Abstract
Ischemia reperfusion injury (IRI) is a leading cause of acute kidney injury with high morbidity and mortality due to limited therapy. Here, we examine whether sesamin attenuates renal IRI in an animal model and explore the underlying mechanisms. Male mice were subjected to right renal ischemia for 30 min followed by reperfusion for 24 h with sesamin (100 mg/kg) during which the left kidney was removed. Renal damage and function were assessed subsequently. The results showed that sesamin reduced kidney ischemia reperfusion injury, as assessed by decreased serum creatinine (Scr) and Blood urea nitrogen (BUN), alleviated tubular damage and apoptosis. In addition, sesamin inhibited neutrophils infiltration and pro-inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β production in IR-preformed kidney. Notably, sesamin promoted the expression of CD39, A2A adenosine receptor (A2AAR), and A2BAR mRNA and protein as well as adenosine production. Furthermore, CD39 inhibitor or A2AR antagonist abolished partly the protection of sesamin in kidney IRI. In conclusion, sesamin could effectively protect kidney from IRI by inhibiting inflammatory responses, which might be associated with promoting the adenosine-CD39-A2AR signaling pathway.
Keywords: Renal ischemia reperfusion injury, sesamin, adenosine, CD39, A2 adenosine receptors (A2AR)
Introduction
Acute kidney injury (AKI) is a clinical challenge often leading to multiple organ dysfunctions, collapse of the circulatory system and death [1,2]. Renal ischemia reperfusion injury (IRI) is one of the leading cause of AKI occurred in many clinical settings, including renal transplantation, shock, and cardiothoracic and vascular surgery [3,4]. It is manifested by a local inflammatory response caused by an initial ischemia and followed by reperfusion, which elicits to rapid neutrophil infiltration, pro-inflammatory cytokines release, oxidative stress generation, and subsequent proximal tubule apoptosis/necrosis [3-5]. Due to limited therapeutic modalities available, renal IRI is associated with extremely high morbidity and mortality [4]. Therefore, novel and effective therapeutic strategies are required desperately.
In recent some years, the biologically active substances derived from natural plants have been paid attentions on the prevention and treatment of acute and chronic diseases. Sesamin (Figure 1A), the major lignan extracted from sesame seed and oil, has been found to exert various pharmaceutical functions, including anti-hypertensive, hypocholesterolemic, neuroprotective, anti-fibrotic, anti-oxidative, anti-tumor, and anti-inflammatory actions [6-12]. In fact, our and other studies have shown that sesamin reduced production of inflammatory mediators, alleviated inflammatory responses, and improved survival in various inflammatory cells and animal models [13-16]. In addition, some previous studies have indicated that sesamin protected from brain and hepatic ischemia-reperfusion injury [17,18]. Furthermore, Wu et al reported that sesamin exerted renoprotective effects in renovascular hypertensive rats [6]. Thus, we hypothesized that sesamin might be a possible candidate for treating acute renal IRI.
Figure 1.

Sesamin improved kidney dysfunction caused by ischemic reperfusion. C57/BL6 mice (n = 6) were treated with vehicle or sesamin (100 mg/kg) every 8 h one time for three times within 24 h before surgery. Twenty-four hours after operation, blood was collected for renal function tests. A. The chemical structure of sesamin. B. Concentration of serum creatinine. C. Concentration of blood urea nitrogen (BUN). Data are expressed as the mean ± SD. *P<0.05, **P<0.01.
Here, the aims of the present study were to examine whether sesamin protects against acute renal IRI in a mouse model and, if so, to identify its underlying mechanisms. The results showed that sesamin protected kidneys from IRI by inhibiting inflammation and inducing the endogenous production of adenosine.
