have been reported of potentially therapeutic effects on various diseases, including colitis [14] and some types of cancer [15-24], although the underlying mechanisms are largely unknown. Moreover, whether GRh2 may be an effective treatment for alleviating CRC-associated depression has not been investigated.
Here, we studied the role of GRh2 in the control of depression in CRC using a mouse model. CRC was induced in mice through orthotopic implantation. GRh2 or control vehicle was then given to the mice twice per week for 4 weeks, after which the mice were subjected to a forced swim test (FST), a tail suspension test (TST) and a sucrose intake test (SIT). We found that the mice that received GRh2 treatment significantly improved their behaviors in all FST, TST and SIT tests, seemingly through decreases in the depression-associated cytokines, interleukin 6 (IL-6), IL-18 and tumor necrosis factor-alpha, consistent with previous report [25].
Materials and methods
Animals
All experimental protocols were approved by the Research Bureau of Hangzhou First People’s Hospital. All mouse experiments were approved by the Institutional Animal Care and Use Committee at Hangzhou First People’s Hospital (Animal Welfare Assurance). The methods regarding animals were carried out in “accordance” with the approved guidelines. Mouse surgeries were performed in accordance with the Principles of Laboratory Care, supervised by a qualified veterinarian. The methods were carried out in accordance with the approved guidelines. All efforts were made to minimize pain and suffering. Female NOD/SCID mice of 12 weeks of age were used in the current study. Ten mice were analyzed in each experimental condition.
CRC cell line
Caco-2 is a human epithelial CRC cell line [26], which was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Caco-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (Invitrogen, Carlsbad, CA, USA).
Orthotopic implantation
Caco-2 cells were prepared in normal saline and 105 cells were injected via s.c. in the flank of 12-week-old NOD/SCID mice. After 6 weeks, the mice were euthanized and the tumors were removed and cut into pieces of 1.5 mm in diameter. Twelve-week-old female mice were used as recipients. These mice were anesthetized with isoflurane, and the abdomen was scrubbed for surgery. A small incision was made parallel to the linea alba, and the cecum was exteriorized. One piece of the prepared tumor was implanted on the top of the serosal surface. A surgical suture was used to attach the tumor to the serosal wall without penetrating the inner layers of the intestine. The organ was returned to the abdominal cavity, and the abdominal wall was closed. The analyses on mice were performed 4 weeks after tumor implantation.
GRh2 administration
Ten mice were analyzed in each experimental condition. Gastric irrigation with GRh2 (Weikeqi Bioscience, China) was performed as has been described previously [14,27], and the frequency is twice per week for 4 weeks, till analyses. GRh2 was given at 0.2 mg/kg body weight, 1 mg/kg body weight, and 5 mg/kg body weight, respectively.
Forced swim test (FST)
Forced swim test (FST) assesses despair which is a depressive-like behaviors comprising struggling time vs immobile time. In this study, each mouse was individually placed in a glass barrel (height: 25 cm; diameter: 15 cm) containing 20 cm water (25°C). All the mice were allowed to swim for 6 min and recorded by videos to score subsequently. The immobility time, defined as the moment when a mouse lacks other activities except for movements to keep its head above the water, was scored during the last 4 min.
Tail suspension test (TST)
In this study, mouse tails were wrapped with tape from the base to the tip and fixed upside down on the hook. Sessions were recorded and scored subsequently for the immobility time, defined as the moment of absence of active attempts to escape during the last 4 min.
Sucrose intake test (SIT)
1% sucrose intake was measured for 1 hour between 19:00 and 20:00 every Monday. All mice (including the control group) were deprived of water and food 12 hours before the SIT. Mice continued freely eating and drinking after the test.
ELISA
The serum levels of depression-associated cytokines, interleukin 6 (IL-6), IL-18 and tumor necrosis factor-alpha (TNF-α) in mice were analyzed using corresponding ELISA kit (R&D Systems, Los Angeles, CA, USA), according to the manufacturer’s instruction.
