Abstract
Infrarenal aortic cross-clamping (IAC) is commonly used during infrarenal vascular operations. Prolonged IAC causes ischemia-reperfusion injury to local tissues, resulting in the release of inflammatory cytokines and acute lung injury (ALI). Pentoxifylline (PTX) is a clinically used drug for chronic occlusive arterial diseases and exerts protective effects against ALI induced by various factors in experimental models. In this study, we evaluated the protective effects of PTX in a rat model of IAC. Wistar rats underwent IAC for 2 h, followed by 4 h reperfusion. PTX alone, or in combination with ZM-241385 (an adenosine receptor A2A antagonist) or CGS-21680 (an A2A agonist), was pre-administered to rats 1 h prior to IAC, and the severity of lung injury and inflammation were examined. Administration of PTX significantly attenuated ALI induced by IAC, evidenced by reduced histological scores and wet lung contents, improved blood gas parameters, decreased cell counts and protein amounts in bronchoalveolar lavage fluids, and inhibition of MPO activity and ICAM-1 expression in lung tissues, and lower plasma levels of TNF-α, IL-6, IL-1β and soluble ICAM-1. ZM-241385 significantly abrogated, while CGS-21680 slightly enhanced, the effects of PTX in ameliorating ALI and inhibiting pulmonary inflammation. In exploration of the mechanisms, we found that PTX stimulated IL-10 production through the phosphorylation of STAT3, and A2A receptor participated in this regulation. The study indicates PTX plays a protective role in IAC-induced ALI in rats by inhibiting pulmonary inflammation through A2A signaling pathways.
Keywords: Infrarenal aortic cross-clamping, pentoxifylline, ischemia reperfusion injury, tumor necrosis factor-α, acute lung injury, adenosine A2A receptor
Introduction
Infrarenal aortic cross-clamping (IAC) is a very common procedure during infrarenal vascular operations. IAC induces ischemia-reperfusion (IR) injury to local tissues whose blood is supplied by arteries from the infrarenal aorta, particularly when long-term ischemic periods are unavoidable in surgical interventions [1]. IAC-induced IR injury can also provoke the release of inflammatory mediators or even cytokine cascade, a potentially fatal immune reaction that often leads to multiple organ dysfunction syndrome (MODS) [2]. The lungs suffer from the first round of cytokine attack due to its anatomic features [1]. Acute lung injury (ALI) is characterized by alveolar-capillary injury and inflammation with leukocyte accumulation, and release of pro-inflammatory cytokines [3]. ALI can cause persistent respiratory failure and increase the susceptibility to MODS, and the mortality related to these conditions is reported to be around 40% [4]. Therefore, it is essential to seek potential agents to protect lungs from IAC-induced ALI.
In searching for candidates that possess this ability, pentoxifylline (PTX, C13H18N4O3), a methylxanthine compound, had drawn our attention. PTX is currently used for the treatment of patients with intermittent claudication due to chronic occlusive arterial diseases of the limbs [5]. PTX has hemorheologic effects and vasodilating properties for peripheral blood vessels, displays anti-inflammatory activities by inhibiting the production of tumor necrosis factor (TNF)-α, and improves the recovery of adenosine triphosphate (ATP) [5,6]. Recent studies reveal that PTX is a phosphodiesterase inhibitor and demonstrates more pharmaceutical activities. PTX has been clinically used to treat alcoholic hepatitis [7], severe acute pancreatitis [8], and pediatric sepsis and necrotizing enterocolitis [9], and to prevent bronchopulmonary dysplasia in preterm infants [10]. These clinical trials and systemic reviews demonstrate that PTX is well tolerated without adverse effects, and encourage researchers to undertake large, well-designed and multicentered clinical trials to confirm its effectiveness [7-10].
More importantly, PTX has been shown to inhibit IR-induced injuries to the heart [11], liver [12], intestine [13,14] and kidneys [15], and attenuate ALI induced by nitrogen mustard [16], hyperoxia [17], intestinal IR [18] and phosgene exposure [19] in experimental animals. However, the underlying mechanisms of PTX accounting for its protective effects against ALI are yet to be defined. Two recently published studies suggest that the anti-inflammatory effects of PTX may be mediated via adenosine receptor 2A (A2A)-dependent pathways in vitro [20] and in an animal model of pulmonary inflammation [21]. PTX increases the activation of A2A without affecting the expression of the receptor itself [20,21]. Therefore, the present study was designed to investigate whether PTX could attenuate ALI, and whether the A2A signaling participates in its effects in a rat model of IAC.
Materials and methods
Animals and experiment design
Male Wistar rats (200-250 g) were supplied by the Animal Research Center of the First Affiliated Hospital, Harbin Medical University, China. This study was approved by the Animal Ethics Committee of Harbin Medical University, in compliance with Experimental Animal Regulations by the National Science and Technology Commission in China. Animals maintained under standard conditions were fed rodent chow and water. Animals were randomly assigned to sham operation and IAC groups (n=10). Animals in the IAC groups were treated with vehicle, PTX, PTX + ZM-241385 (an adenosine receptor A2A antagonist) or PTX+CGS-21680 (an A2A agonist), respectively. Three doses of PTX (10, 50 and 100 mg/kg) were tested based on published reports [11-21]. ZM-241385 and CGS-21680 were both given at a dose of 1 mg/kg [21]. PTX, ZM-241385 and CGS-21680 (Sigma-Aldrich, Shanghai, China) were dissolved in dimethyl sulfoxide (DMSO) to make stock solutions (20 mg/ml each), which were further diluted in normal saline to prepare therapeutic solutions. Normal saline containing the same concentration of DMSO as therapeutic solutions served as a vehicle. The vehicle and therapeutic solutions (1.5 ml of each) were intraperitoneally injected 1 h prior to the commencement of IAC. Sham-operated rats and vehicle-treated IAC rats received the vehicle solution, and the other rats, respective therapeutic solutions.
