Abstract
The interleukin (IL)-12 family, composed of heterodimeric cytokines including IL-12 (formed by IL-12p35 and IL-12p40 subunits), IL-23 (formed by IL-23p19 and IL-12p40 subunits), IL-27 (formed by IL-27p28 and EBI3 subunits) and IL-35 (formed by IL-12p35 and EBI3 subunits), establishes a link between innate and adaptive immunity that involves different immune effector cells and cytokines to tumors. However, the role of IL-12 family in breast cancer (BC) progression and prognosis remains unclear. In the present study, we demonstrated evidence indicating that EBI3, IL-12p35 and IL-12p40 but not IL-23p19 or IL-27p28 were highly expressed in BC tissues, suggested that tumor derived EBI3, IL-12p35 and IL-12p40 were associated with tumor progression. Circulating IL-12 and IL-23 low expressed, but IL-27 and IL-35 high expressed in BC patients, especially circulating IL-23 associated with IL-35 to mediate BC tumor resection. Ki-67, p53 and EGFR expression on BC tissues, as well as CA125, CA153 and CA199 levels on BC bloods increased when circulating IL-23: IL-35 ratio decreased. Together, for the first time, our data suggest that circulating IL-23: IL-35 ratio may be an important indicator association with BC progression and prognosis. However, further research should be carried out to assess the implications of circulating IL-23: IL-35 ratio in a larger sample size.
Keywords: Breast cancer, interleukin (IL)-12, IL-23, IL-27, IL-35
Introduction
Recently, breast cancer (BC) is the most prevalent cancer among women worldwide, withan incidence rate of approximately 1.7 million cases per year and 0.5 million deaths per year [1]. Traditional prognostic parameters such as histological type, lymph node stage, nottingham prognosis index and serum tumor biomarkers are used in the assessment of BC outcomes. However, the survival outcomes of BC patients are still not optimistic. Early-stage BC has a favorable prognosis with a 5-year survival rate up to 90%, while this rate declines to 20% upon tumor spreading to distant organs [2]. Therefore, it has been necessary to identify an effective biomarker for more accurately predicting the prognosis of BC patients. Inflammation within the tumor microenvironment correlates with increased invasiveness and poor prognosis in many types of cancer, including BC [3]. Moreover, lots of clinical and experimental evidences indicate that the outcome of an immune response, tumor rejection or promotion, toward an evolving BC is largely determined by the type of immune response , chronic inflammation or acute inflammation elicited [4]. In recent decades much attention has focused on the uncovering of the role of cytokines in BC.
The interleukin (IL)-12 family, which is composed of heterodimeric cytokines including IL-12, IL-23, IL-27 and IL-35, establishes a link between innate and adaptive immunity that involves different immune effector cells and cytokines to tumors. However, the role of IL-12 family in BCprogression and prognosisremains unclear.
IL-12, formed by IL-12p35 and IL-12p40 subunits, is produced by activated antigen-presenting cells with an antitumor via inducing IFN-γ production by NK and T cells [5], shifting differentiation of naive Th0 cells toward the Th1 phenotype [6] and enhancing antibody dependent cellular cytotoxicity against tumor cells [7]. IL-12p35 subunit also can form IL-35 with Epstein-Barr virus-induced gene 3 (EBI3) subunit. However, IL-35 appears to have aprotumor rolethroughexpanding Tregs and inhibiting CD4+CD25- effector T cells [8], stimulating IL-35-producing CD1dhighCD5+ B cells mediated tumor cell proliferation [9], inhibiting apoptosis [10,11] and enhancing myeloid cell accumulation [12]. Similarly, IL-12p40 subunit also can form IL-23 with IL-23p19 subunit. IL-23 has also been reported to play a protumor role by promoting tumor cell epithelial-mesenchymal transition (EMT) [13], enhancing anti-apoptotic and drug resistance [14] and inducing tumor cell migration and invasion [15]. Notablely, IL-27, build by EBI3 and IL-27p28, has pleiotropic functions in the regulation of immune responses with both pro-inflammatory and anti-inflammatory properties. Therefore, IL-27 acts with a double-edged sword, both antitumor and protumor effects of IL-27 are conceivably expected depending on the type of cells that IL-27 stimulates and the tumor context [16,17].
Accordingly, to uncover the pleiotropic functions of IL-12 family cytokines within the BC tumor microenvironment and peripheral blood, our study was designed to evaluate prospectively the independent prognostic importance of circulating IL-12 family cytokines in patients with BC and the potential association with early cancer detection or disease monitoring.