Statistical analysis
All statistical analyses were carried out using the SPSS 18.0 statistical software package. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s Exact Test. Mouse survival was analyzed using Kaplan-Meier curves. All values are depicted as mean ± standard error and are considered significant if p < 0.05.
Results
Experimental design
In order to evaluate the effects of GRh2 on CRC-associated depression, we set up a mouse model. First, NOD/SCID mice were subcutaneously injected with Caco-2 cells to form tumor. Then orthotopic implantation of an identical size of tumor was performed in NOD/SCID mice to create CRC. After tumor implantation, the mice received GRh2 at different doses for 4 weeks. The CRC-mice that received saline only were used as controls. Serum cytokine levels were measured at the end of the experiment and the mice were subjected to 3 different behavior tests to evaluate the levels of depression. The mice were also kept to evaluate the lifespan without further treatment (Figure 1).
Figure 1.
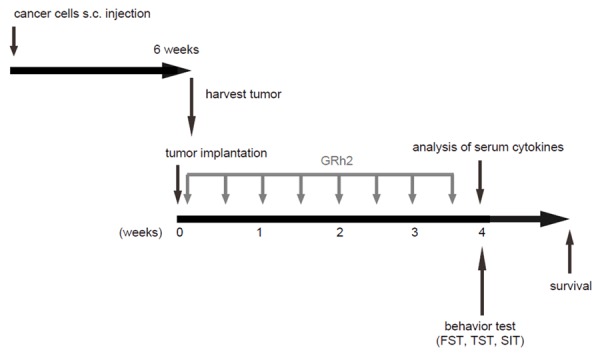
The schematic of the experiment. NOD/SCID mice were subcutaneously injected with Caco-2 cells to form tumor. Then orthotopic implantation of an identical size of tumor of 1 mm diameter was performed in NOD/SCID mice to create CRC. After tumor implantation, the mice received GRh2 at different doses for 4 weeks. The CRC-mice that received saline only were used as controls (Control). Serum cytokine levels were measured at the end of the experiment and the mice were subjected to 3 different behavior tests to evaluate the levels of depression. The mice were then kept to evaluate the lifespan without further treatment.
GRh2 significantly improves the score of FST of CRC-mice
We found that compared to control mice, GRh2 dose-dependently decreased the immobility times in the FST, suggesting that GRh2 may have improved the status of depression in CRC-mice (Figure 2).
Figure 2.
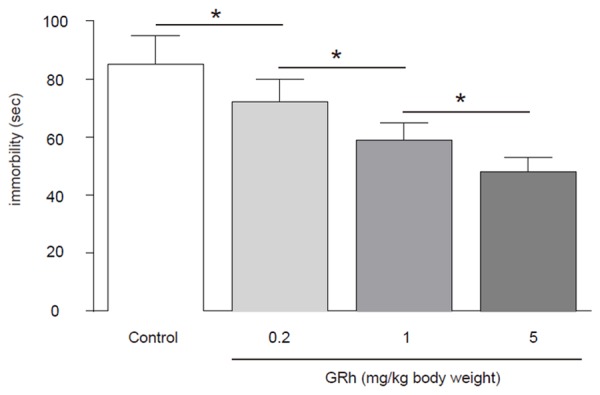
GRh2 significantly improves the score of FST of CRC-mice. Compared to control mice, GRh2 dose-dependently decreased the immobility times in the FST, suggesting that GRh2 may have improved the status of depression in CRC-mice. *p < 0.05. N = 10.
GRh2 significantly improves the score of TST of CRC-mice
We found that compared to control mice, GRh2 dose-dependently decreased the immobility times in the TST, suggesting that GRh2 may have improved the status of depression in CRC-mice (Figure 3).
Figure 3.