Materials and methods
Reagents
Sesamin (C20H15O6, FW=354.34, purity ≥98%) was purchased from Aladdin Industrial Corporation (Shanghai, China). POM-1 (the CD39 specific inhibitor) was purchased from Santa Cruz Biotechnology (CA, USA). SCH-58261 (the A2AAR antagonist), PSB1115 (the A2BAR antagonist) was purchased from Sigma-Aldrich (USA). Mouse tumor necrosis factor (TNF)-α and interleukin (IL)-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from Bender MedSystems (Vienna, Austria). Blood urea nitrogen (BUN), creatinine, and myeloperoxidase (MPO) detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The caspase 3 colorimetric assay kit was from Beyotime institute of biotechnology (Nanjing, China). In Situ Cell Death Detection Kit was from Roche Applied Science (Basel, Switzerland). The bicinchoninic acid (BCA) protein assay kit was purchased from Pierce (Rockford, IL, USA). Anti-CD73, anti-CD39, anti-A2AAR, and anti-A2BAR and anti-GAPDH antibodies were purchased from Abcam (Cambridge, MA, UK).
Animals
Male C57/BL6-mice (6-8 weeks old, weight 18-22 g) were obtained from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). Mice were housed in a specific pathogen-free (SPF) laboratory under optimum conditions (25 ± 2°C, 55% humidity, and a 12 h light/dark cycle) and fed a standard laboratory diet and water. Mice were acclimatized for at least 1 week before use. All experimental procedures involving animals were approved by the Animal Care and Use Committee of Chongqing Medical University.
Experimental protocol
Renal IRI was induced by performing a left nephrectomy followed by ischemic to the remaining right kidney. Briefly, the mice were anesthetized by intraperitoneal injection of a mixture of ketamine and xylazine (45 mg/kg and 8 mg/kg, respectively) and placed on a temperature-controlled heating table. A flank incision was performed using a coagulation electrode to prevent bleeding. The left kidney was removed without interfering with the adrenal vessels and the right renal pedicle was then clamped for 30 min. Subsequently, the clamp was released and the kidney was monitored for color changes to confirm blood reflow before the incision was closed. Sham control animals were subjected to the same procedure without clamping of the renal pedicle.
Mice were give oral gavage of sesamin (100 mg/kg, dissolved in 0.5% carboxylmethylcellulose sodium salt in 0.9% normal saline, respectively) every 8 h one time for three times within 24 h before IR. The dose of sesamin (100 mg/kg) was chosen for our previous preliminary experiment and study [13]. To inhibit or antagonize CD39 or A2AAR, A2BAR, POM-1 (5 mg/kg) or SCH-58261 (10 mg/kg), PSB115 (10 mg/kg) was administered intraperitoneally 30 min before IR, respectively. Each experiment group had 6 mice. After surgery, the mice were kept on a warming blanket for 24 h and allowed food and water ad libitum. At the end of the 24 h reperfusion period, all animals were sacrificed by injecting a high dose of pentobarbital sodium. The blood and kidney tissues were then harvested.
Measurement of creatinine and BUN
After reperfusion for 24 h, blood samples were collected and renal function was monitored by measuring the concentration of creatinine and BUN in the serum using the commercial kits according to the manufacturer’s suggestions.
Hematoxylin and eosin (HE) staining
Kidneys were harvested and fixed with 4% formaldehyde prior to paraffin embedding. Paraffin-embedded tissues were sectioned (5 μm thick) and stained with hematoxylin and eosin (H&E). The degree of kidney injury was scored by a pathologist in a blinded fashion on a 5-point scale: 0 = no damage, 1 ≤ 10%, 2 = 10-25%, 3 = 25-50%, 4 = 50-75%, 5 = more than 75% of the corticomedullary junction injured, as described by Jablonski et al [19].
TUNEL assay
Deparaffinized and dehydrated sections were were incubated for 15 min at 37°C with proteinase K. The sections were rinsed twice (5 min/each) with PBS, and the area around the sections was dried. TUNEL reaction mixture (50 µl) was added to the samples, which were then incubated for 60 min at 37°C in a humidified atmosphere. The sections were rinsed three times with PBS. Horseradish-peroxidase-labeled streptavidin was used to bind the biotinylated nucleotides. The sections were then colored with DAB and counterstained with hematoxylin.
Immunofluorescence
Neutrophils were identified by immunofluorescence using a rat anti-mouse Ly6G Ab followed by AlexaFluora 488-labelled goat anti-rat IgG. Goat IgG was used as an isotype control.
