Abstract
Objective: Anaplastic thyroid cancer (ATC) is one of the most lethal human malignancies. However, the molecular mechanisms of ATC invasion are poorly understood. The transforming growth factor-beta (TGF-β) signaling pathway plays a critical role in promoting tumor metastasis. TGF-β1 was found to be overexpressed in anaplastic thyroid cancer (ATC). We therefore tested our hypothesis that targeted down-regulation of TGF-β1 inhibits invasion of ATC cells. Methods: Effects of TGF-β1 stimulation or TGF-β1 sliencing by small interfering RNA (TGF-β1 siRNA) on invasion in 8505C and SW1736 cells in vitro was detected. Using siRNAs and inhibitors to examine the TGF-β1 signaling pathway. Results: TGF-β1 siRNA inhibits cell migration and invasion in vitro, followed by inactivation of pSMAD2, S100A4 and MMP-2/9. TGF-β stimulation activated pSMAD2-dependent S100A4 and MMP-2/9 expression, and increased cell migration and invasion. The depletion of pSMAD2 or S100A4 or MMP-2/9 expression inhibited TGF-β signaling pathway. Moreover, it significantly weakened the proinvasive effects of TGF-β on ATC cells. Conclusions: Therapies targeting the TGF-β1 inhibits invasion of ATC cells by impeding the SMAD2-dependent S100A4-MMP-2/9 signalling in vitro.
Keywords: Anaplastic thyroid cancer, TGF-β1, metastasis
Introduction
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive human malignancies. Clinical presentation of ATC is frequently characterized by a rapidly growing neck mass and displays highly invasive behavior with an 5 year survival rate < 5% [1,2]. Surgery, chemotherapy and radiotherapy are the conventional therapeutic strategies performed in the attempt to improve survival. Unfortunately, very often they do not succeed any clinical benefit but only palliative [3]. Given our poor ability to control ATC progression with conventional modalities, investigation of novel antimetastasis and gene therapies are needed for treating this disease.
TGF-β (transforming growth factor-β) is a prototypical member of a multifunctional cytokine family that regulates a wide variety of cellular functions. TGF-β is both a tumor promoter and a tumor suppressor [4,5]. TGF-β functions as a tumor promoter in epithelial cells through increasing tumor cell invasion and metastasis [6-8]. There are 3 isoforms of TGF-β ligand (TGF-β 1-3). TGF-β signalling is propagated via cell surface serine/threonine kinases, TGF-β type I receptor (TβRI) and TGF-β type II receptor (TβRII) [9], which in turn phosphorylate and activate the Smad family of signal transducers. Once activated, Smad2 and Smad3 associate with Smad4 and translocate to the nucleus, where, together with cofactors, it binds DNA and alters the expression of many genes [10].
Many studies has reported that introduction of dominant-negative TGF-β receptors into metastatic cancer cells has been shown to suppress epithelial-to-mesenchymal transdifferentiation (EMT), invasiveness and motility, supporting the tumor promoter role in TGF-β in fully transformed cells [11-13]. In addition, excess production and/or activation of TGF-β can contribute to tumor progression [14-16], and TGF-β silencing can inhibit tumor progression [17-19]. All this evidence suggests an interatomic nature of the TGF-β1 induced metastatic process, which provided a rationale in favor of blockade of TGF-β signaling in human cancers with a therapeutic intent.
TGF-β can induce the progression and metastasis via both canonical and noncanonical pathways. Ras/MAPK, PI3K/Akt and Rho/ROCK signalin are critical effectors of the TGF-β-induced cell invasion and metastasis in certain contexts [20-23]. S100A4 is a critical mediator of invasion in endometrial cancer ,which is also upregulated by the TGF-β signaling pathway [24,25].
Our recent study has found that elevated plasma levels of TGF-β1 was closely related to invasiveness and lympy node metastasis in thyroid cancer. In mouse models of skin papillomas, TGF-β1 overexpression may rapidly induced metastasis, and knockdown of TGF-β1 signaling components could reverse the effect. In breast cancer [26,27] and pancreatic cancer [28] xenograft model, targeting TGF-β1 signaling could effectively inhibit metastasis and tumorigenesis.
In this study, we use TGF-β1 gene-silenced and overexpressing ATC cells to determine if TGF-β1 sliencing can inhibits the migration and invasion of ATC cells in vitro. The results of these studies indicate that small interfering RNA (siRNA)-mediated silencing of TGF-β1 in the ATC cells decreased the migration and invasion in vitro. TGF-β/Smad2/S100A4/MMP-2/9 are involved in the mechanisms that TGFβ1 promotes the migration and invasion of ATC cells.
Materials and methods
Cell line and culture
The human anaplastic thyroid cancer cell lines SW1736 and 8505C cell lines was purchased from DSMZ (Beijing, China) The cells were cultured as the DSMZ’s instruction. Briefly, the cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin, sodium pyruvate, and non-essential amino acids. Adherent monolayer cultures were maintained on plastic and incubated at 37°C in 5% carbon dioxide and 95% air. The cultures were free of Mycoplasma species. In all of the assays, a monolayer of cells that was 50-70% confluent was used. All the methods used were according to the manufacture’s instruction.
Agents
The following primary antibodies were used from Santa Cruz Biotechnology: SMAD2, pSMAD2 (Ser465), TGF-β1-3, TGF-βRI, TGF-βRII, S100A4, MMP-9, TGF-β1 siRNA, Smad2 siRNA, S100A4 siRNA, MMP-9 siRNA, control siRNA and β-actin. BB-94 (Batimastat), a MMP inhibitor was purchased from ApexBio. Human recombinant TGF-β1 (rh TGF-β1) was obtained from Cell Signaling Technology (Danvers, USA). TGF-β1: 10 ng/ml and BB-94: 10 μM. Cells incubated with culture medium or culture medium with DMSO served as controls.
