Abstract
Recent researches indicate that the mechanism of anesthetic induce loss of consciousness (LOC) is related to dopamine dysfunction in the media prefrontal cortex (mPFC). Given GABAA receptors are the main target for commonly intravenous anesthetic propofol, in this study, we test whether that propofol induced LOC mediate by GABAA receptors in mPFC through altering the dopamine and its metabolites. In the present study, we use Loss of righting reflex (LORR) and Recovery of righting reflex (RORR) as measure to respectively reflect the status of unconsciousness and consciousness recovery in rats. We imitate the clinical anesthesia process, found the minimum of induction and maintenance concentration of propofol respectively was 11 mg/kg and 40 mg/kg per hour. Then, microdialysis technique was used to observe the change of dopamine (DA), metabolites 3, 4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) before and after intravenous infusion of propofol from caudal vein of freely moving rats. The results showed that propofol can increase the level of DOPAC except HVA, and reduced the level of DA in mPFC during unconsciousness of rats. DOPAC and DA return to the baseline level when the rats began to regain consciousness. Local reverse dialysis infusion of GABAA receptor antagonist GABAzine (50 uM) in mPFC can promote the time of LORR, reduce the time of RORR, and increase the basal level of DOPAC. With this condition, propofol increased HVA instead of DOPAC, whereas the DA was still reduced. These results suggest that propofol may induce unconsciousness by directly inhibiting dopamine release in the mPFC, and this effect does not be mediated by GABAA receptor in mPFC.
Keywords: Propofol, media prefrontal cortex, dopamine, metabolites, GABAA receptor, microdialysis
Instruction
Propofol is a commonly intravenous anesthetic that has been used in clinical for anesthetization during surgical procedures. The critical feature of it is inducing LOC for patients [1]. Although the mechanisms of general anesthesia inducing loss of consciousness (LOC) have been studied since the 19th century, at the moment it is still unclear.
There is a view that propofol is working by influence on multiple distinct brain regions. It was evidence showed that when propofol immediately induced LOC, the neuronal activity was depressed, while thalamic activity seemed to be exsited [2]. Subsequent research demonstrated that a direct effect of propofol on cortical dynamics by a decrease in backward connectivity from frontal to parietal cortices [3]. More importantly, in human, study during anesthesia performed with functional near-infrared system (fNIRS) have suggested that PFC (prefrontal cortex) is associated with suppression and emergence of consciousness. In a more in-depth analysis of PFC regions, only mPFC (media PFC) showed significant changes during both induction and emergence phases [4]. Therefore, mPFC may be the important target area of propofol to induce LOC by affecting integration of brain information.
In addition, studies showed that the neurotransmitter dopamine (DA) and its metabolites are associated with general anesthesia. For example, the intracerebroventricularly injection of orexin-A decreased the time of emergence from propofol anesthesia associated with increases the levels of dopamine in the central [5]. Propofol intravenous infusion significantly increased the somatosensory cortex extracellular levels of the DA metabolites, 3, 4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) of rats [6]. Thus, it follows that dopamine activity is association with consciousness switch of anesthesia.
Besides, because researcher proved that the mPFC could modulates DA neuronal function of ventraltegmental area (VTA) through excitatory or inhibitory innervations on VTA DA neurons [7]. Therefore, we have reason to think anesthetic may be through complex mechanisms to modulate the dopamine activity of mPFC to regulate VTA nuclei activity. However, until now, no one literature demonstrate that the effects of propofol on the dopamine system involving neurotransmitters DA, metabolites DOPAC and HVA in mPFC during the whole process from induction to recovery phase, and whether dopamine activity changes of mPFC have relationship with LOC.
Since it is known that GABAA receptors is the main action for propofol [8,9]. There was research by reverse dialysis of bicuculline (10-100 microM) increased dopamine efflux, which indicated that GABAA receptors may regulate dopamine system [10]. We first aimed to observe the effects of propofol on neurotransmitters DA, DOPAC and HVA in mPFC during the whole process of anesthesia, and investigate that whether the effects is correlate with GABAA receptors of intra mPFC by using reverse microdialysis technique in this study. We hope to provide new clue for the mechanism of general anesthesia inducing unconsciousness.