Measurement of caspase-3 activity
Caspase-3 activity in the liver tissue was measured using a caspase-3 colorimetric assay kit, according to the manufacturer’s instructions. Briefly, after homogenization of whole liver tissue in cell lysis buffer, homogenates were centrifuged for 1 min at 10,000 g, and the supernatant (100 mg protein) was incubated with Ac-DEVD-pNA substrate for caspase 3, and reaction buffer for 90 min at 37°C. Absorbance was measured at 405 nm as caspase protease activity.
MPO assays
Kidney tissues were thawed and homogenized in phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. MPO activity was measured using a commercial kit, according to the manufacturer’s instructions.
Measurement of TNF-α and IL-1β production
Mouse kidneys were homogenized in phosphate buffer. TNF-α and IL-1β were measured by commercial ELISA kits as manufacturer described.
Measurement of adenosine concentration
After renal ischemic and reperfusion, mouse kidneys were pulverized in liquid nitrogen and then sonicated in 0.6 N ice-cold perchloric acid to extract the proteins. The extracts were then neutralized by addition of 0.6 M potassium phosphate tribasic. Tissue adenosine levels were determined by high performance liquid chromatography-ultraviolet (HPLC-UV) as previously described [20].
Real-time reverse transcriptase PCR
Total RNA was extracted from cells and tissues using Trizol (Invitrogen, CA). RNA samples were reverse transcribed into complementary DNA (cDNA) using a PrimeScript RT reagent kit (Takara, Dalian, China). The transcripts were amplified from the cDNA with PCR. Real-time PCR was performed with SYBR Premix Ex Taq (Takara). The specific primers used were as follows: GAPDH 5’-AGGTCGGTGTG AACGGATTTG-3’ (sense), 5’-TGTAGACCATGTAGTTGAGGTCA-3’ (antisense); CD73 5’-GGACATTTGACCTCGTCCAAT-3’ (sense), 5’-GGGCACTCGACACT TGGTG-3’ (antisense); CD39 5’-AAGGTGAAGAGATTTTGCTCCAA-3’ (sense), 5’-TTTGTTCTGGGTCAGTCCCAC-3’ (antisense); A2AAR 5’-GCCATCCCATT CGCCATCA-3’ (sense), 5’-GCAATAGCCAAGAGGCTGAAGA-3’ (antisense); A2BAR 5’-AGCTAGAGACGCAAGACGC-3’ (sense), 5’-GTGGGGGTCTGT AATGCACT-3’ (antisense). The relative levels of expression were quantified and analyzed by the heat map.
Western blot analysis
Protein extracts were prepared according to the method described in the protein extract kit (Piece Biotechnology, Rockford, USA). Protein concentrations were measured using the BCA Protein assay kit. 40 μg proteins were separated by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis and transferred to nitrocellulose membrane. The membranes were incubated with primary antibody at 4°C overnight. HRP-conjugated secondary antibody was added and the blots were developed with the ECL plus kit.
Statistical analysis
All data were analyzed using Student’s t test or analysis of variance (ANOVA) as appropriate. Results were expressed as the mean ± S.D. P values ≤ 0.05 were considered significant.
Results
Sesamin attenuated renal damage and dysfunction after kidney ischemia-reperfusion
In order to evaluate the effect of sesamin on renal IRI, C57/BL6 mice were subjected to sham operation or warm ischemia (renal artery occlusion for 30 minutes) followed by a 24 h reperfusion after treatment with either PBS or sesamin (100 mg/kg). As expected, compared with control, IR induced significantly acute kidney injury, characterized by marked increases in serum creatinine (Scr) and BUN. Pretreatment with sesamin in IR-operated mice led to a remarkable reduction in the levels of Scr and BUN compared with the IR model group (Figure 1B and 1C).
Sesamin ameliorated renal damage and apoptosis after kidney ischemia-reperfusion
Kidney tissue morphology (H&E stain) was shown in Figure 2A. In the IR model, tubular dilatation, necrotic cells and brush border loss were clearly seen. However, pretreatment with sesamin obviously alleviated these pathological changes (Figure 2B). TUNEL staining and caspase 3 activity analysis showed that a significantly higher proportion of TUNEL-positive cells and increased caspase 3 activity were observed in the kidney tissues of IR-operated mice compared with control sham mice, whereas little TUNEL-positive cells and lower caspase 3 activity were detected in the kidney tissues of sesamin-treated mice after IR operation (Figure 2C and 2D).