Plasmids
Short chain oligonucleotide was designed according to the TGF-β1 mRNA sequence provided by Genebank. The two oligonucleotides were selected as: forward, 5’-GATCCCCTGCCGCTGCTGCTACCttcaagagaGGTAGCA; GCGGCAGCATTTTTGGAAA-3’; reverse, 3’-AGCTTTTCCAAAAATGCTGCTGCCGCTGCTGCTACCtctctt; gaaGGTAGCAGCGGCAGCAGGG-5’. It was chemosynthesized by Shgong.com. It was ligated and then insert the two oligonucleotides above into the pcDNA3.1 plasmid (which encodes GFP as a reporter protein). The recombinant TGF-β1 shRNA expression vector was evaluated by using enzyme cutting. The negative control plasmid had a sequence inserted at the same place using the following two oligonucleotides: 5’-GCTACGCCTTCATAAGGCACGTGCTTCAAACGGGCATGCGCCATATGCAGTCTTTTTTGTCGACA-3’; reverse, 3’-GGCTAAGATTTCCGCGGACGAAGCCTTG CCGTACCCCGAGCACTTCACGAAAAACAGCTGCGAGA-5’. The recombinant TGF-β1-shRNA plasmid was confirmed by digestion and gene sequencing. Plasmid pcDNA3.1 was as the control plasmid.
Transient/stable siRNA transfection
Cells were seeded in six-well plates, grown to 50-80% confluence. The cell density is not too high and that the cells are in optimal physiological condition on the day of transfection. 8505C cells were transfected with TGF-β1 siRNA (2 μg) in OptiMEM (Gibco, BRL) for 24-72 hs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. For stable TGF-β1 siRNA transfection, 24 hs after TGF-β1 siRNA or control siRNA transfection, the cells were split into 96-well plates and subjected to the G418 (1 mg/ml) selection for 2 weeks. The transcriptional silencing TGF-β1 protein and mRNA was screened using reverse transcription-PCR (RT-PCR) and western blot as described below. Different agents used in the transfection assay had no effect on aim protein expression. All transfection experiments were done at least three times.
Stimulation of 8505C and SW1736 cells with TGF-β1
8505C and SW1736 cells were cultured in 96-well plates (3 × 104 per well) and stimulated with TGFβ1 (0-10 ng/mL) in DMEM supplemented with 10% FBS and antibiotics and harvested 24 hours. To determine the signaling pathways involved in the production of MMP-2/9, 8505C and SW1736 cells were preincubated with BB-94: 10 μM or DMSO 6 h before the addition of TGFβ1. To determine the signaling pathways involved in the production of SMAD2 and S100A4, 8505C and SW1736 cells were transfected with SMAD2 siRNA or S100A4 siRNA or control siRNA 24 h (using Lipofectamine 2000 according to the manufacturer’s instructions above) before the addition of TGFβ1.
Western blot assay
For total protein extraction, cells were washed once with phosphate-buffered saline (PBS) and lysed with RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 1% Nonidet P-40, supplemented with complete protease inhibitor tablets; Roche Diagnostics) for 30 minutes on ice. For in vivo study, the tumor tissues were homogenized for tissue lysate extraction. Both cell lysate and tissue lysate were centrifuged and the supernatants were collected. Nuclear and cytoplasmic extracts were prepared using the Nuclear Extract Kit (Active Motif), according to manufacturer’s instructions. Protein concentration was quantified with Coomassie Plus (Bradford) Protein Assay Reagent according to manufacturer’s instructions. Extracts (40 μg) were resolved on 10% SDS-PAGE and transferred to Hybond-C Extra nitrocellulose membrane (GE Healthcare; Germany). Membranes were probed with primary antibodies against TGF-β1, TGF-β2, TGF-β3, TGF-βRI, TGF-βRII, S100A4, MMP-2, MMP-9, SMAD, pSMAD2, followed by incubation for 1 hour at room temperature with HRP-conjugated anti-rabbit IgG or anti-goat IgG, respectively. Immunoblotting for β-actin served as protein loading control. All experiments were performed at least three independent times.
Reverse-transcriptase polymerase chain reaction (RT-PCR)
RNA from transfection or inhibitors treated cells was extracted using Purification Kit ( Roboklon, Germany). We used 2 µg of total RNA for cDNA synthesis with the Reverse Transcriptase Kit (Promega, Shanghai, China). The resulting cDNA was used for RT-PCR analysis with the G-Taq Kit according to the manufacturer’s instructions. RT-PCR was performed using appropriate gene-specific forward and reverse primers, which were selected using the Blast Primer tool. The results were analyzed using the ImageJ software. The relative change in the ratio of the target protein to the DMSO control was determined.
Matrix metalloproteinases (MMPs) zymography
8505C and SW1736 cells were treated with TGF-β1 for 24 hs, and 8505C cells were transfected with TGF-β1 siRNA or control siRNA for 48 hs. Zymography was performed as described [30]. Briefly, an equal number of cells (1 × 106) were homogenized in lysis buffer. The insoluble and soluble extracts were separated by centrifugation and stored at -20°C. This lysate was mixed with SDS sample buffer [0.185 M Tris-HCl (pH 6.8), 30% glycerol, and 6% SDS] and analyzed by gelatin zymography on 12% SDS-polyacrylamide gels containing 0.5 mg/ml gelatin. After electrophoresis, SDS was removed from the gels by soaking twice for 20 min in 2.5% Triton X-100 and washed for 20 min in rinse buffer [50 mM Tris-HCl (pH 7.4) and 0.1 M NaCl]. The gels were incubated for 20 h at 37°C in gel incubation buffer (50 mM Tris-HCl, 10 mM CaCl2, and 0.02% NaN3), stained in 0.5% Coomassie Blue, and destained in a solution of 5% methanol and 7.5% acetic acid.