Surgical procedures of IAC, gas analysis and sampling
The procedures have been described previously [22]. Briefly, anesthesia was induced by an intraperitoneal injection of a mixture of ketamine (90 mg/kg) and xylazine (20 mg/kg), and maintained by intravenous injections of 25 mg/kg/h of ketamine and 2.5 mg/kg/h of xylazine via the right jugular vein. Heparin (60 IU/kg) was administered 5 min prior to occlusion of the aorta. A midline laparotomy was performed on the rat placed on a heating pad. The infrarenal part of the aorta was isolated and occluded with an atraumatic clip above the bifurcation of the abdominal aorta. The abdominal cavity was temporally closed with clips, and the wound was covered with a plastic wrap. The clip was removed 2 h later, and reperfusion was initiated. The abdominal wall was closed with a continuous suture, and reperfusion was allowed for 4 h. Aortic occlusion and reperfusion were corroborated by the loss and resurrection of the pulsation on the distal aorta. Sham-operated rats underwent the same procedures except for clamping the aorta.
Blood gas analysis was performed from the left carotid artery at 0, 2 and 4 h after the commencement of reperfusion. At the end of reperfusion, rats underwent a median sternotomy. Blood samples were collected from the right ventricle, and centrifuged at 1000 × g for 10 min; and plasma decanted and stored at -80°C. The left lung was ligated and a bronchoalveolar lavage (BAL) was performed using 5 ml of normal saline via a 16-gauge tracheal catheter into the right lung [22,23]. The BAL fluid was collected, and centrifuged at 1000 × g for 10 min at 4°C. The protein content in the supernatant was measured (Pierce BCA Protein Assay, Thermo Scientific, Rockford, USA). The pellet was resuspended in lavage buffer and cell numbers counted. BAL fluid cytospins were stained with Diff-Quik stain (Dade Behring Inc. USA), and differential cell counts enumerated. The upper lobe of the right lung was fixed in 10% buffered formalin, and the remaining part of the right lung was snap-frozen in liquid nitrogen and stored at -80°C. The wet/dry ratio (%) was calculated from the left lung.
Lung histological scoring
Formalin-fixed lung specimens were embedded, sectioned (3 μm), stained with hematoxylin-eosin, and examined by an independent pathologist in a blinded manner. Evaluation of lung injury was performed with a histological score as described previously [22,24]. Briefly, the severity of lung injury was expressed as the sum of the individual score grades of 0 (no symptoms), 1 (mild), 2 (moderate), to 3 (severe) for each of the following 5 symptoms: alveolar edema, atelectasis, hemorrage, infiltration of leukocytes, and vascular congestion. In addition, the thickness of alveolar walls was measured in 20 high-power fields (magnification × 1000) randomly selected from each sample.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α, interleukin (IL)-6, IL-1β, IL-10 and soluble intercellular adhesion molecule (ICAM)-1 in plasma samples and/or BAL fluids were measured with ELISA kits (R&D Systems, Shanghai, China) according to the manufacturer’s instruction. Absorbance was read at a wavelength of 450 nm using an ELISA reader.
Myeloperoxidase (MPO) activity
A lung fraction was homogenized with 1 ml of PBS containing 0.5% of hexadecyl-trimethylammonium bromide and 5 mM EDTA, pH 6.0, and then centrifuged at 30,000 × g for 10 min. Aliquots of homogenates (10 μl) were incubated for 5 min with a solution containing H2O2 (0.1%) and orthodianisidine. The reaction was stopped by the addition of 1% NaNO3. The absorbance was determined at 450 nm using a microplate reader. The absorbance was normalized to lung weight, and results are expressed as units of MPO activity/gram tissue.
Immunohistochemistry and western blot analysis
The methods have been described previously [25]. Briefly, lung cryosections (4 μm) were blocked and incubated overnight with the primary antibody (Ab). They were subsequently incubated for 30 min with the secondary Ab, and developed with FAST DAB and CoCl2 enhancer tablets (Sigma-Aldrich, Shanghai, China). Sections were counterstained with hematoxylin, and examined by microscopy. Lung homogenates prepared in protein lysate buffer were centrifuged to remove debris. Protein concentrations were determined, and lysates were resolved on SDS-PAGE gels, electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked, incubated overnight with primary Abs, subsequently with alkaline phosphatase-conjugated secondary Abs, and developed with BCIP/NBT (Tiangen Biotech Co. Ltd., Beijing, China). The density of each band was measured using a densitometric analysis program (FR200, Shanghai, China). In preliminary experiments, serial dilutions of lysates were blotted, band densities were measured and plotted against protein amounts to generate a standard curve, and the amount of protein for each blot was determined. Abs used in this study included primary Abs against A2A receptor, STAT3, p-STAT3Tyr705 (phosphorylated STAT3) and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), TNF-α and IL-10 (Boster Biological Technology, Ltd., Wuhan, China), ICAM-1 (Cell Signaling Technology, Danvers, USA), and secondary horseradish peroxidase-conjugated Abs (Zhongshan Golden Bridge Biotechnology Co. Ltd, Beijing, China).
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
The methods have been described previously [21,25]. Briefly, total RNA was extracted from lung tissues using TRIzol reagent (Invitrogen Life Technolgies, Beijing, China). RNA concentration and quality were verified, and reversely transcribed to produce complementary DNA (cDNA). The qRT-PCR reaction mixtures containing cDNA, PCR mix and A2A primers (5’-GAAGCAGATGGAGAGCCAAC-3’ and 5’-GAGAGGATGATGGCCAGGTA-3’) were prepared, and analyzed by MX3000P Real-time PCR systems (Stratagen, USA). Experiments were performed in triplicate, and data were calculated by ∆∆Ct methods.
Statistical analysis
Results were expressed as mean values ± standard deviation. Comparisons among multiple groups were made with a one-way analysis of variance (ANOVA) followed by the post hoc Dunnett’s t-test. P < 0.05 was considered to be statistically significant.