Materials and methods
Subjects
A total of 65 BC patients with pathologically confirmed were collected at the Department of Surgery of Xiaolan Hospital of Southern Medical University, between December 2010 and July 2013. According to the World Health Organization guidelines, the tumor-node-metastasis (TNM) system of tumor stage and histological grade were performed, 53 patients (81.5%) were in T2, 4 patients (6.2%) were in T1, 5 patients (7.7%) were in T3 and 3 patients (4.6%) were in T4, and the demographic and clinical characteristics of the selected subjects were summarized in Table S1. Both cancer and normal tissues (>2 cm away from cancer tissues) were obtained from operative, and fixed in 10% buffered formalin and/or frozen immediately in liquid N2, stored at -80°C until use. Blood samples of BC patients were collected pre-operation and post-operation according Samy et al. reported [18]. For normal controls, 40 healthy volunteers (HV) were organized by the Medical Examination Center of Xiaolan Hospital of Southern Medical University, between December 2010 and July 2013. Serum aliquots (200 μL for each) were rapidly frozen and stored in a -80°C freezer until further analysis. There was no significant difference in age and gender between BC patients and control subjects. Patients who were male gender, diagnosed with no definitive surgery or excisional biopsy and received preoperativeblood transfusion, chemotherapy or hormonal therapy were excluded. The study was approved by the Internal Review and Ethics Boards of Guangdong Medical University, Xiaolan Hospital of Southern Medical University, and Dongguan Hospital Affiliated to Medical College of Jinan University, and informed consent was obtained from the parents.
Immunohistochemistry
Immunohistochemistry of 65 BC and matched normal tissues were performed to detect the expression of EBI3, IL-23p19, IL-27p28, IL-12p35, IL-12p40, Ki-67, p53 and EGFR. Briefly, tissue sections (4 μm) were prepared from formalin-fixed paraffinembedded tissue blocks were dewaxed in xylene and rehydrated in graded ethanols, and immersed in 0.3% Hydrogen peroxide solution for 10 min at room temperature to block the endogenous peroxidase activity, and washed by Phosphate Buffered Saline (PBS) solution. Antigenic epitopes were next retrieved by heating for 2 min in 10 mmol/L citrate buffer (pH 6.0). Then, the sections were incubated with the primary antibody for EBI3 (sc-32868, Santa Cruz Biotech, Inc.), IL-23p19 (sc-21083, Santa Cruz Biotech, Inc.), IL-12p35 (sc-7925, Santa Cruz Biotech, Inc.), IL-27p28 (sc-27487, Santa Cruz Biotech, Inc.), IL-12p40 (sc-7926, Santa Cruz Biotech, Inc.), Ki-67 (NCL-L-Ki67-MM1, Leica Novocastra), p53 (NCL-L-p53-DO7, Leica Novocastra) and EGFR (NCL-L-EGFR-384, Leica Novocastra) for 30 min at room temperature as our previously reported. Next, the sections were washed with PBS and followed by a goat anti-rabbit and mouse IgG-HRP (Kit-0015, Maixin Biotech, Fuzhou, China) secondary antibody for one hour at room temperature at 1:500 dilutions. In the end, the sectionswere visualized using DAB Detection Kit (Enhanced Polymer) (Kit-0015, Maixin Biotech, Fuzhou, China) and chromogenic reaction was controlled under a microscope (Nikon). After immunostaining, sections were immersed into hematoxylin for nuclear staining, then dehydrated through gradient concentrations of ethanol, cleared with xylene, and covered with neutral balsam. The score of immunohistochemical sections were assessed by two pathologists in a blinded fashion to the clinical status of the patients by Michalski and colleaguesas previously reported [19]. The immunoreactive area (percentage of positive staining cells) and intensity scores of proteins were evaluated.
Western blotting
Western blot analysis was performed to determine the expression levels of IL-12p35, IL-12p40, IL-23p19, IL-27p28 and EBI3 in BC tissues and matched normal tissues. Frozen tissues were ground in liquid N2 and lysed with RIPA lysis buffer (Beyotime, China) containing 0.5 M DTT, 0.1 M PMSF and 20× phosphatase inhibitor for 30 min on ice, then centrifuged at 12,000 g for 5 min at 4°C. The protein concentration in the supernatant was determined by BCA Protein Assay Kit (Beyotime, China). Equal amounts of total protein (~150 μg) were separated on 10% SDS-PAGE electrophoresis and transferred on to polyvinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, USA). The membranes were blocked in 5% nonfat milk, and hybridized with primary antibody overnight at 4°C, identified with a secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature. Immunoblots were visualized by enhanced chemiluminescence detection reagents (Millipore, Massachusetts, USA), with β-actin as a loading control.
ELISA analysis
Serum samples were collected from venous blood at room temperature and stored at -80°C until use. Serum IL-12, IL-23, IL-27 and IL-35 levels were measured using the Human IL-12 (p70) ELISA MAX™ Deluxe (Biolegend, San Diego, CA, USA), Pre-coated LEGEND MAX Human IL-23 ELISA Kit (Biolegend, San Diego, CA, USA), Pre-coated LEGEND MAX™ Human IL-27 ELISA Kit (Biolegend, San Diego, CA, USA), Pre-coated LEGEND MAX Human IL-35 ELISA Kit (Biolegend, San Diego, CA, USA), respectively, according to the manufacturer’s instructions. CA125, CA153 and CA199 were analyzed on UniCelDxI 800 immunoassay system (Beckman Coulter, Brea, CA).