ELISA
8505C cells were transfected with TGFβ1 siRNA or control siRNA for 48 h. 8505C and SW1736 cells were treated with TGFβ1 (10 ng/ml) for 24 h. To determine the signaling pathways involved in the production of MMP-2 and MMP-9, 8505C and SW1736 cells were preincubated with BB-94: 10 μM or DMSO 6 h before the addition of TGFβ1 (10 ng/ml). The supernatants were collected and analyzed for MMP-2 and MMP-9 using an MMP-2 and MMP9-specific ELISA kit from Amersham Bioscience and following the vendor’s protocol.
Transwell migration and matrigel invasion assays
Transwell migration and matrigel invasion assays were performed using a transwell membrane (8-μm pore size, 6.5-mm diameter) in a 24-well plate according to the manufacturer’s instructions. Briefly, a matrigel matrix was coated in the transwell membrane and used for the cell invasion assay. The lower chamber of the transwell plates was filled with 600 μl DMEM medium containing 10% FBS. 8505C cells were transfected with control siRNA or TGFβ1 siRNA for 48 hs. 8505C and SW1736 cells were treated with TGFβ1 (10 ng/mL) for 24 hs. 8505C and SW1736 cells were transfected with SMAD siRNA or S100A4 siRNA for 24 h or treated with BB94 for 6 hs before stimulating with TGFβ1 (10 ng/mL) for 24 hs. The cells were then detached from the tissue culture plates and resuspended in DMEM medium containing 1% FBS and then loaded to the upper side of the chamber (300 μl/well, 2 × 105 cells/well). The cells were placed in incubators at 37°C for 24 hs. Unmigrated cells on the upper side were removed using a cotton swab and cells that had migrated to the other side of the membrane were stained with 0.1% Crystal Violet, 20% ethanol and 1% formaldehyde. The number of migrated cells was counted for quantification.
Wound-healing assays
For the wound-healing assays, the cells above were plated in six-well plates at a density of 4 × 105 cells/well and incubated overnight. The media was then aspirated, and the cells were scratched with a sterile tip. Three representative images were collected. After treatment for 24-48 h, images of the same regions were collected, and the cell motility ratio for each experimental condition was quantified. The tests were repeated in triplicate.
Statistical analysis
Data are expressed as the mean ± SD. SPSS 11.0 software was used for analysis. The statistical significance of a difference between two groups was assessed using Student’s t-tests (two-tailed). ANOVA was used when more than two groups were involved, and then Student’s t-test was further applied to analyze difference between groups. P < 0.05 was considered to indicate a statistically significant result. All experiments were repeated at least three times.
Results
Effect of siRNA on TGF-β1 expression in 8505C cells
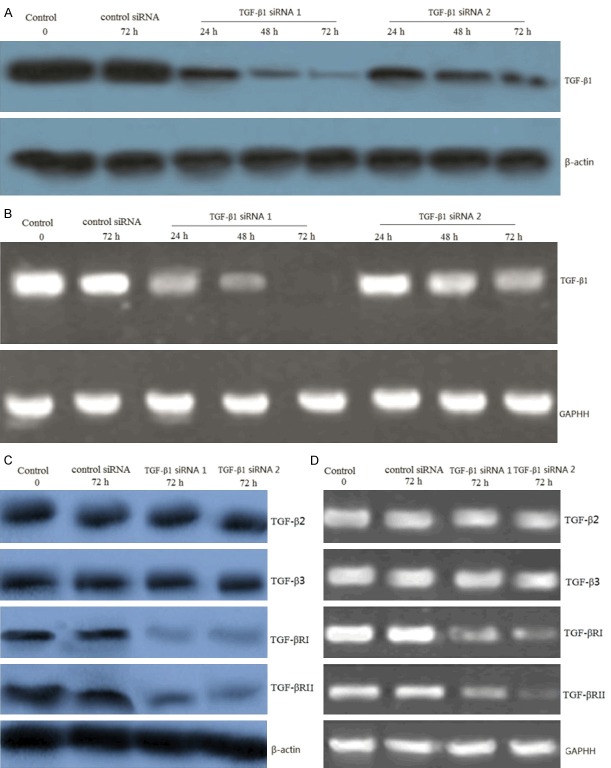
8505C cells were transfected with TGF-β1 siRNA and control siRNA for 24-72 hs. Western blot and RT-PCR analysis was used to detect the TGF-β1 protein and mRNA level after transfection. Cells transfected with TGF-β1 siRNA displayed a time-dependent reduction in the expression levels of TGF-β1 protein (Figure 1A) and mRNA (Figure 1B). Control siRNA did not exhibit any effect on protein levels of TGF-β1 (Figure 1A) and mRNA levels of TGF-β1 (Figure 1B). These data confirmed the suppression effect of siRNA and established the efficiency of siRNA transfection. We also detected TGF-β2 and TGF-β3 protein and mRNA expression before and after siRNA transfection. Results from our in vitro experiments showed no change in expression for the two isoforms TGF-β2 and TGF-β3, illustrating the specificity of the siRNA sequence designed for this study (Figure 1C, 1D). However, TGF-βRI and TGF-βRII protein and mRNA expression was inhibited after siRNA transfection (Figure 1C, 1D).
Figure 1.
TGF-β1 gene knockdown by siRNA transfection in 8505C cells. 8505C cells were transfected with TGF-β1 siRNA and control siRNA for 24-72 hs. A. Representative images showing expression of TGF-β1 protein in control siRNA and TGF-β1 siRNA transfected cells as analyzed by Western blot. B. Representative images showing expression of TGF-β1 mRNA in control siRNA and TGF-β1 siRNA transfected cells as analyzed by RT-PCR. C. Representative images showing expression of TGF-β2, TGF-β3, TGF-βRI and TGF-βRII protein in control siRNA and TGF-β1 siRNA transfected cells (72 h transfection) as analyzed by Western blot. D. Representative images showing expression of TGF-β2, TGF-β3, TGF-βRI and TGF-βRII mRNA in control siRNA and TGF-β1 siRNA transfected cells (72 h transfection) as analyzed by RT-PCR. β-actin and GAPDH was as a control.