Results
PTX attenuates lung injury and dysfunction induced by IAC
Compared with sham-operated rats, lungs from vehicle-treated IAC rats had significantly higher histological scores and wet/dry ratios, which were slightly but insignificantly reduced by treatment of PTX at 10 mg/kg (Table 1). However, PTX at 50 and 100 mg/kg significantly reduced the histological scores and wet/dry ratios of lungs, compared with those in IAC rats treated with vehicle or PTX at 10 mg/kg (Table 1). The results were supported by the arterial blood gas analysis. Arterial pO2 was decreased, and arterial pCO2 increased in vehicle-treated IAC rats, compared with sham-operated rats (Table 1). PTX at 10 mg/kg only improved the pO2 4 h after reperfusion, and had no significant effects on pO2 and pCO2 at the other time points, compared with vehicle-treated rats. However, PTX at 50 and 100 mg/kg significantly ameliorated the reduction of pO2 and the increase of pCO2 by IAC at most time points, compared with vehicle- and PTX (10 mg/kg)-treated rats (Table 1).
Table 1.
Parameters of lung injury and plasma cytokines in IAC rats receiving different doses of PTX and the effects of A2A activation
| Sham | IAC | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Vehicle | PTX (10 mg/Kg) | PTX (50 mg/Kg) | PTX (100 mg/Kg) | PTX (50 mg/Kg) + ZM241385 | PTX (50 mg/Kg) + CGS21680 | ||
| Parameters of lung injury | |||||||
| Histological scores | 2.1±0.3 | 10.3±1.1** | 8.2±1.3 | 5.4±0.9‡‡,# | 4.9±0.8‡‡,# | 7.9±1.1φ | 5.1±0.8 |
| Wet/dry ratio (%) | 57.4±3.5 | 66.4±2.7** | 64.1±2.8 | 62.2±2.4‡‡,# | 59.8±3.1‡‡,# | 65.1±3.3φ | 60.9±3.1 |
| pO2 (mmHg) | |||||||
| 0 h after reperfusion | 94.2±7.4 | 75.2±3.6** | 76.8±5.6 | 79.7±6.8‡ | 81.3±7.1‡‡,# | 75.1±5.3 | 80.2±6.7 |
| 2 h after reperfusion | 96.9±3.5 | 80.3±3.5** | 84.9±4.4 | 90.2±6.4‡‡,# | 94.7±5.1‡‡,# | 81.5±6.2φ | 93.5±7.8 |
| 4 h after reperfusion | 98.1±2.6 | 82.1±5.8** | 86.2±3.9‡ | 95.6±7.2‡‡,# | 96.5±6.3‡‡,# | 82.3±4.5φφ | 97.7±6.5 |
| pCO2 (mmHg) | |||||||
| 0 h after reperfusion | 38.1±3.6 | 45.5±2.8** | 43.8±3.6 | 43.2±4.5 | 42.9±3.4 | 45.8±3.7 | 42.1±4.1 |
| 2 h after reperfusion | 37.7±1.9 | 44.8±5.1** | 42.6±4.2 | 38.1±3.6‡,# | 37.9±4.2‡‡,# | 44.6±2.9φ | 37.4±2.5 |
| 4 h after reperfusion | 39.9±4.0 | 45.1±3.7* | 41.2±3.1 | 38.9±2.5‡‡ | 39.2±3.3‡‡ | 45.2±4.3φ | 39.0±5.2 |
| Plasma inflammatory cytokines | |||||||
| TNF-α (ng/L) | 14.2±4.9 | 112.5±16.8** | 72.9±12.9‡‡ | 42.4±6.8‡‡,# | 37.8±5.5‡‡,# | 81.7±9.5φφ | 38.1±2.5φ |
| IL-6 (ng/L) | 25.9±6.3 | 242.7±22.5** | 200.9±30.4 | 133.5±19.6‡‡,## | 110.6±14.7‡‡,## | 212.8±20.7φφ | 107.6±11.3φ |
| IL-1β (ng/L) | 9.2±3.6 | 32.9±4.6** | 28.1±4.3 | 22.6±4.3‡‡,# | 19.8±4.8‡‡,# | 26.2±3.5φ | 20.4±3.9 |
| sICAM-1 (ng/L) | 72.6±10.7 | 219.8±37.4** | 156.5±25.3‡ | 102.8±14.3‡‡,# | 91.5±11.6‡‡,# | 169.1±19.5φφ | 89.6±8.7φ |
Notes: A2A, adenosine receptor 2A; IAC, infrarenal aortic cross-clamping; IL, interleukin; PTX, pentoxifylline; sICAM, soluble intercellular adhesion molecule; TNF-α, tumor necrosis factor-α. ZM-241385 is an adenosine receptor A2A antagonist, and CGS-21680, an A2A agonist.
P < 0.05 indicate a significant difference vs. Sham;
P < 0.001 indicate a significant difference vs. Sham;
P < 0.05, a significant difference vs. vehicle-treated IAC rats;
P < 0.001, a significant difference vs. vehicle-treated IAC rats;
P < 0.05, a significant difference vs. PTX (10 mg/kg)-treated IAC rats;
P < 0.001, a significant difference vs. PTX (10 mg/kg)-treated IAC rats;
P < 0.05, a significant difference vs. PTX (50 mg/kg)-treated IAC rats;
P < 0.001, a significant difference vs. PTX (50 mg/kg)-treated IAC rats.
PTX inhibits systemic inflammation induced by IAC
The plasma levels of TNF-α, IL-6, IL-1β and soluble ICAM-1 in vehicle-treated IAC rats were significantly higher than those in sham-operatedrats (Table 1). PTX at 10 mg/kg significantly reduced the levels of TNF-α and soluble ICAM-1, but not IL-6 or IL-1β, compared with vehicle, in IAC rats (Table 1). IAC rats treated with PTX at 50 and 100 mg/kg had significantly lower levels of all the above 4 pro-inflammatory factors, than those treated with vehicle or PTX at 10 mg/kg (Table 1).
As shown above, most of the parameters in IAC rats treated with 50 and 100 mg/kg of PTX were significantly different from those treated with 10 mg/kg of PTX, indicating that PTX has a dose-dependent protective effect on IAC-induced lung injury and inflammation. There was no significant difference in the parameters between IAC rats treated with 50 mg/kg and 100 mg/kg of PTX, suggesting that PTX at 50 mg/kg would be an ideal dose for this animal model, and was selected for the following experiments.