Materials and methods
Animals
Adult male Sprague - Dawley rats (280-350 g) were housed 3-4 per cage in a controlled 12:12 h light-dark cycle and had free access to food and water at 22°C. After implanting guide for microdialysis experimental, the rat was housed one per cage. All experimental procedures were approved by the Animal Ethics Committee of the University of Zunyi Medical College, China. The animals were cared for in accordance with the “Guide for the care and use of laboratory animals” in China.
The criterion of LORR and RORR
In this study, we use Loss of righting reflex (LORR) and Recovery of righting reflex (RORR) as primary measure to respectively reflect the status of suppression and emergence of consciousness in rat. The rat was considered to have LORR if it did not turn itself prone onto all four limbs within 30 s [11]. The RORR is the opposite of LORR.
Propofol concentration of induction and maintenance
Rats (n = 90) were used to determine the minimum concentration of LORR (Loss of righting reflex) of propofol induction through caudal vein. The concentration group was beginning at 7 mg/kg and increment by 1 until 15 mg/kg. And on the basis of finding out the minimum effective concentration of propofol for LORR, we further choose rats (n = 60) to find out the minimum effective maintenance concentration of propofol. The maintenance concentration group was beginning at 20 mg/kg/h and increment by 10 mg/kg/h until 60 mg/kg/h. Each concentration group includes ten rats.
Surgical preparation
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.). Under this condition, each rat was stereotaxically implanted with a guide cannual (CXG-4, Eicom, Japan) into the mPFC (+ 3.2 mm anterior and + 0.6 mm lateral to bregam, 2.0 mm below dura) according to the Brain Atlas of the Paxinos and Watson [12] and fixed with two screws and dental cement. The matching dummy guide (CXD-4, Eicom, Japan) was used to seal the cannual. Subcutaneous injection of penicillin 800,000 units for prevention of infection.
Microdialysis
We performed all microdialysis experiments between 9:00 and 16:00 when the rat was recovery two days after operation and under the condition of quiet at 22°C. Each rat was only for once experiment. According to whether intravenous infusion (iv) of propofol or mPFC local reverse dialysis GABAA receptor antagonist GABAzine (50 uM), Microdialysis rats were randomly divided into three groups (n = 6 in each group). They respectively were saline group, propofol group and GABAzine propofol group. At the beginning of the experiment, we placed the animal in a Plexiglas box (Eicom, Japan) to accommodate the environment. Rat can move freely in the box. Microdialysis probe (CX-I- 4-3, cutoff 50,000 Daltons, Eicom, Japan) was then inserted through the guide cannual under light anesthesia with 2% isoflurane in oxygen and caudal vein was cannulated for intravenous infusion of drugs in 5 min. The probe of saline group and propofol group was constant perfused with ACSF (artificial cerebrospinal fluid, 147 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 0.8-1.2 mM MgCl2, adjust PH = 7.4 with NaOH) at the flow rate of 2 ul/min using pump (ESP-32, Eicom, Japan) . The GABAzine propofol group was perfused with 50 uM GABAzine until the experiment is over. After the probe was equilibrate for 2 h, consecutive four dialysate samples were collected every 30 min to measure the mean values of DA, DOPAC and HVA of awareness. The mean values as the basal values. Next, recording the time of LORR after the rat received an intravenous induction 11 mg/kg of propofol followed by a continuous infusing an hour of 40 mg/kg/h propofol and collect two samples to observe dopamine activity dynamic changes in mPFC. Then, stopping infusion propofol and recording the RORR time. Meanwhile, continue collecting three samples after emergence to compare the level of dopamine and metabolites with awareness and RORR.
HPLC electrochemical detection
Dialysates were collected every 30 min as described above, and immediately absorbed by autosampler (ESA - Model 542) and analyzed by high-performance liquid chromatography with electrochemical detector (ESA - Coulochem III) using a C18 column (Quattro 150 × 2.1 mm). We set guard cell potential at + 300 mV, graphite electrode at + 220 mV. The mobile phase include the following (mM): 90 NaH2PO4·H2O, 50 monohydrate citric acid, 1.7 1-Octanesulfonic acid sodium salt, 0.05 EDTA, 8.75% acetonitrile, adjust PH to 3.0 with phosphoric acid. The flow rate was 0.21 ml/min. The whole dialysate (30 ul) of each sample was injected to the column. The minimum detectable concentration of ESA is 10 pg/ml. We used 0.005 ug/ml DHBA as internal standard by pump (ESP-32, Eicom, Japan) and mixed with dialysate through three-way pipe in the end. 0.0001, 0.001, 0.025, 0.005, 0.05 ug/ml concentrations DOPAC, DA and HVA mixed with HClO4 solution were utilized to establish the calibration curve. The recovery of microdialysis probe was obtained with using vitro dialysis method by dividing the concentrations after dialysis by the concentration that we knew before dialysis.