Effect of TGF-β1 gene targeting on invasive and migratory capability of 8505C cells
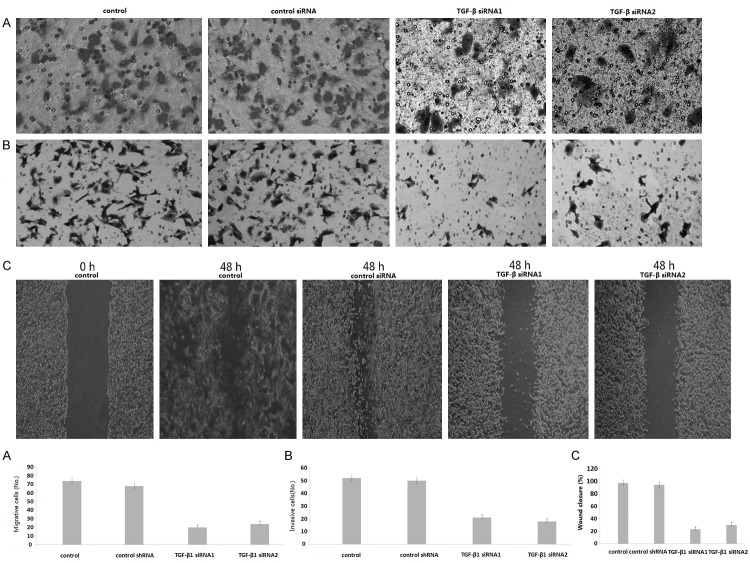
In vitro studies were done to determine the effects of TGF-β1 silencing on both migration and invasion of the 8505C cell clones. The migration and invasion of TGF-β1/siRNA1 cells were markedly inhibited, compared with control siRNA (P < 0.01) (Figure 2A, 2B). The migration and invasion of TGF-β1/siRNA2 cells were also markedly inhibited compared with control siRNA (P < 0.01). These results suggest that TGF-β silencing inhibits cell migration and invasion. Cell migration was also determined using a wound-healing assay in which cells were scratched and allowed to migrate into the wound area. The amount of wound closure was enumerated 48 hours after disruption. Compared with the non-transfected cells that showed 95% wound closure by 48 hours, clones expressing the TGF-β1/siRNA1 showed 20% wound closure in the same period (Figure 2C), and 25% wound closure in the same period in the TGF-β1-siRNA2 clones (Figure 2C).
Figure 2.
Knockdown of TGF-β1 inhibits invasion and migration in 8505C cells. A. Cells were detected by transwell migration assay. Migrated cells were counted in five random fields of each filter under a microscope using a 200 × magnification. The scale bar indicates 200 μm. Bars represent the average number of migrated cells. B. The migration of TGF-β1-knockdown 8505C cells. Cells were detected by matrigel invasion assay. Cells that invaded across the matrigel of the transwell were counted in five random fields of each filter under a microscope using a 200 × magnification. The scale bar indicates 200 μm. Bars represent the average number of invaded cells. C. Cells were detected by wound-healing assay. The photographs were taken by microscope using a 100 × magnification at the same area at 0 h and after 48 h of incubation of five random fields. The scale bar indicates 50 μm. Bars represent the average number of invaded cells. *P < 0.01 compared with respective controls.
TGF-β1 promotes the migration and invasion of 8505C and SW1736 cells
To determine the effect of TGF-β1 on the migration and invasion of ATC cells, 8505C and SW1736 cells were treated with TGF-β1 (10 ng/ml) for 24 h, matrigel invasion, transwell migration, and wound-healing assays were performed. As shown in Figure 3A, 3B, TGF-β1 significantly promoted migration and invasion in SW1736 cells (p < 0.05, respectively). Treatment with TGF-β1 (10 ng/ml) for 24 h showed about 75-85% wound closure in SW1736 cells, which was higher than the SW1736 (45%) cells at 24 h (p < 0.05, Figure 3C).
Figure 3.
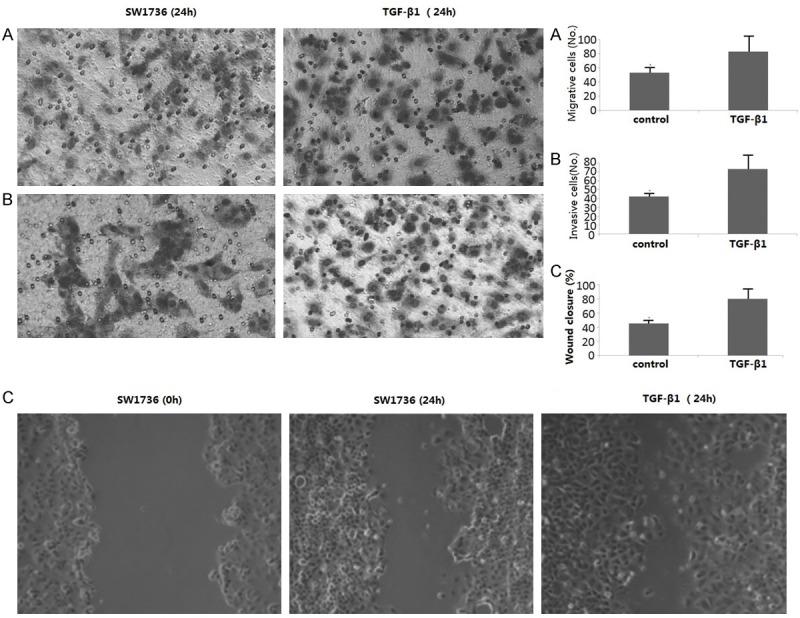
TGF-β1 promotes the migration of SW1736 cells. The SW1736 cells were treated with TGF-β1 for 24 h. A. Cells were detected by transwell migration assay. B. Cells were detected by matrigel invasion assay. C. cells were detected by wound-healing assay. Bars represent the average number of invaded cells. *P < 0.05 compared with respective controls.