Figure 2.

Sesamin reduced kidney damage and apoptosis induced by ischemic reperfusion. C57/BL6 mice (n = 6) were treated with vehicle or sesamin (100 mg/kg) every 8 h one time for three times within 24 h before surgery. Twenty-four hours after reperfusion, kidneys were collected for histological examination. A. Representative images of damages in the kidney (H&E staining, × 200). B. Statistical analysis of pathological damage scores. C. Representative images of apoptosis in the kidney (TUNEL staining, × 200). D. The activity of caspase-3 in the kidney. Each value is mean ± SD. *P<0.05, **P<0.01 compared with ischemic reperfusion.
Sesamin reduced renal inflammation after kidney ischemia-reperfusion
Because renal inflammation contributes to kidney IRI, which involves in excessive neutrophil infiltration and pro-inflammatory cytokine production, we assessed renal neutrophils and cytokines in each experimental group. Immunofluorescence with Ly6G antibody showed that massive Ly6G+ neutrophils appeared in the kidney tissues of mice performed by IR. However, sesamin pretreatment decreased the IR-induced infiltration of Ly6G+ neutrophils (Figure 3A). In addition, the MPO activity, the other marker of neutrophil, was also detected. It was markedly higher in IR group than that in the control group. Pretreatment with sesamin reduced the IRI-induced MPO activation (Figure 3B). Further, using the ELISA method, we evaluated the levels of TNF-α and IL-1β in the kidney tissues. As shown in Figure 3C and 3D, IR induced the significant increases in the levels of TNF-α and IL-1β in the kidney, which were markedly inhibited by sesamin.
Figure 3.
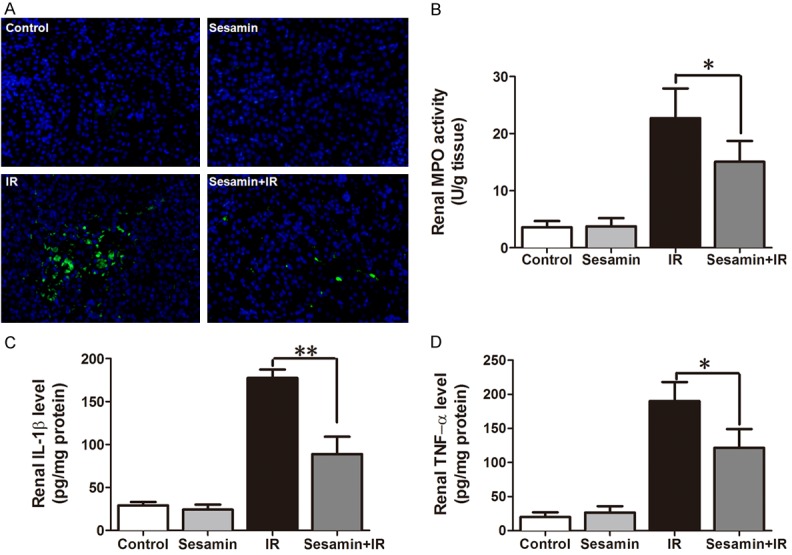
Sesamin alleviated neutrophil infiltration and proinflammatory cytokines production in the kidney of mice operated by ischemia reperfusion. C57/BL6 mice (n = 6) were pretreated with vehicle or sesamin (100 mg/kg) every 8 h one time for three times within 24 h before surgery. Twenty-four hours after reperfusion, kidneys were collected for Ly6G staining, MPO activity, and cytokines production. A. Representative images of Ly6G+ neutrophil in the kidney (immunofluorescence staining, × 200). B. MPO activity in the kidney tissues. C. The level of IL-1β in the kidney. D. The level of TNF-α in the kidney. Each value is mean ± SD. *P<0.05, **P<0.01 compared with ischemic reperfusion.