Statistical analysis
The results are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Student’s t-test and Chi-square test were employed for analysis the differences of categorical variables. Pearson correlation was used to measure the degree of dependency between variables. A value of P<0.05 was deemed significant, and values of P<0.01 and P<0.001 were considered as highly significant.
Results
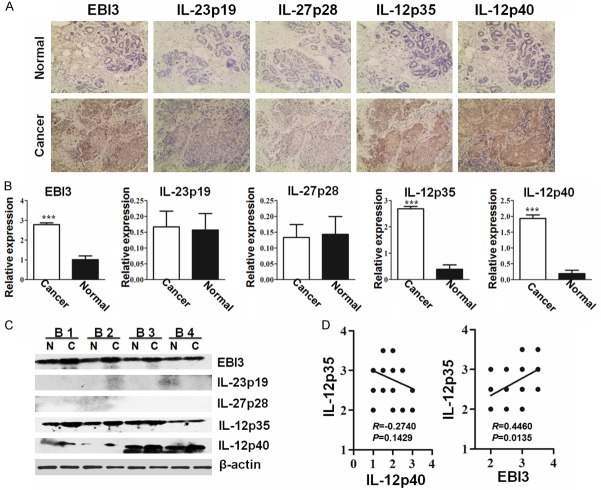
EBI3, IL-12p35 and IL-12p40 but not IL-23p19 or IL-27p28 are highly expressed in BC tissues
Our previously study showed that high expression of EBI3 and IL-12p35 are correlated to the severity of malignancy and the clinical stage of colorectal cancer [19]. However, the role of EBI3 and IL-12p35 expression on BC were not be elaborated. Therefore, we systemic evaluated EBI3 and IL-12p35 expression collaborative with IL-23p19, IL-27p28 and IL-12p40 expression on BC and matched normal tissues by immunohistochemical analysis. Results showed cancer sections from all patients displayed positive reactivity for EBI3, IL-12p35 and IL-12p40, but low or loss expression of IL-23p19 and IL-27p28 (Figure 1A). For quantitative analysis, the expression levels for EBI3, IL-12p35 and IL-12p40 in cancer tissues were shown signification higher expression than normal tissues (Figure 1B). However, there is no difference of IL-23p19 and IL-27p28 expression, respectively, between cancer and normal tissues. In order to further determine EBI3, IL-23p19, IL-27p28, IL-12p35 and IL-12p40 expression on cancer and normal tissues, western blot analysis was performed on 8 cases cancer and 8 cases matched normal tissues. In line with the above results, EBI3, IL-12p35 and IL-12p40 expression on cancer tissues were higher than matched normal tissues (Figure 1C). Of noted, no visible IL-23p19 and IL-27p28 expression were detected both on cancer and normal tissues (Figure 1C), suggesting tumor derived EBI3, IL-12p35 and IL-12p40, but not IL-23p19 or IL-27p28 associated with BC progression. Furthermore, IL-12p35 was noted to be positively correlated to EBI3 but not to IL-12p40 in BC tissues after Pearson correlation analysis (Figure 1D), suggesting tumor derived IL-35 but not IL-12 associated with BC progression. This results were consistent with the findings in colorectal cancer, pancreas cancer, gastric cancer that tumor derived IL-35 were associated with tumor progression [10,12,19-21].
Figure 1.
Quantitative analysis of EBI3, IL-23p19, IL-27p28, IL-12p35 and IL-12p40 expression in BC and normal tissues. Representative images (200×) for the immunohistochemical staining of EBI3, IL-23p19, IL-27p28, IL-12p35 and IL-12p40 in BC and normal tissues (A). Bar graphic figures showing the relative expression levels of EBI3, IL-23p19, IL-27p28, IL-12p35 and IL-12p40 assessed in all tissues (B). The expression of EBI3, IL-23p19, IL-27p28, IL-12p35 and IL-12p40 on normal tissues, shown with “N”, and cancer tissues, shown with “C” from representative BC patients (B1, B2, B3 and B4) were confirmed by western blot analysis (C). Results for correlation analysis of IL-12p35 with IL-12p40 and EBI3 in BC tissues (D). *, P<0.05; **, P<0.01; ***, P<0.001.