Histology
After the microdialysis experiment, animals were anesthetized with isoflurane and transcardially perfused with 0.9% saline probably 500 milliliter (ml), followed by paraformaldehyde (4%) at PH = 7.4 until the caudal to harder. Brain then fixed in 4% paraformaldehyde overnight at 4°C. Gradient auto dehydrated were performed by Leica TP1020. Next, brain was embedded, sectioned, and HE stained. The placement of the guide cannual and the microdialysis probe in the mPFC is illustrated in the Figure 1. The data will be excluded if the probe position is not in the mPFC.
Figure 1.
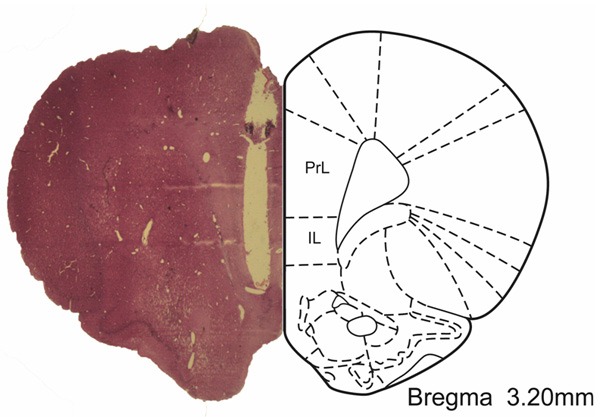
Representative histological stain of the placement of the guide cannual and the microdialysis probe in brain slice (2 um thickness). The mPFC is shown in the picture.
Drug
Propofol was obtained from AstraZeneca of Italy. GABAzine, NaH2PO4·H2O, monohydrate citric acid, 1-Octanesulfonic acid sodium salt, EDTA, phosphoric acid were purchased from Sigma (St Louis, MO, USA). Acetonitrile was purchased from Tedia of U.S.A. DOPAC, DA, HVA were purchased from Fluka.
Statistical analysis
The incidence of LORR was compared between different concentrations groups with Chi-Square Tests followed by Fisher’s Exact test. The times of LORR and RORR and the basal mean values between the groups by using the independent samples t test. The basal mean values were taken as 100%. And all the following values were related to these averaged. The means values within the group were analyzed by ANOVA for repeated measure with Dunnett post hoc tests. All data were presented as mean ± SD, P < 0.05 was considered statistically significant.
Results
Propofol induce the incidence of LORR at different concentrations
We found that from 7 to 11 (mg/kg) propofol dose-dependently increased the incidence of LORR in rats. The incidence of LORR about 10 mg/kg group was 50% compared with 100% of 11 mg/kg group (P = 0.033) (Figure 2A). So, we further chose propofol 11 mg/kg as the induction concentration (iv), observed that propofol concentration-dependently maintain the incidence of LORR more than one hour from 20 to 40 mg/kg/h (P < 0.001). There were no significantly between the groups 40, 50 and 60 (mg/kg/h). The incidence of LORR reached 100% at minimum effective maintenance concentration 40 mg/kg/h (Figure 2B). Because the mortality increased with the increase of concentration of propofol, therefore, we imitate the clinical anesthesia process, using the minimum concentration of 11 mg/kg and 40 mg/kg/h for induction and maintenance to observe the changes of DA, DOPAC, and HVA during the whole process of propofol inducing unconsciousness.
Figure 2.
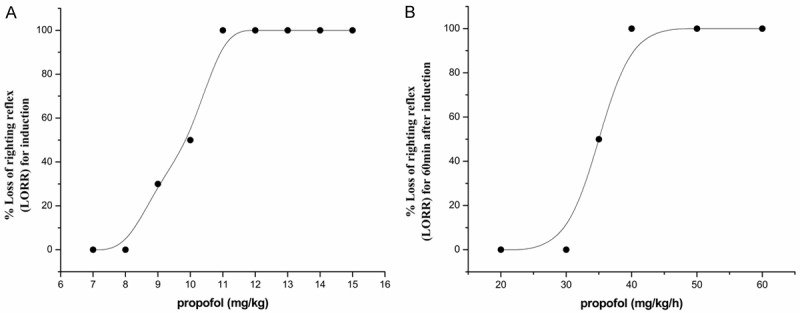
Effects of propofol on the incidence of LORR at different induced concentrations (A) and maintenance concentrations (B). Results are the form of rate value. Each concentration group n = 10.