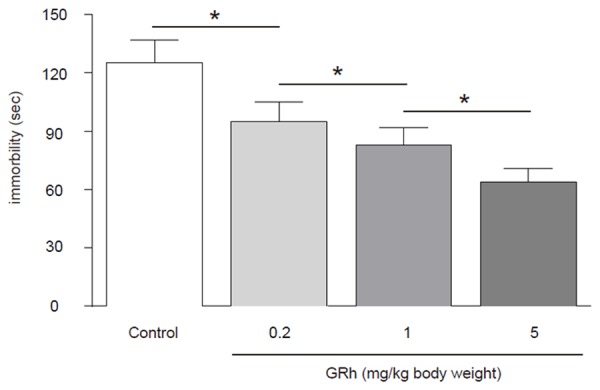
GRh2 significantly improves the score of TST of CRC-mice. Compared to control mice, GRh2 dose-dependently decreased the immobility times in the TST, suggesting that GRh2 may have improved the status of depression in CRC-mice. *p < 0.05. N = 10.
GRh2 significantly improves the score of SIT of CRC-mice
We found that compared to control mice, GRh2 dose-dependently increased the sugar intake in the SIT, suggesting that GRh2 may have improved the status of depression in CRC-mice (Figure 4).
Figure 4.
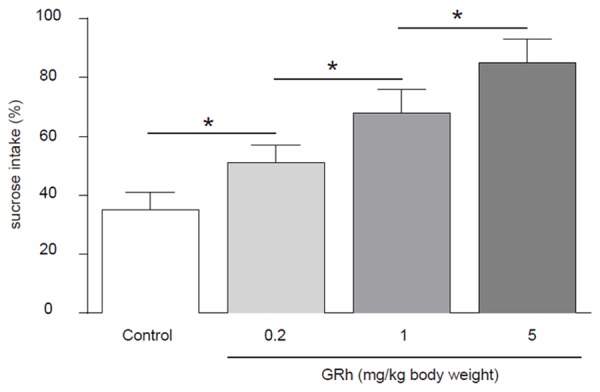
GRh2 significantly improves the score of SIT of CRC-mice. Compared to control mice, GRh2 dose-dependently increased the sugar intake in the SIT, suggesting that GRh2 may have improved the status of depression in CRC-mice. *p < 0.05. N = 10.
GRh2 significantly reduces the levels of the depression-associated cytokines
In order to understand the mechanisms underlying the GRh2-induced alleviation of depression in CRC-mice, we analyzed the serum levels of depression-associated cytokines, interleukin 6 (IL-6), IL-18 and TNF-α. We found that compared to control mice, GRh2 dose-dependently decreased the serum levels of IL-6, IL-18 and TNF-α (Figure 5). These data suggest that the anti-depression role of GRh2 may result from reduction in the serum levels of the depression-associated cytokines in CRC-mice.
Figure 5.
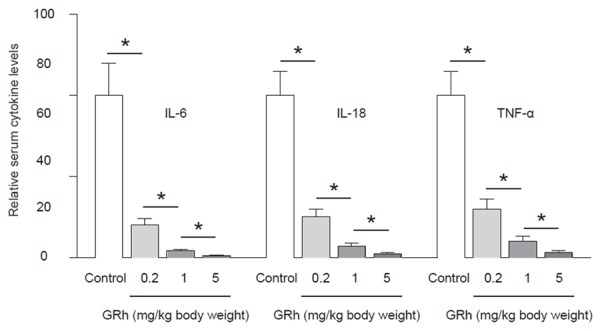
GRh2 significantly reduces the levels of the depression-associated cytokines. The serum levels of depression-associated cytokines, interleukin 6 (IL-6), IL-18 and tumor necrosis factor-alpha (TNF-α) were analyzed, showing that compared to control mice, GRh2 dose-dependently decreased the serum levels of IL-6, IL-18 and TNF-α. These data suggest that the anti-depression role of GRh2 may result from reduction in the serum levels of the depression-associated cytokines in CRC-mice. *p < 0.05. N = 10.
GRh2 treatment significantly increases the survival time of CRC-mice
Finally, we investigated whether GRh2 treatment may have an effect on the survival of the CRC-mice. Kaplan-Meier curves were performed, showing that GRh2 treatment significantly increased the survival time of CRC-mice (Figure 6), demonstrating a potential role of GRh2 in contradicting the CRC-associated depression.