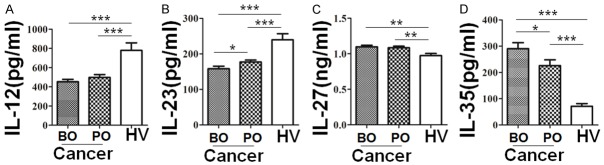
Circulating IL-12 and IL-23 low expressed, but IL-27 and IL-35 high expressed in BC patients
The above expression data prompted us to examine IL-12, IL-23, IL-27 and IL-35 levels in BC patients. For this purpose, we conducted ELISA analysis of IL-12, IL-23, IL-27 and IL-35 using bloods. In line with the expression data, IL-12 and IL-23 levels were lower in BC patients than that of control (Figure 2A and 2B), but IL-27 and IL-35 levels were higher in BC patients than that of health control (Figure 2C and 2D). More importantly, a significantlyrise for serum IL-23 but reduction for serum IL-35 were noted in all patientsafter tumor resection (Figure 2B and 2D). Altogether, those data suggested that serum IL-23 and IL-35 could be a valuable biomarker for assessing BC progression.
Figure 2.
Circulating IL-12, IL-23, IL-27 and IL-35 were detected in BC and healthy individuals serum by ELISA. Bar graphic figures showing circulating IL-12 (A), IL-23 (B), IL-27 (C) and IL-35 (D) levels in serum of 65 cases preoperative (BO) and postoperative (PO) BC patients, and 60 healthy volunteers (HV). *, P<0.05; **, P<0.01; ***, P<0.001.
Circulating IL-23 associated with IL-35 to mediate BC progression
Above results suggested that serum IL-23 and IL-35 could mediate BC progression. Next, the correlation between serum IL-23 and IL-35, also and the correlation among serum IL-12, IL-23, IL-27 and IL-35 both on BC patients and health control were analyzed by Pearson correlation analysis. Results shown serum IL-35 levels was noted to be negatively correlated to serum IL-23 levels in patient blood before surgery. However, this relationship was broken after tumor resection (Figure 3A). Conversely, positively correlation of serum IL-12 levels with serum IL-23 levels was broken in preoperative BC patients (Figure 3A). Of noted, there was no significantly correlation between serum IL-12 levels and serum IL-27 levels, serum IL-27 levels and serum IL-23 levels, serum IL-27 levels and serum IL-35 levels, and serum IL-12 levels and serum IL-35 levels both on preoperative and postoperative BC patients or healthy volunteers (Figure 3B and 3C). This results suggested that IL-23 may be associated with IL-35 to mediate BC progression.
Figure 3.
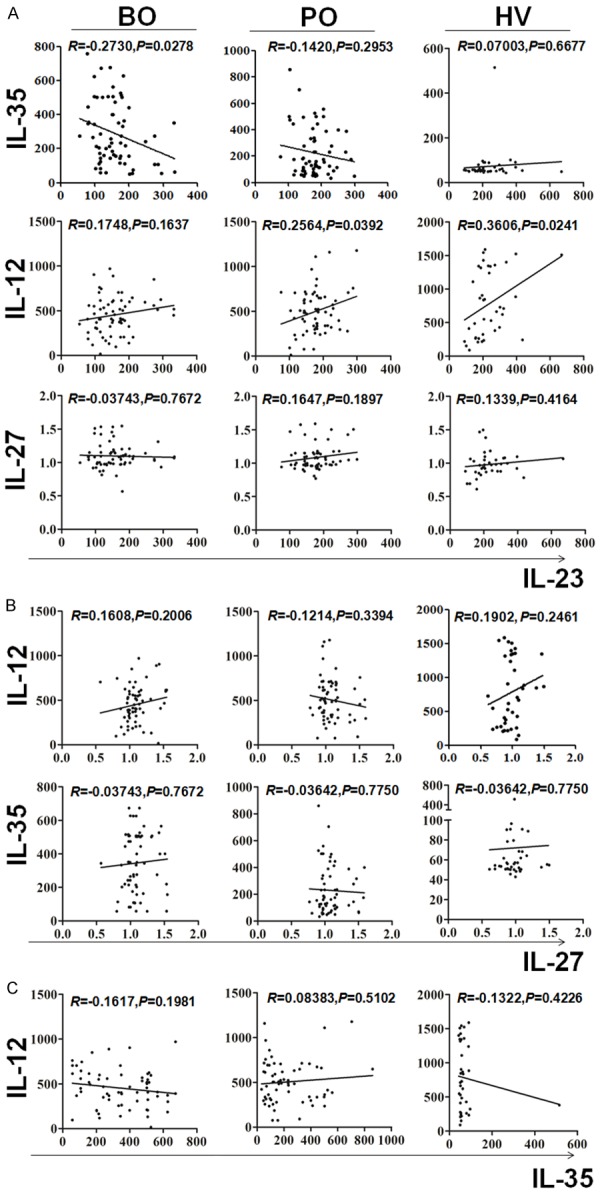
Relationship among the levels of IL-12, IL-23, IL-27 and IL-35 in serum of BC patientsand healthy individuals. Correlations among the levels of IL-23 with IL-12, IL-27 and IL-35 (A), the levels of IL-27 with IL-12 and IL-35 (B), and the levels of IL-35 with IL-12 (C) in preoperative (BO) and postoperative (PO) BC patients, and healthy volunteers (HV) were analyzed by Pearson correlation.