Sesamin promoted renal adenosine signaling after kidney ischemia-reperfusion
It has been reported that adenosine protects renal from IR injury. Thereby, we determined adenosine level in kidney tissue by HPLC. Although ischemia-reperfusion slightly induced the production of adenosine, this induction was markedly increased by sesamin. The level of adenosine was also induced by sesamin in the sham-operated kidneys compared to control mice (Figure 4A). Meantime, these key limit enzymes of adenosine production, such as CD39 and CD73, and its receptors A2AR were determined by qRT-PCR and western blotting. The results displayed that IR inhibited the expression of CD39, A2AAR, and A2BAR mRNA and protein, pretreatment with sesamin markedly promoted the expression of CD39, A2AAR, and A2BAR mRNA and protein in the kidney. However, there are no significant difference in the expression of CD73 mRNA and protein in four different groups (Figure 4B and 4C).
Figure 4.

Sesamin promoted adenosine production and upregulated the expression of CD39 and A2AR in the kidney after ischemic reperfusion. C57/BL6 mice (n = 6) were pretreated with vehicle or sesamin (100 mg/kg) every 8 h one time for three times within 24 h before surgery. Twenty-four hours after reperfusion, kidney tissues were collected for next analysis. A. The concentration of adenosine in the kidney was measured by HPLC. B. The mRNA expression of CD39, CD73 and A2AR in the kidney was evaluated by qRT-PCR. C. The protein expression of CD39, CD73 and A2AR in the kidney was detected by western blotting. Data are expressed as the mean ± SD. *P<0.05; **P<0.01.
CD39-Adenosine-A2AR contributed to the protective actions of sesamin on IRI
To examine whether adenosine signaling participated in the renalprotective action of sesamin in IR injury, we administered injection of the CD39 inhibitor or the A2AR antagonists, respectively, before IR surgery. Renal function analysis showed that these inhibitors or antagonists partly abrogated the beneficial actions of sesamin on the serum creatinine and BUN in IR-mice (Figure 5A and 5B). Consistence with these results, the improvement of sesamin on the pathological damage scores and caspase 3 activity was also reversed partly by the CD39 inhibitor or the A2AR antagonists, respectively (Figure 5C and 5D).
Figure 5.

CD39 inhibitor or A2AR antagonist reversed the protective actions of sesamin in IR-operated mice. C57/BL6 mice (n = 6) were treated with sesamin (100 mg/kg) every 8 h one time for three times within 24 h before surgery with or without POM-1 (CD39 inhibitor, 5 mg/kg), SCH-58261 (the A2AAR antagonist, 10 mg/kg), PSB115 (A2BAR antagonist, 10 mg/kg) 30 min before IR. Twenty-four hours after IR, blood and kidney tissues were collected. A. Concentration of serum creatinine. B. Concentration of BUN. C. The tubular damage scores. D. The caspase 3 activity. Data are expressed as the mean ± SD. *P<0.05; **P<0.01.
Discussion
IR-induced acute kidney injury (AKI) remains an unfailing clinical problem associated with high health care costs as well as very high mortality and morbidity [3,21]. There are no effective drugs or therapies for the treatment of AKI. Therefore, therapies to prevent or accelerate renal recovery during and after ischemic AKI are critically needed. The preliminary experiments suggested that sesamin is a promising preventive drug for IR-induced AKI. In this study, we found that sesamin significantly improved IR-induced kidney injury, as assessed by reduced Scr and BUN concentrations, ameliorated renal pathological damages, and alleviated tubular cell apoptosis, suggesting that sesamin may be a potential therapeutic agent for the treatment/prevention of acute renal IRI.
Renal IR injury leading to AKI involves a complex series of events, including renal tubular and endothelial necrosis and apoptosis, neutrophil infiltration, and pro-inflammatory cytokines release and production [3,5,22,23]. It is well known that renal tubular cell death and inflammation are hallmark traits of IRI [3]. Thereby, we explored the effects of sesamin on tubular cell death and inflammatory response caused by IR. The results showed that sesamin significantly not only alleviated tubular cell necrosis and apoptosis, but also inhibited renal neutrophil infiltration and pro-inflammatory cytokines production, which is consistent with published reports describing that sesamin exerts cytoprotective, anti-apoptosis, and anti-inflammatory properties [8,14,24].