The different effects between the propofol group and GABAzine group on the time of LORR and RORR
To observe the effect of propofol on the consciousness state of rats, and investigate the participating role of the mPFC GABAA receptor. Rats were local mPFC reverse dialyzed with ACSF or GABAzine before propofol infusion. Our results showed that GABAzine can significantly delayed the induction time of propofol anesthesia (35.67 ± 7.41 s vs 21.17 ± 4.57 s, P < 0.001) (Figure 3A), and shorten the time of the emergence from propofol. (376.5 ± 59.21 s vs 638.00 ± 102.06 s, P < 0.001) (Figure 3B).
Figure 3.
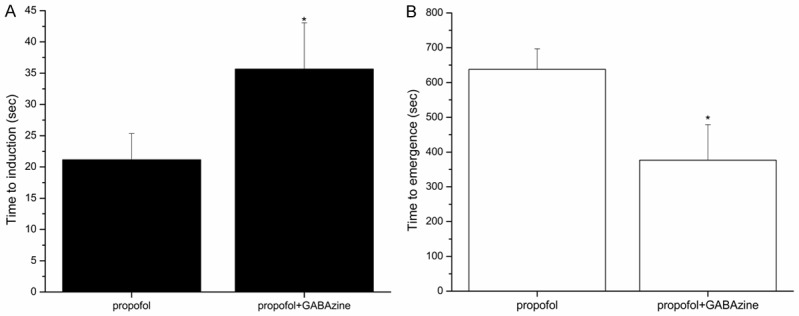
Effects of GABAA antagonist, GABAzine (50 uM), on the induction (A) and emergence (B) time of propofol anesthesia. Reverse dialyzed GABAzine in Local mPFC of rat, increased the time of induction and decrease the time of emergence from anesthesia. Results are the mean ± SD, n = 6 in each group. *P < 0.05.
Effects of propofol or plus GABAzine on the DA, DOPAC, HVA in the mPFC
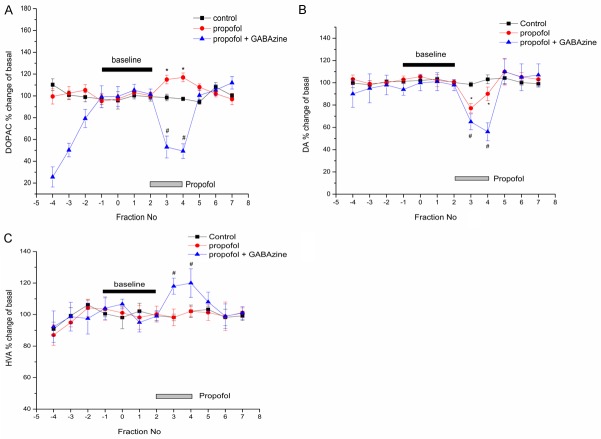
DOPAC, DA and HVA standard calibration curve is order (Figure 4). We calculate the neurotransmitters basal level in mPFC by the following formula: Area ratio = As/Ar, Concentration ratio = Cs/Cr, f = Area ratio/Concentration ratio. Ci = f × Ai/(As/Cs). As: the peak area of internal standard DHBA; Ar: the peak area of standard concentration substance; Cs: the concentration of internal standard DHBA; Cr: the concentration of standard concentration substance; Ci: the concentration of neurotransmitter in mPFC; Ai: the peak area of neurotransmitter in mPFC; f is the correction factor. The results showed that DA, DOPAC, HVA relative basal value in mPFC (Table 1). We found that the rat with GABAzine before propofol increased extracellular DOPAC by about 131.14% ± 22.35% compared to ACSF (P < 0.001). However, GABAzine failed to alter the basal values of DA and HVA (P > 0.05).
Figure 4.