TGF-β1 treatment also significantly promoted migration and invasion in 8505C cells (Figure 5E-G). Treatment with TGF-β1 (10 ng/ml) for 24 h showed about 80-90% wound closure in 8505C cells, which was higher than the 8505C (50%) cells at 24 h (p < 0.05) (Figure 5E-G). Taken together, these results indicate that TGF-β1 promotes the migration and invasion of ATC cells.
Figure 5.
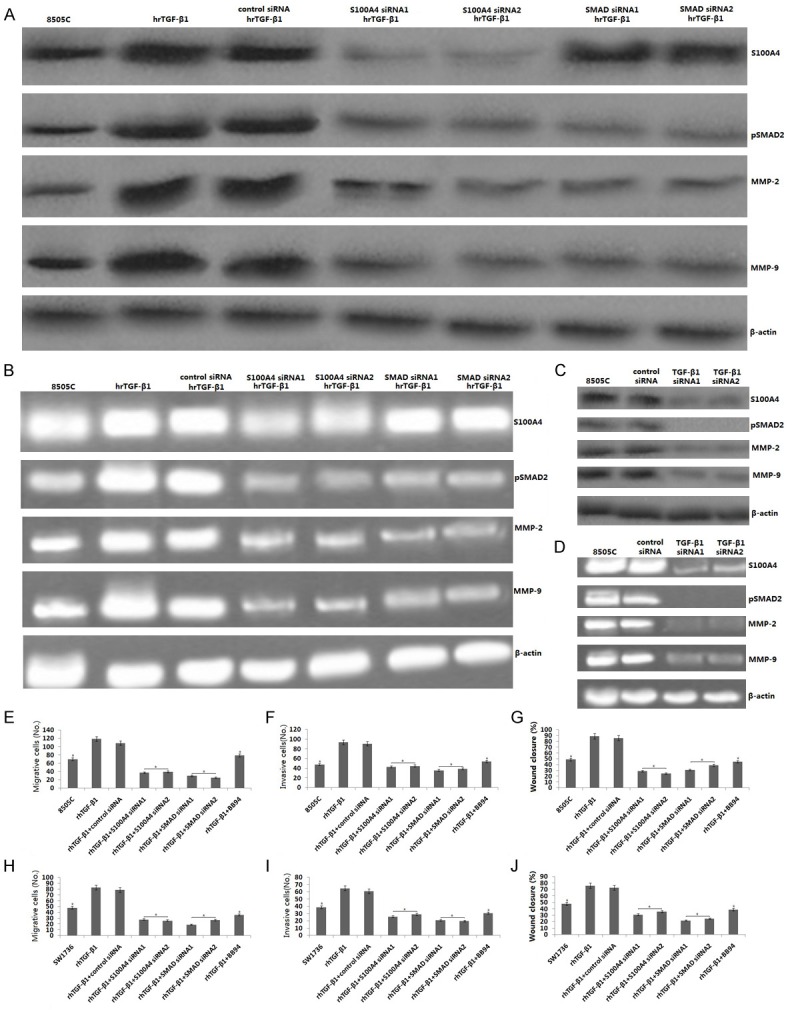
TGF-β1 promotes migration and invasion of 8505C and SW1736 cells by SMAD2/S100A4/MMPs signaling. 8505C cells were treated with hrTGF-β1, or transfected with SMAD siRNA, S100A4 siRNA or exposure to BB94, then treated with hrTGF-β1. A. pSMAD2, S100A4, MMP-2,9 protein was detected by western blot assay. B. pSMAD2, S100A4, MMP2,9 mRNA was detected by RT-PCR assay. C. 8505C cells were transfected with TGF-β1 siRNA, pSMAD2, S100A4, MMP-2,9 protein was detected by western blot assay. D. pSMAD2, S100A4, MMP2,9 mRNA was detected by RT-PCR assay. 8505C cells and SW1736 cells were treated with hrTGF-β1, or transfected with SMAD siRNA, S100A4 siRNA or exposure to BB94, then treated with hrTGF-β1. E, H. Transwell migration assay. F, I. Matrigel invasion assay. G, J. Wound healing assay was used to detect invasion and metastasis.
TGF-β1-induced MMP-2,9 promotes the migration and invasion of 8505C and SW1736 cells
Increased MMP activity is considered very important for the invasion and metastasis of cancer cells. To study the mechanism of TGF-β1-enhanced ATC cell migration and invasion, the protein expression patterns of MMP-2,9 were examined by analyzing culture supernatants using the enzyme-linked immunosorbent assay (ELISA), gelatin zymography and western blot assay.
8505C cells were transfected with TGF-β1/siRNA and control siRNA for 48 hs. The supernatants was collected and analyzed for MMP-2 and MMP-9. As shown in Figure 4A, TGF-β1/siRNA transfection significantly inhibited the production of MMP-9, compared to the control siRNA in the 8505C cells (p < 0.01). TGF-β1/siRNA transfection also inhibited the product of MMP-2 in the 8505C cells (data not shown).
Figure 4.