IL-23: IL-35 ratio in serum maybe a prognostic factor on BC patients
Given that circulating IL-23 may be associated with IL-35 to mediate BC progression, value of IL-23: IL-35 ratio was further evaluated. Meanwhile, value of IL-12: IL-23 ratio, IL-23: IL-27 ratio, IL-12: IL-27 ratio, IL-12: IL-35 ratio and IL-35: IL-27 ratio were also evaluated (Figure 4A-F). Results showed IL-23: IL-35 ratio (Figure 4B), IL-23: IL-27 ratio (Figure 4C), IL-12: IL-35 ratio (Figure 4D) and IL-12: IL-27 ratio (Figure 4E) in peripheral blood of BC patients were lower, but IL-35: IL-27 ratio (Figure 4F) were higher, than that of in healthy volunteers, respectively. Notably, only IL-23: IL-35 ratio had a guiding change in peripheral blood of BC patients after tumor resection (Figure 4B), suggesting IL-23: IL-35 ratio may play animportant value in tumor treatment. In addition, Ki-67, p53 and EGFR expression on cancer tissues from 16 cases BC patients with IL-23: IL-35 ratio decreased, and 16 cases BC patients with IL-23: IL-35 ratio >1.6 and increased 2-fold were retrospective analyzed (Figure 5A). As expected, cancer tissues from patients with IL-23: IL-35 ratio decreased showed higher expression of Ki-67, p53 and EGFR than that from patients with IL-23: IL-35 ratio >1.6 and increased 2-fold (Figure 5B), suggesting IL-23: IL-35 ratio may relate to BC progression and prognosis. Besides, CA125, CA153 and CA199 levels on BC bloods also show a increasing trend when IL-23: IL-35 ratio decreased, especially for CA125 (Figure 5C).
Figure 4.
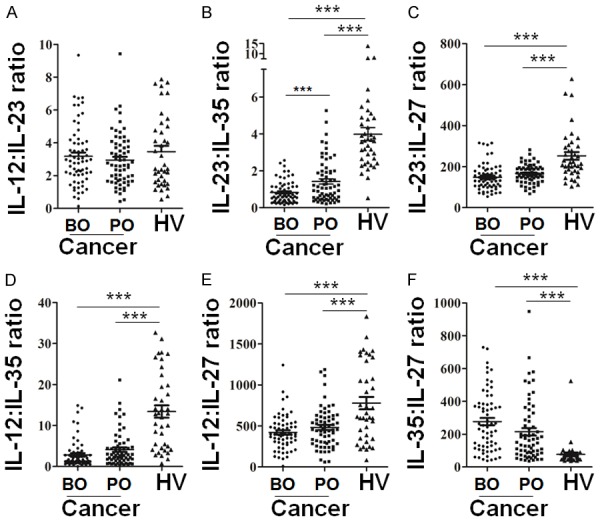
The expression ratio among IL-12, IL-23, IL-27 and IL-35 levels in BC patients and healthy individuals. The expression ratio of IL-12 to IL-23 (A), IL-23 to IL-35 (B), IL-23 to IL-27 (C), IL-12 to IL-35 (D), IL-12 to IL-27 (E), IL-35 to IL-27 (F) in preoperative (BO) and postoperative (PO) BC patients, and healthy volunteers (HV) were analyzed. *, P<0.05; **, P<0.01; ***, P<0.001.
Figure 5.

Ki-67, p53 and EGFR expression on BC tissues, as well as CA125, CA153 and CA199 levels on BC bloods increased when circulating IL-23: IL-35 ratio decreased. Before-after graph of 16 cases preoperative (BO) matched postoperative (PO) BC patients with IL-23: IL-35 ratio decreased, or with IL-23: IL-35 ratio >1.6 and increased 2-fold were used to display circulating IL-23: IL-35 ratio (A). Ki-67, p53 and EGFR expression between patients with IL-23: IL-35 ratio decreased (n=16) and patients with IL-23: IL-35 ratio >1.6 and increased 2-fold (n=16) were analyzed by immunohistochemical staining (B). CA125, CA153 and CA199 levels between patients with IL-23: IL-35 ratio decreased (n=16) and patients with IL-23: IL-35 ratio >1.6 and increased 2-fold (n=16) were also evaluated (C). *, P<0.05; **, P<0.01; ***, P<0.001: NS, no signification.
Together, our data suggested that tumor derived IL-35 associated with BC progression, and circulating low IL-23: IL-35 ratio promotes BC progression associated with poor prognosis.