Recent studies suggest that adenosine, an endogenous signaling molecule, plays a key role in protecting the kidney from ischemia-induced damage [25,26]. Adenosine is derived from the phosphohydrolysis of adenosine triphosphate (ATP) and adenosine monophosphate (AMP) by ecto-nucleoside triphosphate diphosphohydrolase-1 (CD39) and ecto-5’-nucleotidase (CD73) [26-31]. Extracellular adenosine signals are activated through G-protein coupled purinergic adenosine receptors (ARs), which includes A1AR, A2AAR, A2BAR and A3AR [32,33]. As a critical cytoprotective molecule, adenosine could inhibit renal tubular cell death including necrosis and apoptosis, and relieve inflammatory response in ischemic AKI. Previous studies have shown that ischemic AKI was worse in CD39, or CD73 deficient mice, alleviated in overexpression or activation of CD39 or CD73 [27,28,34], indicating that these key limit enzymes controlling adenosine production play important role in protecting from renal IRI. In addition, it has been found that A2AR could effectively alleviate inflammatory response and protect from tissues damages in some inflammatory diseases, and activation of A2AAR or A2BARs prevents against IR injury in several organs, including the kidney [35-38]. Thereby, the modulation of adenosine signal pathway might be major targets of novel drug for prophylactic or therapeutic intervention in IRI [26,39]. In fact, a previou study has reported that sesamin exhibits anti-apoptosis effect via the mechanisms that involve adenosine signaling [24]. In this study, we intriguingly found that sesamin pretreatment increased both adenosine production and CD39 and A2AR expression. Moreover, inhibition or antagonism of CD39, A2AAR and A2BAR reversed the beneficial effects of sesamin on IR-induced acute kidney injury. Thus, it is reasonable to assume that sesamin prevents kidney IRI by promoting adenosine signaling pathway.
In conclusion, the current findings demonstrate that sesamin protect the kidney from IRI by inhibiting tubular cell death and inflammatory response, further confirming that upregulating CD39-adenosine-A2AR signal contributes to the protection of sesamin.
Acknowledgements
This study is funded by National Science Foundation of China (No. 81200540).
Disclosure of conflict of interest
None.
References
- 1.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusch A, Hoff U, Bubalo G, Zhu Y, Fechner M, Schmidt-Ullrich R, Marko L, Muller DN, Schmidt-Ott KM, Gurgen D, Blum M, Schunck WH, Dragun D. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol (Oxf) 2013;208:25–40. doi: 10.1111/apha.12089. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J, Chen J, Xie L, Wang J, Feng C, Song J. Protective properties of sesamin against fluoride-induced oxidative stress and apoptosis in kidney of carp (Cyprinus carpio) via JNK signaling pathway. Aquat Toxicol. 2015;167:180–190. doi: 10.1016/j.aquatox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Kong X, Wang GD, Ma MZ, Deng RY, Guo LQ, Zhang JX, Yang JR, Su Q. Sesamin Ameliorates Advanced Glycation End Products-Induced Pancreatic beta-Cell Dysfunction and Apoptosis. Nutrients. 2015;7:4689–4704. doi: 10.3390/nu7064689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro EM, Chibli LA, Yamamoto CH, Pereira MC, Vilela FM, Rodarte MP, Pinto MA, do Amaral Mda P, Silverio MS, Araujo AL, de Araujo Ada L, Del-Vechio-Vieira G, de Sousa OV. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients. 2014;6:1931–1944. doi: 10.3390/nu6051931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu XQ, Kong X, Zhou Y, Huang K, Yang JR, Li XL. Sesamin exerts renoprotective effects by enhancing NO bioactivity in renovascular hypertensive rats fed with high-fat-sucrose diet. Eur J Pharmacol. 2012;683:231–237. doi: 10.1016/j.ejphar.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Yokota T, Matsuzaki Y, Koyama M, Hitomi T, Kawanaka M, Enoki-Konishi M, Okuyama Y, Takayasu J, Nishino H, Nishikawa A, Osawa T, Sakai T. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–1453. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujikawa T, Kanada N, Shimada A, Ogata M, Suzuki I, Hayashi I, Nakashima K. Effect of sesamin in Acanthopanax senticosus HARMS on behavioral dysfunction in rotenone-induced parkinsonian rats. Biol Pharm Bull. 2005;28:169–172. doi: 10.1248/bpb.28.169. [DOI] [PubMed] [Google Scholar]