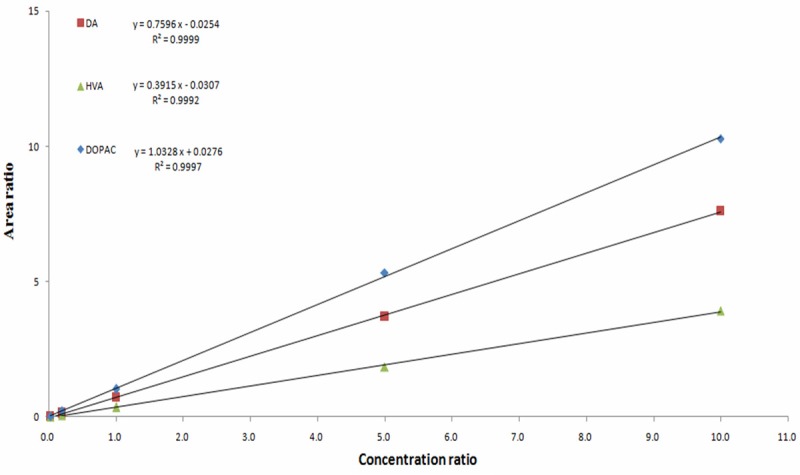
0.0001, 0.001, 0.025, 0.005, 0.05 ug/ml concentrations DOPAC, DA and HVA mixed with HClO4 solution and 0.005 ug/ml inter standard DHBA were utilized to establish the calibration curve. The picture showed that DOPAC, DA and HVA standard calibration curve is order.
Table 1.
Extracellular DOPAC, DA and HVA basal levels in the mPFC of rats
| DOPAC (nM) | DA (nM) | HVA (nM) | |
|---|---|---|---|
| Dialysis values (ACSF group) | 25.85 ± 7.94 | 1.41 ± 0.35 | 115.41 ± 22.76 |
| Dialysis values (GABAzine group) | 56.69 ± 6.58 | 1.37 ± 0.69 | 132.76 ± 34.76 |
| Mean Probe recovery (%) | 12.0 | 14.1 | 11.4 |
| Basal values (ACSF group) | 215.42 ± 66.17 | 10.00 ± 2.48 | 1012.37 ± 119.65 |
| Basal values (GABAzine group) | 472.42 ± 54.83* | 9.71 ± 4.89 | 1164.56 ± 304.91 |
Data shown are the mean ± SD. We obtained the probes recovery by using 3 mm membrane length and at the 2 ul/min flow rate. Basal values = dialysis values/mean probe recovery. Significant difference between GABAzine- and ACSF- treated rats;
P < 0.05.
Comparing with saline group, Intravenous infusion of propofol increased extracellular DOPAC level by 10.30 ± 2.57% (P = 0.027) (Figure 5A), decreased DA level by 21.86 ± 3.35% (P = 0.003) (Figure 5B). When we stopped infusion propofol, the DOPAC and DA return to the baseline level. The DOPAC and DA had no significant difference during the periods of awaking compared to emergence (P = 0.996). Meanwhile, we didn’t find any significant change of HVA among these three different periods (P > 0.05).
Figure 5.
Effects of propofol on the extracellular concentration of DA and its metabolites DOPAC and HVA. Each graph showed the concentration of neurotransmitters expressed as the percentage of baseline. We defined the baseline concentration is the mean of four consecutive values before intravenous infusion of propofol. X-axis represents the number of collecting dialysate. Dialysate fraction was collected every 30 min. *p < 0.05 against saline group value at each fraction. Values are expressed as mean ± SD, and n = 6 per each group.
However, within GABAzine group, propofol can decrease extracellular DOPAC (Figure 5A) and DA (Figure 5B) values by 46.91 ± 3.77% (P < 0.001) and 39.71 ± 3.67 (P < 0.001). The HVA values were raised about 14.53 ± 5.7% (P = 0.029) by propofol (Figure 5C). After stopping infusion of propofol, the level of DOPAC and DA was increased by 54.59 ± 5.12% (P < 0.001) and 49.85 ± 8.34 (P = 0.001) versus to propofol times. At the same time, the HVA decreased to baseline level.
Discussion
It is well known that neuromodulator in extracellular fluid modulate the state of neuronal circuits with temporal and spatial specificity [13]. Given dopamine has been believed as an important neurotransmitter for modulating the anesthesia and arousal [14], in this study, we utilized the microdialysis technique first to demonstrate that the effect of propofol on dopamine and its metabolism levels of extracellular fluid in mPFC in one study. We found that propofol induced unconsciousness with the reduction DA and increase the extracellular fluid DOPAC level. Moreover, this effect has disappeared when we remove the propofol. This change was correlated with consciousness state transformation. We believed that propofol inducing unconsciousness is association with increasing the catabolism of DA into DOPAC.