Discussion
In the present study, we demonstrated evidence indicating that (1) EBI3, IL-12p35 and IL-12p40 but not IL-23p19 or IL-27p28 were highly expressed in BC tissues, suggested that tumor derived EBI3, IL-12p35 and IL-12p40 were associated with tumor progression. (2) Circulating IL-12 and IL-23 low expressed, but IL-27 and IL-35 high expressed in BC patients, especially circulating IL-23 associated with IL-35 to mediate BC tumor resection. (3) Ki-67, p53 and EGFR expression on BC tissues, as well as CA125, CA153 and CA199 levels on BC bloods increased when circulating IL-23: IL-35 ratio decreased.
Currently, the role of IL-23 in tumor progression is controversial [13,22-25]. It has been reported that IL-23 was known to be essential for Th17 cell survival and expansion, and for making pathogenic Th17 cells [26,27], which have antitumor effects by attracting CTL and NK cell migrating into the tumor suppressing tumor growth and metastasis [28]. On the other hand, it has also been reported that IL-23 contributed to EMT through the Wnt/β-catenin pathway [13], induced de novo gut tumorigenesis, through activation of innate lymphoid cells [29], promoting tumor migration and invasion [15,25]. In here, we established IL-12p40, a subunit of the IL-23 was high expression on BC tissues. However, IL-23p19, another subunits of IL-23 was low expression or could not be detected on BC tissues, suggesting IL-23 may play an antitumor effects in BC. Similar result has been reported inhuman lung adenocarcinoma and oral squamous cell carcinoma [30,31]. Low expression of circulating IL-23 in BC patients also supported this speculation. Meanwhile, Heckel et al. demonstrated that established breast tumor cell lines produced IL-12p40 to form monomer/homodimer, but not to form a heterodimer with the IL-12p35 subunit to build IL-12 [32]. Of noted, we also found IL-12p35 was noted to be positively correlated to EBI3 but not to IL-12p40 in BC tissues, consistent with our found in colorectal cancer [19]. It is different from low expression of circulating IL-23 in BC patients of our demonstrated, Gangemi et al.found IL-23 was significantly higher expression in peripheral blood of BC patients compared with the healthy controls [33]. These differences may be associated with the choice of specimens. 26% (13/50) patients, Sebastian et al. selected, were prior to receive first line chemotherapy [33], but the patients who received chemotherapy or hormonal therapy were excluded, and 81.5% (53/65) patients were in T2 stage in our study. We have observed circulating IL-23 was significantly elevated after tumor resection. Besides circulating IL-23, we also found circulating IL-12 had significantly lower expression but no relationship to tumor resection.
Different from controversial IL-23, now all present evidence supported tumor derived IL-35 promoting tumor growth and angiogenesis [10,12,19-21]. Here, we demonstrated EBI3 associated with IL-12p35, both of subunits of IL-35, were highly expressed in tumor tissues and mediated BC progression. Circulating IL-35 were also higher expression, similar to our previous reported in colorectal cancer [19], Jin et al. reported in pancreatic ductal adenocarcinoma [34], and Gu et al. reported in non-small cell lung cancer [35]. Additional, in contrast to the IL-23, circulating IL-35 was significantly decreased after tumor resection. Conversely, high level of the circulating IL-27 was no significant differences between preoperative and postoperative. Notably, circulating IL-35 levels was noted to be negatively correlated to circulating IL-23 levels in patient blood before surgery, and this relationship was broken after tumor resection, suggesting IL-23 associated with IL-35 to mediate BC progression. The IL-23: IL-35 ratio was significantly lower in serum of BC patients versus healthy volunteers and significantly rise by tumor resection. Although this study also found that IL-23: IL-27 ratio, IL-12: IL-35 ratio and IL-12: IL-27 ratio in peripheral blood of BC patients were lower, and IL-35: IL-27 ratio were significantly higher than that of in healthy volunteers, respectively, all had nothing to do with surgery treatment.
Interestingly, Ki-67, p53 and EGFR expression on cancer tissues from patients with IL-23: IL-35 ratio decreased were significantly higher than that from patients with IL-23: IL-35 ratio >1.6 and increased 2-fold. High expression of Ki-67, p53 and EGFR shows a more aggressive behavior and poor prognosis in BC [36-39]. Therefore, we demonstrated that circulating low IL-23: IL-35 cytokine ratio promotes progression associated with poor prognosis in BC. Meantime, tumor biomarkers CA125, CA153 and CA199 levels, for BC aided diagnosis on bloods [40], also increased when circulating IL-23: IL-35 ratio decreased in our observed.