PTX inhibits pulmonary inflammation and leukocyte migration into the lung induced by IAC
Compared with sham-operated rats, lungs from vehicle-treated IAC rats had significantly more partial atelectasis and thicker alveolar walls, which could be attenuated by administration of PTX (Figure 1A and 1B). PTX also significantly reduced cell counts in BAL fluids, which were highly elevated by IAC (Figure 1C). The differential analysis of leukocytes in the BAL fluids showed that PTX treatment decreased the numbers of neutrophils, macrophages/monocytes and lymphocytes that migrated into the lungs in IAC rats (Figure 1D). The results were further supported by the alteration of MPO activity (Figure 1E), a marker for leukocyte infiltration in ALI [26].
Figure 1.
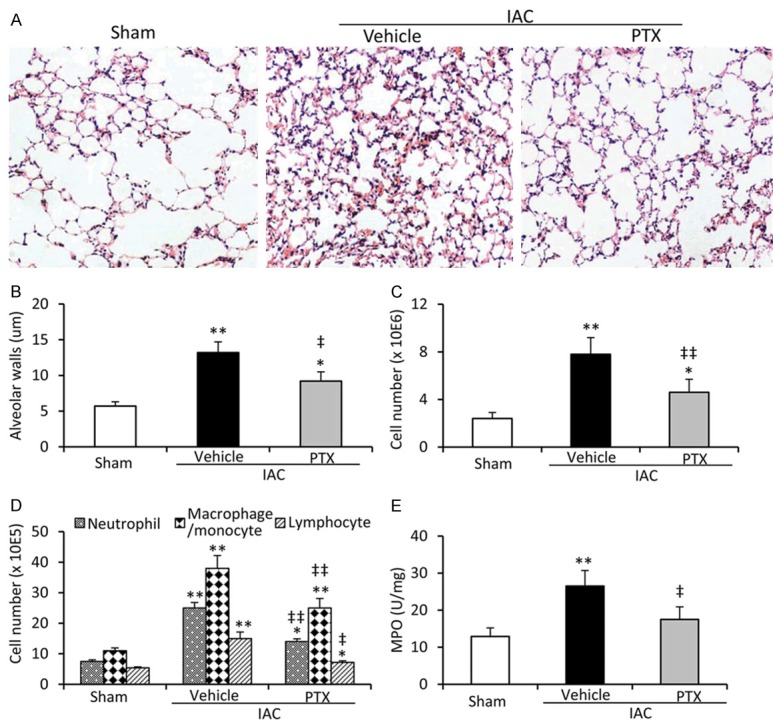
Assessment of lung injury and inflammation. A. Representative images were hematoxylin/eosin-stained lung sections from sham-operated, vehicle- or PTX (50 mg/Kg) -treated IAC rats (magnification × 200). B. The thickness of alveolar walls was measured. C. The number of cells in BAL fluids was counted. D. The number of neutrophils, macrophages/monocytes and lymphocytes in BAL fluids were counted. E. MPO activity in lung tissues was assessed. *P < 0.05 and **P < 0.001 indicate a significant difference from sham-operated rats. ‡P < 0.05 and ‡‡P < 0.001 indicate a significant reduction from vehicle-treated IAC rats.
PTX decreases vascular permeability and ICAM-1 expression and in lung tissues
Impairment of tissue fluid homeostasis is crucial in the pathogenesis of ALI. Compared with sham-operated rats, protein contents in BAL fluids were significantly increased in vehicle-treated IAC rats, and PTX treatment significantly attenuated this increase (Figure 2A). The BAL fluids collected from lungs of vehicle-treated IAC rats had significantly higher levels of TNF-α than sham-operated rats, and PTX treatment significantly attenuated this elevation (Figure 2B). In addition, lung tissues from vehicle-treated IAC rats had a higher expression of ICAM-1 than those from sham-operated rats as examined by immunohistochemical and Western blot analyses, while PTX administration resulted in the down regulation of ICAM-1 in lungs from IAC rats (Figure 2C and 2D).
Figure 2.
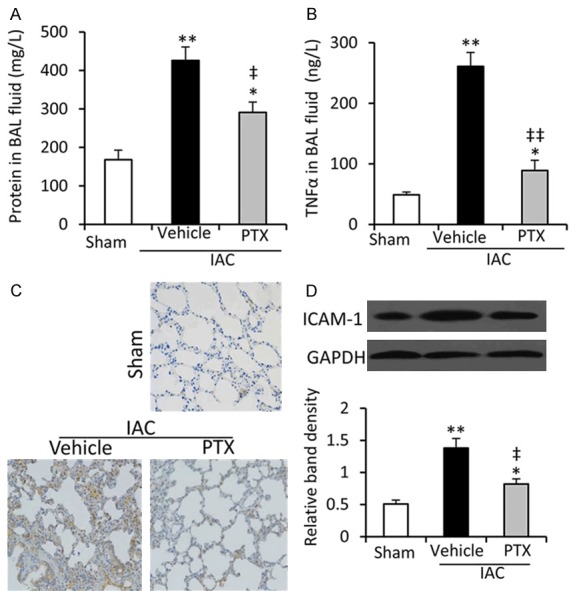
Vascular permeability and ICAM-1 expression of lung tissues. (A, B) The contents of total protein (A) and TNF-α (B) in BAL fluids collected from rats in Figure 1 were measured. (C, D) The expression of ICAM-1 in lung tissues were examined by immunohistochemistry (C) and Western blot analysis (D). The density of each band was normalized to GAPDH. *P < 0.05 and **P < 0.001 indicate a significant difference from sham-operated rats. ‡P < 0.05 and ‡‡P < 0.001 indicate a significant reduction from vehicle-treated IAC rats.