- 12.Nakano D, Itoh C, Ishii F, Kawanishi H, Takaoka M, Kiso Y, Tsuruoka N, Tanaka T, Matsumura Y. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol Pharm Bull. 2003;26:1701–1705. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Gong X, Kuang G, Jiang R, Chen R, Wan J. Sesamin ameliorates lipopolysaccha-ride/d-galactosamine-induced fulminant hepatic failure by suppression of Toll-like receptor 4 signaling in mice. Biochem Biophys Res Commun. 2015;461:230–236. doi: 10.1016/j.bbrc.2015.03.154. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh CC, Kuo CH, Kuo HF, Chen YS, Wang SL, Chao D, Lee MS, Hung CH. Sesamin suppresses macrophage-derived chemokine expression in human monocytes via epigenetic regulation. Food Funct. 2014;5:2494–2500. doi: 10.1039/c4fo00322e. [DOI] [PubMed] [Google Scholar]
- 15.Wu WH, Wang SH, Kuan II, Kao YS, Wu PJ, Liang CJ, Chien HF, Kao CH, Huang CJ, Chen YL. Sesamin attenuates intercellular cell adhesion molecule-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in apolipoprotein-E-deficient mice. Mol Nutr Food Res. 2010;54:1340–1350. doi: 10.1002/mnfr.200900271. [DOI] [PubMed] [Google Scholar]
- 16.Lee WJ, Ou HC, Wu CM, Lee IT, Lin SY, Lin LY, Tsai KL, Lee SD, Sheu WH. Sesamin mitigates inflammation and oxidative stress in endothelial cells exposed to oxidized low-density lipoprotein. J Agric Food Chem. 2009;57:11406–11417. doi: 10.1021/jf902876p. [DOI] [PubMed] [Google Scholar]
- 17.Utsunomiya T, Shimada M, Rikimaru T, Hasegawa H, Yamashita Y, Hamatsu T, Yamasaki M, Kaku S, Yamada K, Sugimachi K. Antioxidant and anti-inflammatory effects of a diet supplemented with sesamin on hepatic ischemia-reperfusion injury in rats. Hepatogastroenterology. 2003;50:1609–1613. [PubMed] [Google Scholar]
- 18.Hou RC, Wu CC, Yang CH, Jeng KC. Protective effects of sesamin and sesamolin on murine BV-2 microglia cell line under hypoxia. Neurosci Lett. 2004;367:10–13. doi: 10.1016/j.neulet.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;724:231–238. doi: 10.1016/s0378-4347(98)00580-5. [DOI] [PubMed] [Google Scholar]
- 21.Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66:523–527. doi: 10.1111/j.1523-1755.2004.761_11.x. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Molitoris BA. Transitioning to therapy in ischemic acute renal failure. J Am Soc Nephrol. 2003;14:265–267. doi: 10.1097/01.asn.0000048852.53881.d9. [DOI] [PubMed] [Google Scholar]
- 24.Bournival J, Francoeur MA, Renaud J, Martinoli MG. Quercetin and sesamin protect neuronal PC12 cells from high-glucose-induced oxidation, nitrosative stress, and apoptosis. Rejuvenation Res. 2012;15:322–333. doi: 10.1089/rej.2011.1242. [DOI] [PubMed] [Google Scholar]
- 25.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 27.Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9:135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Ham A, Kim JY, Brown KM, D’Agati VD, Lee HT. The volatile anesthetic isoflurane induces ecto-5’-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013;84:90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 30.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5’-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 31.Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res. 2014;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 32.Rabadi MM, Lee HT. Adenosine receptors and renal ischaemia reperfusion injury. Acta Physiol (Oxf) 2015;213:222–231. doi: 10.1111/apha.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10:2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y, Piras BA, Kron IL, French BA, Yang Z. Adenosine 2B Receptor Activation Reduces Myocardial Reperfusion Injury by Promoting Anti-Inflammatory Macrophages Differentiation via PI3K/Akt Pathway. Oxid Med Cell Longev. 2015;2015:585297. doi: 10.1155/2015/585297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD. Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol. 2002;282:F10–18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- 39.Laubach VE, French BA, Okusa MD. Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin Ther Targets. 2011;15:103–118. doi: 10.1517/14728222.2011.541441. [DOI] [PMC free article] [PubMed] [Google Scholar]