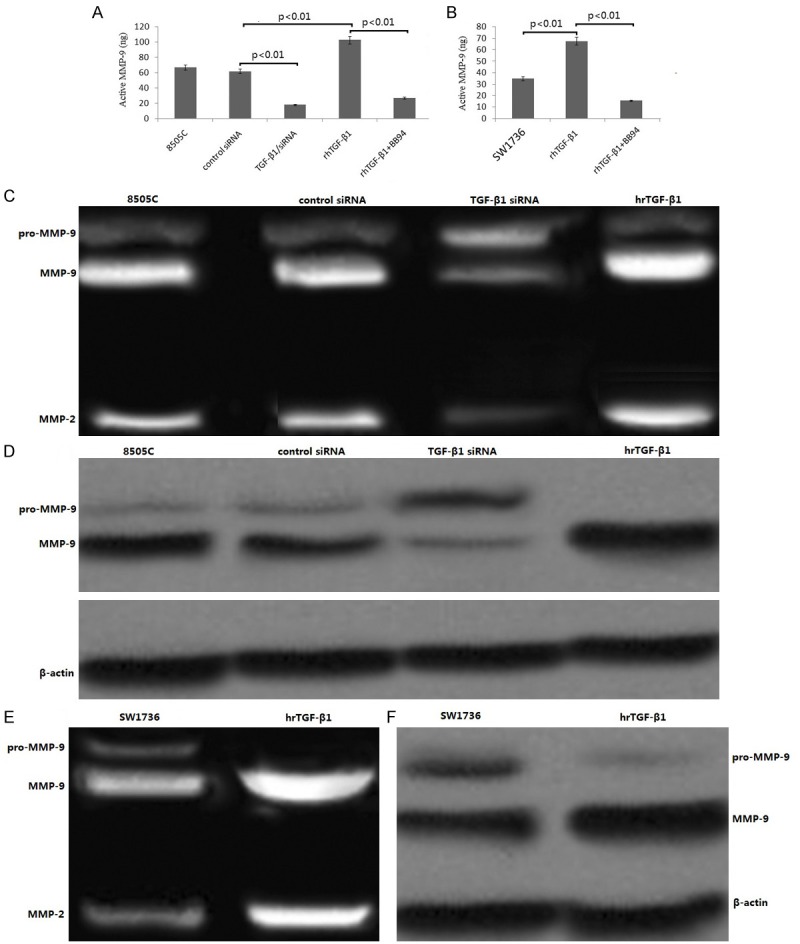
Effect of TGF-β1 on the expression levels of MMP-2,9. (A) Quantification of MMP-9 secretion using MMP-9-specific ELISA done in the culture media of 8505C cells transfected with either control or TGF-β1 siRNA, or treatment with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment. (B) Quantification of MMP-9 secretion using MMP-9-specific ELISA done in the culture media of SW1736 cells treated with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment. (C) MMP-2,9 expression in 8505C cells transfected with either control or TGF-β1 siRNA, or treatment with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment by gelatin zymography (D) MMP-2,9 expression in 8505C cells transfected with either control or TGF-β1 siRNA, or treatment with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment by Western blotting using antibody raised against MMP-9. (E) MMP-2,9 expression in SW1736 cells treated with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment by gelatin zymography. (F) MMP-2,9 expression in SW1736 cells treated with hr TGF-β1, or hr TGF-β1 in combination with BB94 treatment by Western blotting using antibody raised against MMP-9.
SW1736 and 8505C cells were treated with TGF-β1 (10 ng/ml) for 24 h. TGF-β1 significantly stimulated the production of MMP-9 by ELISA assay (Figure 4A, 4B). TGF-β1 (10 ng/ml) for 24 h also stimulated the product of MMP-2 in the 8505C and SW1736 cells (data not shown).
Using gelatin zymography, we found that the expression levels of MMP-9 and MMP-2 were significantly reduced in TGF-β1/siRNA transfected 8505C cells (Figure 4C). The gelatin zymography result on MMP-9 was verified by Western blotting analysis (Figure 4D).
In addition, the expression levels of MMP-9 and MMP-2 were significantly increased in SW1736 cells treated with TGF-β1 (10 ng/ml) for 24 h using gelatin zymography (Figure 4E). The gelatin zymography result on MMP-9 was verified by western blotting analysis (Figure 4F).
BB94, a MMP inhibitors , has been reported to suppress the growth of cancer cells by blocking the MMPs [31,32]. To further verify the effect of the MMP-2,9 on cell migration and invasion, BB94 (10 μM) was used to block the MMPs. The results showed that BB94 inhibited the migration and invasion of the TGF-β1/SW1736 and TGF-β1/8505C cells (Figure 5E-J). BB94 significantly inhibited the production of MMP-9, compared to the TGF-β1 treatment alone (p<0.01, Figure 4A, 4B). BB94 also inhibited the product of MMP-2 in the SW1736 and 8505C cells (data not shown).The findings imply that TGF-β1 affects the migration and invasion possibly by regulation of the expression of MMP-2,9.
TGF-β1-induced MMP-2,9 through activation of S100A4 promotes the migration and invasion of ATC cells
S100A4, a calcium-binding protein is associated with invasion and metastasis of cancer cells. Previous study had shown that TGF-β1 promoted invasion and matastasis via upregulation of S100A4 in colorectal cancer cells [33]. Saleem et al. has found that S100A4 gene controls the invasive potential of human CaP cells through regulation of MMPs and that this association may contribute to metastasis of CaP cells [34]. In the present study, we found that 8505C cells were treated with TGF-β1 (10 ng/ml) for 24 h, the S100A4 protein (Figure 5A) and mRNA (Figure 5B) was increased. SW1736 cells has the same results as 8505C cells (data not shown). In contrast, knockdown of TGF-β1 inhibited S100A4 protein and mRNA expression in the 8505C cells (Figure 5C, 5D).
To test the relation between S100A4 and MMP-2,9, SW1736 and 8505C cells were transfected with S100A4 siRNA1 or S100A4 siRNA2 or control siRNA for 24 hs, then treated with TGF-β1 (10 ng/ml) for 24 h. The results showed that followed by reduced S100A4 protein and mRNA level, the expression levels of MMP-9 and MMP-2 were significantly reduced using western blot and RT-PCR assay in the 8505C cells (Figure 5A, 5B). SW1736 cells has the same results as 8505C cells (data not shown). In addition, the migration and invasion of 8505C/TGF-β1 and SW1736/TGF-β1 cells were obviously suppressed by S100A4 siRNA transfection (Figure 5E-J).