Inhibition of A2A abolishes the effects of PTX in attenuating lunginjury and inflammation
We next investigated the role of A2A activation in the protective effects of PTX against ALI induced by IAC, by co-applying ZM-241385, a highly selective A2A receptor antagonist, and CGS-21680, an A2A receptor agonist [20,21], with PTX (50 mg/Kg) to the IAC rats. First we confirmed that the expression of A2A mRNA (Figure 3A) and protein (Figure 3B) in lung tissues was significantly higher in vehicle-treated IAC rats; but either PTX, or PTX +ZM-241385, or PTX + CGS-21680 had no significant effects on its expressions in lungs from IAC rats. ZM-241385 treatment abrogated the effects of PTX on lung histological scores, wet/dry ratios of lungs, and arterial pO2 and pCO2 (Table 1). However, CGS-21680 treatment only slightly enhanced the effects of PTX on the above 4 parameters, and the differences did not reach significance (Table 1).
Figure 3.
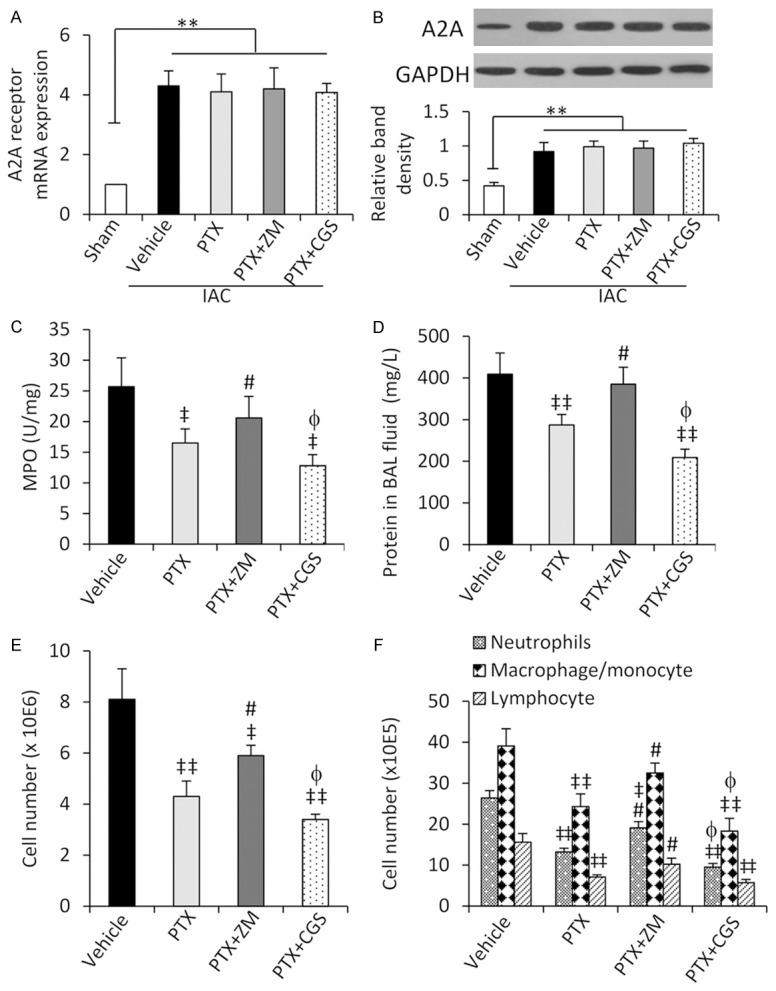
A2A activation affects the action of PTX in attenuating lung injury. Rats were pre-administered with vehicle, PTX (50 mg/Kg), PTX + ZM-241385 (ZM) or PTX + CGS-21680 (CGS), and then underwent IAC. A. The expression of A2A receptor mRNA was examined by qRT-PCR, and the level of mRNA from sham-operated rats was defined as 1. B. The expression of A2A receptor protein was detected by Western blot analysis. The density of each band was normalized to GAPDH. C. MPO activity in lung tissues was measured. D. Protein concentrations in BAL fluids were measured. E. Cell numbers in BAL fluids were counted. F. Numbers of neutrophils, macrophages/monocytes and lymphocytes in BAL fluids were counted. *P < 0.05 and **P < 0.001 indicate a significant difference. ‡P < 0.05 and ‡‡P < 0.001 indicate a significant reduction from vehicle-treated IAC rats; #P < 0.05, a significant increase from PTX-treated IAC rats; and φP < 0.05, a significant reduction from PTX-treated IAC rats.
In addition, ZM-241385 treatment abrogated the reducing effects of PTX on plasma levels of TNF-α, IL-6, IL-1β and soluble ICAM-1 in IAC rats; and CGS-21680 further reduced the plasma levels of TNF-α, IL-6 and soluble ICAM-1 but not IL-1β (Table 1). The results of systemic inflammation were supported by those of pulmonary inflammation. Co-administration of ZM-241385 abrogated the inhibitory effects of PTX on MPO activity of lung tissues (Figure 3C), and protein contents (Figure 3D), total cell counts (Figure 3E) and the numbers of neutrophils, macrophages/monocytes and lymphocytes (Figure 3F) in BAL fluids. In contrast, CGS-21680 showed an opposite effect on most of the above parameters.
Inhibition of A2A abolishes the anti-inflammatory effects of PTX through IL-10 and STAT3 phosphorylation
In the consistent with the plasma levels of TNF-α, PTX treatment significantly reduced the concentration of TNF-α in BAL fluids from IAC rats (Figure 4A). Co-administration of ZM-241385 increased, while CGS-21680 reduced, the concentration of TNF-α in BAL fluids from IAC rats, compared with PTX treatment alone (Figure 4A). PTX treatment significantly increased the concentration of IL-10 in BAL fluids; and co-application of ZM-241385 reduced, while CGS-21680 further increased, the concentration of IL-10 in BAL fluids in IAC rats, compared with PTX alone (Figure 4B). The concentrations TNF-α and IL-10 in BAL fluids were supported by their protein expression in lung tissues (Figure 4C). In investigating the regulatory mechanism of TNF-α by IL-10, we observed that PTX treatment increased the phosphorylation of STAT3 in lung tissues, which could be abolished by co-application of ZM-241385 and further increased by CGS-21680 (Figure 4C). Either PTX alone or in combination with ZM-241385 or CGS-21680 did not have significant effects on the expression of total STAT3 (Figure 4C).