Figure 6.
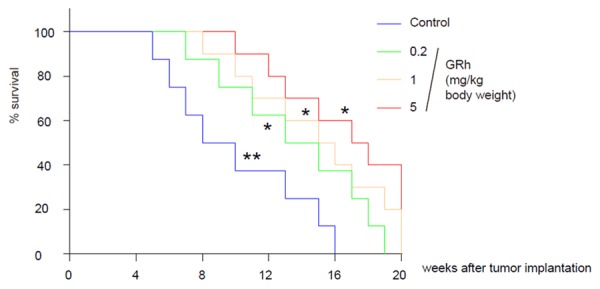
GRh2 treatment significantly increases the survival time of CRC-mice. Kaplan-Meier curves were performed, showing that GRh2 treatment significantly increased the survival time of CRC-mice. **p < 0.01. *p < 0.05. N = 10.
Discussion
CRC is the third leading cause of cancer-related death in the United States, and the number of the affected people continuously increases with time. It has been acknowledged that depression is a concomitant feature of CRC and it is possible that depression itself is a promoter of tumor development or progression [1]. Hence, there is a great need to take good care of CRC patients physiologically and psychologically, to support the medical therapy.
A dysregulated immune response has been proposed to be a causal link between depression and cancer. Animal models suggest an association between proinflammatory cytokines and “depression-like” symptoms [28]. In line with these notions, increased serum levels of cytokines such as IL-6, IL-18 and TNFα are detected in cancer patients [25,29,30]. These cytokines have a demonstrative impact on the hypothalamic-pituitary-adrenal axis and the corticotropin-releasing factor, or act as the fundamental basis of the dexamethason suppression test, in analogy to a paraneoplastic symptom [25,29,30]. Specifically, elevated IL-6 plasma levels are associated with depression in patients in which tumor cells can influence production of serotonin receptor antibodies active in central nervous system [25,29,30].
Since depression is one of the crucial factors impairing life quality of cancer patients, early and adequate antidepressant treatment is critical to an overall supportive care. Depression management requires sufficient pain control and psychological support as well as use of antidepressants [31]. All organic causes of depression like metabolic disorder, infections, drug side effects or brain radiation should be well evaluated before administration of pharmacologic therapy. Nevertheless, an effective medical treatment of CRC-associated depression in patients is so far lacking.
In the current study, we studied the effects of GRh2 on the depression in mice of a CRC model. Mouse models of CRC have played an important role in understanding CRC biology and treatment and have long been instrumental in clarifying the pathobiology of CRC formation and inhibition. Numerous mouse models of CRC have been developed, providing insights into pathogenesis mechanisms, tools for discovery, validation of novel therapeutic targets, and a predictive platform in which to test new chemoprevention strategies. Among these CRC models induced by genetic modification, or chemicals, orthotopic implantation has been used to produce a model more similar to human cancers, which was used in the current study. Here, the implant colon cancer cell line is first put s.c. to the mice to form tumor and then the resected tumor is directly placed on the serosa of the intestine. The advantage of orthotopic implantation is its relevance to human CRC and the disadvantage is the highly technical requirement. We successfully built up the model and used it to test the effects of GRh2 on depression of CRC-mice.
Many Chinese traditional medicine have pronounced therapeutic effects on various diseases, whereas their mixture manner prevents a precise determination of the molecular mechanism by which they exert their effects. GRh2 is a purified item from Chinese traditional medicine, which has been proven to have pronounced therapeutic effect on various diseases. However, whether it may also affect the psychological status in CRC-patients has not been studied. Here, we found that the mice that received GRh2 treatment significantly improved their behaviors in all FST, TST and SIT tests, seemingly through decreases in the depression-associated cytokines, IL-6, IL-18 and TNFα. Moreover, GRh2 significantly increased survival time of the CRC-mice. Together, our data suggest that GRh2 may alleviate tumor-associated depression in mice carrying CRC and highlight GRh2 treatment as a potential beneficial therapy for CRC-associated depression in patients.