TGF-β1 promotes migration and invasion of ATC cells through Smad2-dependent S100A4-MMP-2,9 signaling pathway
TGF-β1 treatment has found in our study to upregulate S100A4, followed by MMP-2/9 upregulation in the ATC cells, suggesting that S100A4 is not one of the early response genes of TGF-β1. Some tertiary or secondary signaling pathways are likely needed to activate S100A4 expression. To verify whether TGF-β1 promoted the migration and invasion of ATC cells through the Smad2/S100A4/MMP-2,9 signal, the Smad2 was blocked by Smad2 siRNA.
As shown in 8505C cells, the phosphorylation of Smad2 and the expression of S100A4 and MMP-2,9 were inhibited by Smad2 siRNA in 8505C cells (Figure 5A, 5B). SW1736 cells has the same results as 8505C cells (data not shown). In addition, the migration and invasion of 8505C/TGF-β1 and SW1736/TGF-β1 cells were obviously suppressed by Smad2 siRNA transfection (Figure 5E-J, p < 0.01).
We also found that knockdown of TGF-β1 by TGF-β1 siRNA inhibited the phosphorylation of Smad2, as well as the S100A4 and MMP-2/9 (Figure 5C, 5D), followed by decreased migration and invasion in the 8505C cells (Figure 5E-G). Taken together, these data indicate phosphorylation of Smad2 is associated with TGF-β1-enhanced cell migration and invasion and the up-regulation of S100A4/MMP-2/9 in ATC cells.
Discussion
Although three mammalian isoforms of TGFβ1, TGFβ2 and TGFβ3 share 60-80% identity at the amino acid level, the promoter regions of these isoforms are highly variable, suggesting that their expression is regulated by distinct mechanisms [35]. Results from our in vitro experiments showed no change in expression for TGFβ2 and TGFβ3 isoforms by TGF-β1 sliencing, illustrating the specificity of the siRNA/shRNA sequence designed for this study. However, TGF-βRI and TGF-βRII protein and mRNA expression was inhibited after siRNA transfection in vitro. Silencing of the TGF-βR expression may be through a negative feedback loop in the cells, suggesting that therapies targeting the ligand may have similar effects as indicated by previous studies, which have shown that blocking TGF-β receptors can decrease tumorigenesis.
To investigate the effect of TGFβ1 on the migration and invasion of ATC cells, we used 8505C and SW1736 cells, and then performed wound-healing, transwell migration, and matrigel invasion assays. The results demonstrated that TGFβ1 stimulating promoted the migration and invasion of both 8505C and SW1736 cells. Knockdown of TGFβ1 by siRNA inhibited the migration and invasion of 8505C/TGFβ1 siRNA1 and 8505C/TGFβ1 siRNA2 cells.
TGF-β plays a tumor suppressive role in normal epithelia, but accelerates the progression through enhancing cell migration and invasion in cancer cells [4]. However, the underlying mechanisms causing this are not entirely clear. S100A4 is a calcium-binding protein, which is associated with invasion and metastasis of cancer cells, including thyroid cancer [36,37]. Many studies has reported that the S100A4 gene controls the invasive potential of human cancer cells through regulation of MMP-9 and that this association may contribute to metastasis of cancer cells [38,39]. Recent study has found that S100A4 is a critical mediator of invasion in endometrial cancer and is upregulated by the TGF-β1 signaling pathway [40]. In the present study, we found that TGF-β stimulation promoted S1004 and MMP-2/9 expression, and vice vaser. In addition, treatment with BB94 weaken the proinvasive effects of TGF-β on ATC cells. The depletion of S100A4 expression by siRNA transfection inhibited the TGF-β-induced MMP-2/9 upregulation and significantly weakened the proinvasive effects of TGF-β on ATC cells. We therefore concluded that TGF-β promoted invasion of ATC cells by S1004/ MMP-2,9 signaling.
Intracellular Smad proteins play a pivotal role in mediating antimitogenic effects of TGF-β, but their function in TGF-β induced invasion and metastasis is unclear. Furthermore, there has been debate whether or not Smads are required for the TGF-β-induced metastasis. In the present study we found that knockdown of SMAD2 inhibited S1004/MMP-2,9 signaling, and significantly weakened the proinvasive effects of TGF-β on ATC cells. We therefore suggested that silencing of TGF-β1 inhibits invasion of human ATC cells by inhibiting the TGF-β1/SMAD2/S100A4/MMP-2,9 signal in vitro.
In conclusion, the present study suggests that therapies targeting TGF-β1 in tumor cells may be effective in decreasing invasion in vitro. The ability of this therapy to decrease invasion in vitro may be related to TGF-β1/TGF-βR-SMAD2-dependent S100A4-MMP-2/9 signals. Future studies will investigate the effect of TGF-β1 in vivo and explore its mechanisms.
Acknowledgements
This study was supported by grants from the National nature scientific research fund, China (No. 81270448) and Natural science research foundation of Shandong Provinc (No. 2014ZRB01198).
Disclosure of conflict of interest
None.