Figure 4.
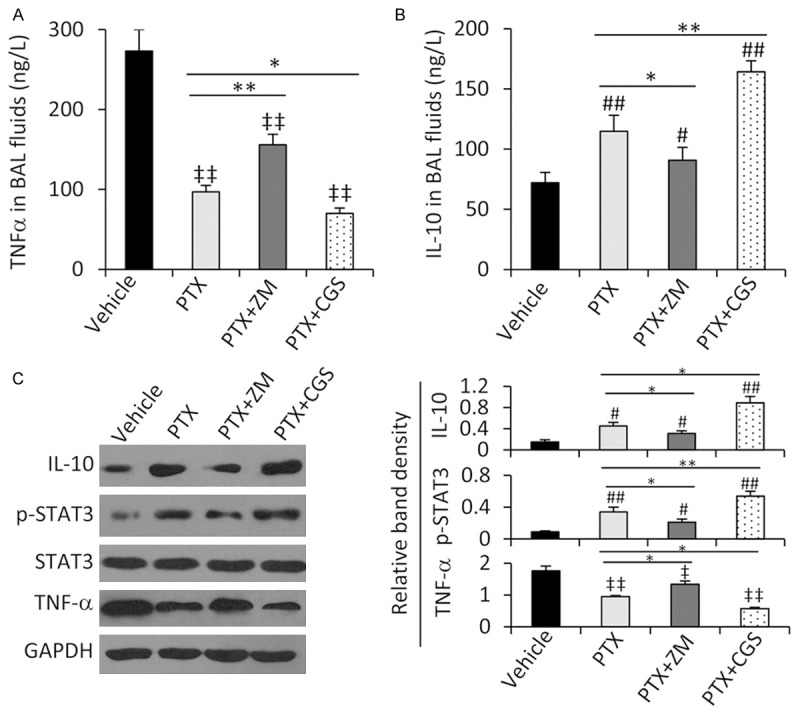
Activation of STAT3 participates in the effects of PTX in attenuating lung injury. (A, B) The levels of TNF-α (A) and IL-10 (B) in BAL fluids collected from rats in Figure 3 were measured. (C) Lung tissues were subjected to Western blot analysis, and the density of each band was normalized to GAPDH. *P < 0.05 and **P < 0.001 indicate a significant difference. ‡P < 0.05 and ‡‡P < 0.001 indicate a significant reduction; while #P < 0.05 and ##P < 0.001, a significant increase from vehicle-treated IAC rats.
Discussion
The present study has demonstrated that PTX has displayed protective activities against IAC-induced ALI as evidenced by reduced lung histological score and wet/dry ratio, improved arterial blood gas parameters, and decreased protein contents and cell counts in BAL fluids. PTX inhibited pulmonary inflammation induced by IAC by reducing production of pro-inflammatory cytokines including TNF-α, IL-6, IL-1β and soluble ICAM-1. The protective effects of PTX seem to be dose-dependent and 50 mg/kg body weight was shown to be an ideal dose for this rat model. Its pharmacological actions are partially dependent on the activation of A2A receptor, and production of IL-10 and phosphorylation of STAT3 are involved in this action.
It is well documented that the anti-inflammatory activity of PTX mainly relies on its inhibitory effect on TNF-α production [6]. The most important mechanism for PTX-mediated lung protection is also dependent of TNF-α inhibition [19,21]. In accord, we have shown here that PTX reduced the release of pro-inflammatory cytokines including TNF-α, IL-6 and IL-1β into the circulation, and the content of TNF-α in BAL fluids. TNF-α has been shown to play a key role in pulmonary vascular permeability, which could lead to lung edema [21]. TNF-α mediates pulmonary edema via ICAM-1-dependent mechanisms [27], and ICAM-1 itself is also a marker for lung injury [28]. PTX has been shown to protect against ALI by down regulating ICAM-1 expression in experimental phosgene-exposure rats [19], and inhibit leukocyte-endothelial interactions by reducing ICAM-1 expression in a rat model of sepsis [28]. In accord, the present study has demonstrated that IAC induced upregulation of ICAM-1 in lungs and elevated the plasma level of soluble ICAM-1, while PTX could attenuate these increases, reducing the protein contents in BAL fluids and pulmonary inflammation. Furthermore, a recent study has shown that phosphodiesterase inhibitors could stabilize capillary leakage in a model of lipopolysaccharide-induced systemic inflammation in rats [29]. Therefore, this mechanism may also contribute to the action of PTX in attenuating pulmonary inflammation as PTX has been regarded as a phosphodiesterase inhibitor [8].
The migration of inflammatory cells across the vascular endothelial barrier is a crucial factor in the pathogenesis of acute lung injury [30]. TNF-α and IL-6 are known to promote neutrophil adhesion to the endothelium [21]. Here we showed that the cell counts in BAL fluids were significantly higher in IAC rats. Numbers of neutrophils, macrophages and lymphocytes were all increased, indicating all the three major leukocytes may participate in the pulmonary inflammation induced by IAC. By inhibiting the release of inflammatory cytokines, PTX was shown to inhibit leukocyte infiltration into the lungs and reduce MPO activity in lung tissues [19,28].
Adenosine signaling has been shown to play an important role in the resolution of experimentally induced ALI, and A2A is known to exert anti-inflammatory effects [31,32]. PTX demonstrates a protective effect in lipopolysaccharide-induced ALI via A2A receptor [21]. PTX induces increase of cAMP and displays its anti-inflammatory activities by activating A2A without affecting the expression of the receptor itself [20]. Here we have shown that IAC markedly induced upregulation of A2A at mRNA and protein levels via the self-defense mechanisms of the host, but PTX had no effect on its expression, in accordance with previous reports [20,21]. Remarkably, inhibition of A2A activation by ZM-241385 abrogated the protective and anti-inflammatory effects of PTX, supported by the alterations of lung histological scores, leukocyte migration into the lungs, MPO activity, TNF-α production and vascular permeability. However, further activation of A2A by CGS-21680 only slightly strengthened the protective effects of PTX in this model.