Disclosure of conflict of interest
None.
References
- 1.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. J. Clin. Oncol. 2005;23:6440–6441. doi: 10.1200/JCO.2005.02.3259. [DOI] [PubMed] [Google Scholar]
- 2.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 3.Hinz A, Krauss O, Hauss JP, Hockel M, Kortmann RD, Stolzenburg JU, Schwarz R. Anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care (Engl) 2010;19:522–529. doi: 10.1111/j.1365-2354.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- 4.East JE, Dekker E. Colorectal cancer diagnosis in 2012: A new focus for CRC prevention--more serration, less inflammation. Nat Rev Gastroenterol Hepatol. 2013;10:69–70. doi: 10.1038/nrgastro.2012.245. [DOI] [PubMed] [Google Scholar]
- 5.Van Schaeybroeck S, Allen WL, Turkington RC, Johnston PG. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 2011;8:222–232. doi: 10.1038/nrclinonc.2011.15. [DOI] [PubMed] [Google Scholar]
- 6.Labianca R, Beretta GD, Mosconi S, Pessi MA, Milesi L. The development of clinical research in CRC. Ann Oncol. 2005;16(Suppl 4):iv37–43. doi: 10.1093/annonc/mdi906. [DOI] [PubMed] [Google Scholar]
- 7.Chai J, Wang S, Han D, Dong W, Xie C, Guo H. MicroRNA-455 inhibits proliferation and invasion of colorectal cancer by targeting RAF proto-oncogene serine/threonine-protein kinase. Tumour Biol. 2015;36:1313–1321. doi: 10.1007/s13277-014-2766-3. [DOI] [PubMed] [Google Scholar]
- 8.Chu D, Zheng J, Li J, Li Y, Zhang J, Zhao Q, Wang W, Ji G. MicroRNA-630 is a prognostic marker for patients with colorectal cancer. Tumour Biol. 2014;35:9787–9792. doi: 10.1007/s13277-014-2223-3. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, Li Q, Han Z, Wang D, Wei H, Gao X, Wang X. MicroRNA-93 suppress colorectal cancer development via Wnt/beta-catenin pathway downregulating. Tumour Biol. 2015;36:1701–1710. doi: 10.1007/s13277-014-2771-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Kuang Y, Shen X, Zhou H, Chen Y, Han Y, Yuan B, Zhou J, Zhao H, Zhi Q, Xue X. Evaluation of miR-720 prognostic significance in patients with colorectal cancer. Tumour Biol. 2015;36:719–727. doi: 10.1007/s13277-014-2697-z. [DOI] [PubMed] [Google Scholar]
- 11.Sehlo MG, Al Ahwal MS. Depression in patients with colorectal cancer. Saudi Med J. 2013;34:341–347. [PubMed] [Google Scholar]
- 12.Medeiros M, Oshima CT, Forones NM. Depression and anxiety in colorectal cancer patients. J Gastrointest Cancer. 2010;41:179–184. doi: 10.1007/s12029-010-9132-5. [DOI] [PubMed] [Google Scholar]
- 13.Girgis A, Breen S, Stacey F, Lecathelinais C. Impact of two supportive care interventions on anxiety, depression, quality of life, and unmet needs in patients with nonlocalized breast and colorectal cancers. J. Clin. Oncol. 2009;27:6180–6190. doi: 10.1200/JCO.2009.22.8718. [DOI] [PubMed] [Google Scholar]
- 14.Ye H, Wu Q, Zhu Y, Guo C, Zheng X. Ginsenoside Rh2 alleviates dextran sulfate sodium-induced colitis via augmenting TGFbeta signaling. Mol Biol Rep. 2014;41:5485–5490. doi: 10.1007/s11033-014-3422-0. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi Y, Sasa H, Kita T, Hirata J, Tode T, Nagata I. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside Rh2 and adjuvant effects to cisplatin in vivo. Anticancer Drugs. 1991;2:63–67. doi: 10.1097/00001813-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Tode T, Kikuchi Y, Hirata J, Kita T, Imaizumi E, Nagata I. [Inhibitory effects of oral administration of ginsenoside Rh2 on tumor growth in nude mice bearing serous cyst adenocarcinoma of the human ovary] . Nihon Sanka Fujinka Gakkai Zasshi. 1993;45:1275–1282. [PubMed] [Google Scholar]