Together, for the first time, our data suggest that circulating IL-23: IL-35 ratio may be an important indicator association with BC progression and prognosis. However, further research should be carried out to assess the implications of circulating IL-23: IL-35 ratio in a larger sample size.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81500007, 81272434); the Science and Technology Project of Guangdong Province (2014A020212298), the Medical Science Foundation of Guangdong Province (A2015206), the Science and Technology Project of Zhongshan (20113A026), the Science and Technology Fund of Guangdong Medical University (M2013046, M2014044), the National College Students’ innovative Entrepreneurial Training Program (201510571017), the College Students’ innovative Entrepreneurial Training Program of Guangdong Province (201510571049, XJ105711547, XJ105711454 and XJ105711459), and the College Students’ innovative experiment projects of Guangdong Medical University (2015ZYDC001, 2015ZYDC002, 2014ZZDC001 and 2014ZYDC007).
Supporting Information
References
- 1.Arteaga CL, Adamson PC, Engelman JA, Foti M, Gaynor RB, Hilsenbeck SG, Limburg PJ, Lowe SW, Mardis ER, Ramsey S, Rebbeck TR, Richardson AL, Rubin EH, Weiner GJ. AACR Cancer Progress Report 2014. Clin Cancer Res. 2014;20:S1–S112. doi: 10.1158/1078-0432.CCR-14-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlberg R, MacNamara B, Andersson M, Zheng C, Svensson A, Holm G, Hansson M, Porwit-MacDonald A, Bjorkholm M, Sundblad A. Stimulation of T-cell cytokine production and NK-cell function by IL-2, IFN-alpha and histamine treatment during remission of non-Hodgkin’s lymphoma. Hematol J. 2003;4:336–341. doi: 10.1038/sj.thj.6200320. [DOI] [PubMed] [Google Scholar]
- 6.Zeimet AG, Widschwendter M, Knabbe C, Fuchs D, Herold M, Muller-Holzner E, Daxenbichler G, Offner FA, Dapunt O, Marth C. Ascitic interleukin-12 is an independent prognostic factor in ovarian cancer. J. Clin. Oncol. 1998;16:1861–1868. doi: 10.1200/JCO.1998.16.5.1861. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Lee FT, Hanai N, Smyth FE, Burgess AW, Old LJ, Scott AM. Cytokine enhancement of in vitro antibody-dependent cellular cytotoxicity mediated by chimeric anti-GD3 monoclonal antibody KM871. Cancer Immun. 2002;2:13. [PubMed] [Google Scholar]
- 8.Tao Q, Pan Y, Wang Y, Wang H, Xiong S, Li Q, Wang J, Tao L, Wang Z, Wu F, Zhang R, Zhai Z. Regulatory T cells-derived IL-35 promotes the growth of adult acute myeloid leukemia blasts. Int J Cancer. 2015;137:2384–2393. doi: 10.1002/ijc.29563. [DOI] [PubMed] [Google Scholar]
- 9.Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL-35 producing B cells promote the development of pancreatic neoplasia. Cancer Discov. 2016;6:247–255. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholl MB, Ledgewood CL, Chen X, Bai Q, Qin C, Cook KM, Herrick EJ, Diaz-Arias A, Moore BJ, Fang Y. IL-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: evidence for a role as an autocrine growth factor. Cytokine. 2014;70:126–133. doi: 10.1016/j.cyto.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun. 2013;430:364–369. doi: 10.1016/j.bbrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol. 2013;190:2415–2423. doi: 10.4049/jimmunol.1202535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Li W, Liu S, Su Y, Han G, Xu C, Liu H, Zheng T, Zhou Y, Mao C. Interleukin-23 promotes the epithelial-mesenchymal transition of oesophageal carcinoma cells via the Wnt/beta-catenin pathway. Sci Rep. 2015;5:8604. doi: 10.1038/srep08604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Q, Su Y, Zhou Y, Zhu H, Yang X, Xu J. Interleukin-23 strengthens the anti-apoptotic and drug resistance of human tongue squamous cell carcinoma through the Wingless-related integration site/beta-catenin pathway. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:249–254. doi: 10.7518/hxkq.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei Z, Chen S, Chen C, Xiao B, Li F, Wang Y, Tao Z. Interleukin-23 Facilitates Thyroid Cancer Cell Migration and Invasion by Inhibiting SOCS4 Expression via MicroRNA-25. PLoS One. 2015;10:e0139456. doi: 10.1371/journal.pone.0139456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Chiba Y, Furusawa J, Xu M, Tsunoda R, Higuchi K, Mizoguchi I. Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci. 2015;106:1103–1110. doi: 10.1111/cas.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MS, Liu Z, Liu JQ, Zhu X, Liu Z, Bai XF. The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy. 2015;7:191–200. doi: 10.2217/imt.14.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: a short follow-up. Cancer Biomark. 2010;6:63–72. doi: 10.3233/CBM-2009-0119. [DOI] [PubMed] [Google Scholar]