Finally, some efforts have been made to improve our understanding of PTX’s action through A2A receptor-mediated pathways. It has been reported that activation of A2A stimulates the production of IL-10 [33], which is a potent anti-inflammatory molecule by dysregulating TNF-α at multiple levels [34]. We have shown herein that PTX stimulated the production of IL-10 in lungs from IAC rats. By inhibiting A2A activation, ZM-241385 abrogated the effect of PTX, resulting in reduced levels of IL-10. It is well known that IL-10 regulates TNF-α production through the activation of STAT3 [35]. Here we also showed that PTX increased the phosphorylation of STAT3, while inhibiting A2A activation by ZM-241385 abrogated this increase.
In conclusion, nowadays the incidences of peripheral arterial diseases and aortic abdominal aneurysms are steadily increasing due to arteriosclerosis in the elderly. Infrarenal vascular operations can cause very severe complications, most of which are linked to or accompanied by systemic inflammation induced by IR [36]. Lungs are among the most likely affected organs by IAC-induced IR injury. The present results provide a preventative approach for alleviating ALI induced by IAC, a very common procedure in infrarenal vascular operations. The protective mechanisms of PTX may rely on its anti-inflammatory activity by inhibiting TNF-α production, which is partially dependent on the A2A receptor-mediated pathways, as summarized in Figure 5. Given that PTX is a clinically used drug for the treatment of patients with chronic occlusive arterial diseases, protects multiple organs against several risk events, and is well tolerated without adverse effects, the present results warrant further investigation by using PTX in preventing ALI during infrarenal vascular surgical interventions.
Figure 5.
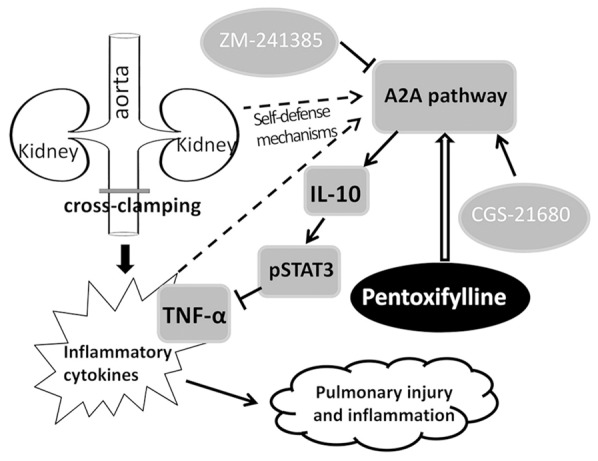
A proposed model of linkages among pulmonary injury and inflammation induced by infrarenal aortic cross-clamping, adenosine receptor A2A pathway, TNF-α and agents used in the study. “→”, Indicates promotion, positive regulation or activation; “^”, Inhibition, negative regulation or blockade.
Acknowledgements
This study was supported in part by grants from the National Natural Scientific Foundation of China (81272467 and 81472321), and Heilongjiang Provincial Scientific Fund for Post-doctoral Researchers (LBH-Q13118) and Heilongjiang Natural Scientific Foundation in China (C201310).
Disclosure of conflict of interest
None.
References
- 1.Garbaisz D, Turoczi Z, Fulop A, Rosero O, Aranyi P, Onody P, Lotz G, Rakonczay Z, Balla Z, Harsanyi L, Szijarto A. Therapeutic option for managing lung injury induced by infrarenal aortic cross-clamping. J Surg Res. 2013;185:469–476. doi: 10.1016/j.jss.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Salhiyyah K, Forster R, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;9:CD005262. doi: 10.1002/14651858.CD005262.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genoves P, Garcia D, Cejalvo D, Martin A, Zaragoza C, Toledo AH, Toledo-Pereyra LH, Lloris-Carsi JM. Pentoxifylline in liver ischemia and reperfusion. J Invest Surg. 2014;27:114–124. doi: 10.3109/08941939.2013.835454. [DOI] [PubMed] [Google Scholar]
- 7.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O’Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 8.Vege SS, Atwal T, Bi Y, Chari ST, Clemens MA, Enders FT. Pentoxifylline Treatment in Severe Acute Pancreatitis: A Pilot, Double-Blind, Placebo-Controlled, Randomized Trial. Gastroenterology. 2015;149:318–320. e313. doi: 10.1053/j.gastro.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Pammi M, Haque KN. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2015;3:CD004205. doi: 10.1002/14651858.CD004205.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Schulzke SM, Kaempfen S, Patole SK. Pentoxifylline for the prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2014;11:CD010018. doi: 10.1002/14651858.CD010018.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azhar A, El-Bassossy HM. Pentoxifylline alleviates cardiac ischemia and dysfunction following experimental angina in insulin resistance. PLoS One. 2014;9:e98281. doi: 10.1371/journal.pone.0098281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha-Santos V, Figueira ER, Rocha-Filho JA, Coelho AM, Pinheiro RS, Bacchella T, Machado MC, D’Albuquerque LA. Pentoxifylline enhances the protective effects of hypertonic saline solution on liver ischemia reperfusion injury through inhibition of oxidative stress. Hepatobiliary Pancreat Dis Int. 2015;14:194–200. doi: 10.1016/s1499-3872(15)60348-4. [DOI] [PubMed] [Google Scholar]