- 17.Tode T, Kikuchi Y, Kita T, Hirata J, Imaizumi E, Nagata I. Inhibitory effects by oral administration of ginsenoside Rh2 on the growth of human ovarian cancer cells in nude mice. J Cancer Res Clin Oncol. 1993;120:24–26. doi: 10.1007/BF01200720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, Kudoh K, Nagata I, Shinomiya N. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998;89:733–740. doi: 10.1111/j.1349-7006.1998.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang XP, Tang GD, Fang CY, Liang ZH, Zhang LY. Effects of ginsenoside Rh2 on growth and migration of pancreatic cancer cells. World J Gastroenterol. 2013;19:1582–1592. doi: 10.3748/wjg.v19.i10.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Shimizu K, Yu H, Zhang C, Jin F, Kondo R. Stereospecificity of hydroxyl group at C-20 in antiproliferative action of ginsenoside Rh2 on prostate cancer cells. Fitoterapia. 2010;81:902–905. doi: 10.1016/j.fitote.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Zhao J, Wang CZ, Searle J, He TC, Yuan CS, Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh M, Choi YH, Choi S, Chung H, Kim K, Kim SI, Kim DK, Kim ND. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–875. doi: 10.3892/ijo.14.5.869. [DOI] [PubMed] [Google Scholar]
- 23.Choi S, Kim TW, Singh SV. Ginsenoside Rh2-mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of cyclin-dependent kinases. Pharm Res. 2009;26:2280–2288. doi: 10.1007/s11095-009-9944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Gao Y, Ma W, Guo W, Zhou G, Cheng T, Liu Y. EGFR signaling-dependent inhibition of glioblastoma growth by ginsenoside Rh2. Tumour Biol. 2014;35:5593–5598. doi: 10.1007/s13277-014-1739-x. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira Miranda D, Soares de Lima TA, Ribeiro Azevedo L, Feres O, Ribeiro da Rocha JJ, Pereira-da-Silva G. Proinflammatory cytokines correlate with depression and anxiety in colorectal cancer patients. Biomed Res Int. 2014;2014:739650. doi: 10.1155/2014/739650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Kleist S, Chany E, Burtin P, King M, Fogh J. Immunohistology of the antigenic pattern of a continuous cell line from a human colon tumor. J Natl Cancer Inst. 1975;55:555–560. doi: 10.1093/jnci/55.3.555. [DOI] [PubMed] [Google Scholar]
- 27.Fish J, Healy J, Gensure R, Choe E, Ferrara J. Effect of peritoneal and gastric irrigation with ozonated saline on arterial and venous blood gas values. Life Sci. 1993;53:1867–1872. doi: 10.1016/0024-3205(93)90025-x. [DOI] [PubMed] [Google Scholar]
- 28.Clark K, Bardwell WA, Arsenault T, DeTeresa R, Loscalzo M. Implementing touch-screen technology to enhance recognition of distress. Psychooncology. 2009;18:822–830. doi: 10.1002/pon.1509. [DOI] [PubMed] [Google Scholar]
- 29.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 30.Allen-Mersh TG, Glover C, Fordy C, Henderson DC, Davies M. Relation between depression and circulating immune products in patients with advanced colorectal cancer. J R Soc Med. 1998;91:408–413. doi: 10.1177/014107689809100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petitto JM, Evans DL. Treatment of Depression in Individuals With Cancer: Implications of Antidepressant Pharmacotherapy. Semin Clin Neuropsychiatry. 1998;3:131–136. [PubMed] [Google Scholar]