References
- 1.Are C, Shaha AR. Anaplastic thyroid carcinoma: Biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 3.Chiacchio S, Lorenzoni A, Boni G, Rubello D, Elisei R, Mariani G. Anaplastic thyroid cancer: prevalence, diagnosis and treatment. Minerva Endocrinol. 2008;33:341–357. [PubMed] [Google Scholar]
- 4.Bachman KE, Park BH. Duel nature of TGF-beta signaling tumor suppressor vs. tumor promoter. Curr Opin Oncol. 2005;17:49–54. doi: 10.1097/01.cco.0000143682.45316.ae. [DOI] [PubMed] [Google Scholar]
- 5.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 6.Tulley S, Chen WT. Transcriptional regulation of seprase in invasive melanoma cells by transforming growthfactor-β signaling. J Biol Chem. 2014;289:15280–1596. doi: 10.1074/jbc.M114.568501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasisby associating with suz12. Clin Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 8.Bruna A, Darken RS, Rojo F, Ocaña A, Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Coppa A, Mincione G, Mammarella S, Ranieri A, Colletta G. “Epithelial rat thyroid cell clones, escaping from transforming growth factor beta negative growth control, are still inhibited by this factor in the ability to trap iodide”. Cell Growth Differ. 1995;6:281–290. [PubMed] [Google Scholar]
- 10.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 11.Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor beta in mammary tumorigenesis and metastaticprogression. Clin Cancer Res. 2005;11:937s–943s. [PubMed] [Google Scholar]
- 12.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massagué J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2013;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Lee J, Lu S, Pettaway CA, Dong Z. Blockade of transforming growth factor-beta signaling suppresses progression of androgen-independent human prostate cancer in nude mice. Clin Cancer Res. 2005;11:4512–4520. doi: 10.1158/1078-0432.CCR-04-2571. [DOI] [PubMed] [Google Scholar]
- 14.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-β-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485–6498. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Valdés N, Basagoiti M, Dotor J, Aranda F, Monreal I, Riezu-Boj JI, Borrás-Cuesta F, Sarobe P, Feijoó E. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFbeta1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Res. 2011;71:812–821. doi: 10.1158/0008-5472.CAN-10-2698. [DOI] [PubMed] [Google Scholar]
- 17.Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, Hartmann G, Endres S, Schnurr M. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 2013;73:1709–1720. doi: 10.1158/0008-5472.CAN-11-3850. [DOI] [PubMed] [Google Scholar]
- 18.Humbert L, Lebrun JJ. TGF-beta inhibits human cutaneous melanoma cell migration and invasion through regulation of the plasminogen activator system. Cell Signal. 2013;25:490–500. doi: 10.1016/j.cellsig.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Moore LD, Isayeva T, Siegal GP, Ponnazhagan S. Silencing of transforming growth factor-beta1 in situ by RNA interference for breast cancer: implications for proliferation and migration in vitro andmetastasis in vivo. Clin Cancer Res. 2008;14:4961–70. doi: 10.1158/1078-0432.CCR-07-4604. [DOI] [PubMed] [Google Scholar]
- 20.Bian Y, Terse A, Du J, Hall B, Molinolo A, Zhang P, Chen W, Flanders KC, Gutkind JS, Wakefield LM, Kulkarni AB. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of thePI3K/Akt pathway. Cancer Res. 2009;69:5918–5926. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–43009. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 22.Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of PKN and p38MAPK. J Biol Chem. 2005;280:31172–1181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- 23.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways intransforming growth factor-beta-mediated Smad-dependentgrowth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2015;280:1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- 24.Xie R, Schlumbrecht MP, Shipley GL, Xie S, Bassett RL Jr, Broaddus RR. S100A4 mediates endometrial cancer invasion and is a target ofTGF-beta1 signaling. Lab Invest. 2009;89:937–947. doi: 10.1038/labinvest.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuura I, Lai CY, Chiang KN. Functional interaction between Smad3 and S100A4 (metastatin-1) for TGF-beta-mediated cancer cell invasiveness. Biochem J. 2010;426:327–335. doi: 10.1042/BJ20090990. [DOI] [PubMed] [Google Scholar]
- 26.Weeks BH, He W, Olson KL, Wang XJ. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001;61:7435–7443. [PubMed] [Google Scholar]
- 27.Tang B, Yoo N, Vu M, Mamura M, Nam JS, Ooshima A, Du Z, Desprez PY, Anver MR, Michalowska AM, Shih J, Parks WT, Wakefield LM. Transforming growth factor-beta can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 2007;67:8643–8652. doi: 10.1158/0008-5472.CAN-07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, Schreiner G, Barnard N, Reiss M. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 29.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Proc Natl Acad Sci U S A. 2014;101:7112–7117. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seki Y, Suico MA, Uto A, Hisatsune A, Shuto T, Isohama Y, Kai H. The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res. 2002;62:6579–6586. [PubMed] [Google Scholar]
- 31.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seki Y, Suico MA, Uto A, Hisatsune A, Shuto T, Isohama Y, Kai H. The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res. 2002;62:6579–6586. [PubMed] [Google Scholar]
- 33.Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, Shao Z, Ding Y, Zhao L. LIM and SH3 protein 1 induces TGFβ-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin Cancer Res. 2014;20:5835–5847. doi: 10.1158/1078-0432.CCR-14-0485. [DOI] [PubMed] [Google Scholar]
- 34.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of MMP- 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Ding W, Neiman J, Mulder KM. Requirement of Smad3 and CREB-1 in mediating transforming growth factor-beta (TGF beta) induction of TGF beta 3 secretion. J Biol Chem. 2006;281:29479–29490. doi: 10.1074/jbc.M600579200. [DOI] [PubMed] [Google Scholar]
- 36.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–285. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 37.Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005;93:1277–1284. doi: 10.1038/sj.bjc.6602856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min HS, Choe G, Kim SW, Park YJ, Park do J, Youn YK, Park SH, Cho BY, Park SY. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod Pathol. 2008;21:748–755. doi: 10.1038/modpathol.2008.51. [DOI] [PubMed] [Google Scholar]
- 39.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw S, Spiegelman VS, Setaluri V, Mukhtar H. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlmann M, Sack U, Herrmann P, Lemm M, Fichtner I, Schlag PM, Stein U. Systemic shRNA mediated knock down of S100A4 in colorectal cancer xenografted mice reduces metastasis formation. Oncotarget. 2012;3:783–797. doi: 10.18632/oncotarget.572. [DOI] [PMC free article] [PubMed] [Google Scholar]