- 13.Brasileiro JL, Ramalho RT, Aydos RD, Silva IS, Takita LC, Marks G, Assis PV. Pentoxifylline and prostaglandin E1 action on ischemia and reperfusion of small intestine tissue in rats. An immunohistochemical study. Acta Cir Bras. 2015;30:115–119. doi: 10.1590/S0102-86502015002000005. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach UA, Atanasov G, Wallenta L, Polenz D, Reutzel-Selke A, Kloepfel M, Jurisch A, Marksteiner M, Loddenkemper C, Neuhaus P, Sawitzki B, Pascher A. Short-term TNF-alpha inhibition reduces short-term and long-term inflammatory changes post-ischemia/reperfusion in rat intestinal transplantation. Transplantation. 2014;97:732–739. doi: 10.1097/TP.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 15.Yang SK, Duan SB, Pan P, Xu XQ, Liu N, Xu J. Preventive effect of pentoxifylline on contrast-induced acute kidney injury in hypercholesterolemic rats. Exp Ther Med. 2015;9:384–388. doi: 10.3892/etm.2014.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunil VR, Vayas KN, Cervelli JA, Malaviya R, Hall L, Massa CB, Gow AJ, Laskin JD, Laskin DL. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp Mol Pathol. 2014;97:89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almario B, Wu S, Peng J, Alapati D, Chen S, Sosenko IR. Pentoxifylline and prevention of hyperoxia-induced lung -injury in neonatal rats. Pediatr Res. 2012;71:583–589. doi: 10.1038/pr.2012.14. [DOI] [PubMed] [Google Scholar]
- 18.Marqui CE, Silva HC, Ferez D, Cavassani SS, Moraes JB, Silva DA, Simoes RS, Lopes CA, Taha MO, Oliveira-Junior IS. Pretreatment with pentoxifylline attenuates lung injury induced by intestinal ischemia/reperfusion in rats. Acta Cir Bras. 2011;26:438–444. doi: 10.1590/s0102-86502011000600006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XD, Hou JF, Qin XJ, Li WL, Chen HL, Liu R, Liang X, Hai CX. Pentoxifylline inhibits intercellular adhesion molecule-1 (ICAM-1) and lung injury in experimental phosgene-exposure rats. Inhal Toxicol. 2010;22:889–895. doi: 10.3109/08958378.2010.493900. [DOI] [PubMed] [Google Scholar]
- 20.Kreth S, Ledderose C, Luchting B, Weis F, Thiel M. Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock. 2010;34:10–16. doi: 10.1097/SHK.0b013e3181cdc3e2. [DOI] [PubMed] [Google Scholar]
- 21.Konrad FM, Neudeck G, Vollmer I, Ngamsri KC, Thiel M, Reutershan J. Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent. FASEB J. 2013;27:3524–3535. doi: 10.1096/fj.13-228122. [DOI] [PubMed] [Google Scholar]
- 22.Tang B, Ma L, Yao X, Tan G, Han P, Yu T, Liu B, Sun X. Hydrogen sulfide ameliorates acute lung injury induced by infrarenal aortic cross-clamping by inhibiting inflammation and angiopoietin 2 release. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Song JA, Yang HS, Lee J, Kwon S, Jung KJ, Heo JD, Cho KH, Song CW, Lee K. Standardization of bronchoalveolar lavage method based on suction frequency number and lavage fraction number using rats. Toxicol Res. 2010;26:203–208. doi: 10.5487/TR.2010.26.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Li F, Wiegman CH, Zhang M, Hong Y, Gong J, Chang Y, Zhang JJ, Adcock I, Chung KF, Zhou X. Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress, and bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2015;52:129–137. doi: 10.1165/rcmb.2013-0415OC. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Zhai B, He C, Tan G, Jiang X, Pan S, Dong X, Wei Z, Ma L, Qiao H, Jiang H, Sun X. Upregulation of HIF-2alpha induced by sorafenib contributes to the resistance by activating the TGF-alpha/EGFR pathway in hepatocellular carcinoma cells. Cell Signal. 2014;26:1030–1039. doi: 10.1016/j.cellsig.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Yao X, Zhang Y, Li W, Kang K, Sun L, Sun X. The protective role of hydrogen sulfide in myocardial ischemia-reperfusion-induced injury in diabetic rats. Int J Cardiol. 2011;152:177–183. doi: 10.1016/j.ijcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Lo SK, Everitt J, Gu J, Malik AB. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J Clin Invest. 1992;89:981–988. doi: 10.1172/JCI115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa NK, Cruz RJ Jr, Aikawa P, Correia CJ, Cruz JW, Mauad T, Zhang H, Rocha-e-Silva M, Sannomiya P. Pentoxifylline attenuates leukocyte-endothelial interactions in a two-hit model of shock and sepsis. J Surg Res. 2015;193:421–428. doi: 10.1016/j.jss.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Schick MA, Wunder C, Wollborn J, Roewer N, Waschke J, Germer CT, Schlegel N. Phosphodiesterase-4 inhibition as a therapeutic approach to treat capillary leakage in systemic inflammation. J Physiol. 2012;590:2693–2708. doi: 10.1113/jphysiol.2012.232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thangavel J, Malik AB, Elias HK, Rajasingh S, Simpson AD, Sundivakkam PK, Vogel SM, Xuan YT, Dawn B, Rajasingh J. Combinatorial therapy with acetylation and methylation modifiers attenuates lung vascular hyperpermeability in endotoxemia-induced mouse inflammatory lung injury. Am J Pathol. 2014;184:2237–2249. doi: 10.1016/j.ajpath.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 32.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 33.Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 34.Brennan FM, Green P, Amjadi P, Robertshaw HJ, Alvarez-Iglesias M, Takata M. Interleukin-10 regulates TNF-alpha-converting enzyme (TACE/ADAM-17) involving a TIMP-3 dependent and independent mechanism. Eur J Immunol. 2008;38:1106–1117. doi: 10.1002/eji.200737821. [DOI] [PubMed] [Google Scholar]
- 35.Chan CS, Ming-Lum A, Golds GB, Lee SJ, Anderson RJ, Mui AL. Interleukin-10 inhibits lipopolysaccharide-induced tumor necrosis factor-alpha translation through a SHIP1-dependent pathway. J Biol Chem. 2012;287:38020–38027. doi: 10.1074/jbc.M112.348599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landry G, Lau I, Liem T, Mitchell E, Moneta G. Open abdominal aortic aneurysm repair in the endovascular era: effect of clamp site on outcomes. Arch Surg. 2009;144:811–816. doi: 10.1001/archsurg.2009.157. [DOI] [PubMed] [Google Scholar]