- 19.Zeng JC, Zhang Z, Li TY, Liang YF, Wang HM, Bao JJ, Zhang JA, Wang WD, Xiang WY, Kong B, Wang ZY, Wu BH, Chen XD, He L, Zhang S, Wang CY, Xu JF. Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol. 2013;6:1806–1816. [PMC free article] [PubMed] [Google Scholar]
- 20.Liao KL, Bai XF, Friedman A. Mathematical modeling of Interleukin-35 promoting tumor growth and angiogenesis. PLoS One. 2014;9:e110126. doi: 10.1371/journal.pone.0110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan YG, Zhai JM, Wang W, Feng B, Yao GL, An YH, Zeng C. IL-35 over-expression is associated with genesis of gastric cancer. Asian Pac J Cancer Prev. 2015;16:2845–2849. doi: 10.7314/apjcp.2015.16.7.2845. [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol. 2010;2010:832454. doi: 10.1155/2010/832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian X, Gu L, Ning H, Zhang Y, Hsueh EC, Fu M, Hu X, Wei L, Hoft DF, Liu J. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J Immunol. 2013;190:5894–5902. doi: 10.4049/jimmunol.1203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Li J, Li L, Zhang J, Wang X, Yang C, Li Y, Lan F, Lin P. IL-23 selectively promotes the metastasis of colorectal carcinoma cells with impaired Socs3 expression via the STAT5 pathway. Carcinogenesis. 2014;35:1330–1340. doi: 10.1093/carcin/bgu017. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zhang L, Zhang J, Wei Y, Li K, Huang L, Zhang S, Gao B, Wang X, Lin P. Interleukin 23 regulates proliferation of lung cancer cells in a concentration-dependent way in association with the interleukin-23 receptor. Carcinogenesis. 2013;34:658–666. doi: 10.1093/carcin/bgs384. [DOI] [PubMed] [Google Scholar]
- 26.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 27.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 28.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S, Ayanoglu G, Chang C, Basham B, Mumm JB, Pierce RH, Yearley JH, McClanahan TK, Phillips JH, Cua DJ, Bowman EP, Kastelein RA, LaFace D. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014;7:842–856. doi: 10.1038/mi.2013.101. [DOI] [PubMed] [Google Scholar]
- 30.Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda M, Ehara M, Suzuki S, Ohmori Y, Sakashita H. IL-23 promotes growth and proliferation in human squamous cell carcinoma of the oral cavity. Int J Oncol. 2010;36:1355–1365. doi: 10.3892/ijo_00000620. [DOI] [PubMed] [Google Scholar]
- 32.Heckel MC, Wolfson A, Slachta CA, Schwarting R, Salgame P, Katsetos CD, Platsoucas CD. Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol. 2011;266:143–153. doi: 10.1016/j.cellimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Gangemi S, Minciullo P, Adamo B, Franchina T, Ricciardi GR, Ferraro M, Briguglio R, Toscano G, Saitta S, Adamo V. Clinical significance of circulating interleukin-23 as a prognostic factor in breast cancer patients. J Cell Biochem. 2012;113:2122–2125. doi: 10.1002/jcb.24083. [DOI] [PubMed] [Google Scholar]
- 34.Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol. 2014;75:29–33. doi: 10.1016/j.humimm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Gu X, Tian T, Zhang B, Liu Y, Yuan C, Shao L, Guo Y, Fan K. Elevated plasma interleukin-35 levels predict poor prognosis in patients with non-small cell lung cancer. Tumour Biol. 2015;36:2651–2656. doi: 10.1007/s13277-014-2887-8. [DOI] [PubMed] [Google Scholar]
- 36.Yin Y, Zeng K, Wu M, Ding Y, Zhao M, Chen Q. The levels of Ki-67 positive are positively associated with lymph node metastasis in invasive ductal breast cancer. Cell Biochem Biophys. 2014;70:1145–1151. doi: 10.1007/s12013-014-0034-1. [DOI] [PubMed] [Google Scholar]
- 37.Li FY, Wu SG, Zhou J, Sun JY, Lin Q, Lin HX, Guan XX, He ZY. Prognostic value of Ki-67 in breast cancer patients with positive axillary lymph nodes: a retrospective cohort study. PLoS One. 2014;9:e87264. doi: 10.1371/journal.pone.0087264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Yuan JP, Wei W, Tu Y, Yao F, Yang XQ, Sun JZ, Sun SR, Li Y. Subtype classification for prediction of prognosis of breast cancer from a biomarker panel: correlations and indications. Int J Nanomedicine. 2014;9:1039–1048. doi: 10.2147/IJN.S58270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Zhang X, Zhao S, Wang Y, Di W, Zhao G, Yang M, Zhang Q. Prognostic value of survivin and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Target Oncol. 2014;9:349–357. doi: 10.1007/s11523-013-0300-y. [DOI] [PubMed] [Google Scholar]
- 40.Wang XF, Wu YH, Wang MS, Wang YS. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev. 2014;15:363–368. doi: 10.7314/apjcp.2014.15.1.363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.