In early 1997, Ming-Hwang Shyr group has assessed the metabolites level of dopamine and serotonin in the somatosensory cortex of rats receiving propofol using microdialysis. In their experiment, the rat was infused intravenously with propofol at a rate of 60 mg·kg-1·h-1 for 40 min. They found that propofol significantly increased DOPAC, HVA. However, we didn’t find any significantly changes of dopamine metabolite HVA in mPFC, perhaps the effect of propofol on the somatosensory cortex is not same as the mPFC and different effects displayed in different brain areas.Moreover, in our study, we gave the induction doses to rat first for clinical imitation, therefore the righting reflex disappeared in a short time that we couldn’t observe the excitation period. We think the effect of propofol on HVA of mPFC may be happened in the excitation period.
Next, we blocked the GABAA receptor in mPFC using reverse dialysis infusion of competitive GABAA receptor antagonist SR 95531, GABAzine. Then we intravenously infused propofol through caudal vein. The time of LORR was increased and RORR was reduced. Because propofol is a GABAergic anesthetic, which mainly act on postsynaptic membrane GABAA receptors. This demonstrates that mPFC GABAA receptor can affect the effect of propofol on consciousness. Our result suggests that propofol induce unconsciousness may need the GABAA receptors of mPFC.
Additionally, we found that the extracellular basal levels of DOPAC except DA or HVA in mPFC was enhanced based on using reverse dialysis infusion of GABAzine. Thus, in the present study, our result indicated that inhibition of mPFC GABAA receptor increased metabolite activity of the dopamine. Similar finding have been reported by administration of GABAzine increased DOPAC concentrations in the median eminence and pituitary [15,16]. But they didn’t investigate the DA level at the same time. DOPAC is the metabolite of DA and DOPAC increasing is accompanied with DA reduction. However, in our study, we did not find a decrease of DA in the mPFC which consistent with research that GABAA receptor in PFC couldn’t change the DA level of PFC [17].
Because propofol also can reduce the DA level in mPFC after we blocked the GABAA receptor of mPFC. This demonstrates that mPFC GABAA receptor is involvement in the mechanism of propofol, yet this area GABAA receptor can’t directly interfere in the effect of propofol on dopamine level of mPFC. The VTA which contains GABAA receptors [18] is major source of DA neurons in the PFC [19]. VTA GABAA receptor may be the first action target for propofol, further interfere in the dopamine level of mPFC. The other reason may be that propofol modulate DA in mPFC by working on the NMDA, D2 or GABAB receptors [20]. Reported showed that NMDA, D2 or GABAB receptors can modulate the DA level in different brain area [17].
The time of LORR was increased and RORR was reduced by propofol before inhibition of GABAA receptors in mPFC, this result suggested that propofol may also modulate the other neurotransmitters [21] rather than dopamine system through GABAA receptor to induce unconsciousness. As propofol couldn’t induce DA transform into HVA, we consider propofol improve DA transform into DOPAC in mPFC under normal state that is the side-effect rather than unconsciousness mechanism.
Given above results, in future we will further investigate whether GABAA receptors of other area, then observe the correlation of unconsciousness induced by propofol. Furthermore, investigating Ach and other amino neurotransmitters, and look for the correlation with GABAA receptors in mPFC is necessary.
In conclusion, we found that propofol reduction of the level of DA and increase the extracellular fluid DOPAC levels in mPFC which may participate propofol induced LOC, although this effect may be not caused by GABAA receptor in mPFC. Given the time of LORR and RORR can be changed by blocking GABAA receptor in mPFC, we suggest that GABAA receptor in mPFC participated in the mechanism of propofol induce LOC. Therefore, GABAA receptor may be not the only way for propofol induce unconsciousness, and propofol can directly reduction of dopamine to induce unconsciousness.
Acknowledgements
The authors thank Professor Hongxin Dong (University of Northwestern University, US) for carefully reading an early version of the manuscript. Financial support and sponsorship: this work was supported by the National Natural Science Foundation of China (81571026).
Disclosure of conflict of interest
None.
References
- 1.Fulton B, Sorkin EM. Propofol: an overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs. 1995;50:636–57. doi: 10.2165/00003495-199550040-00006. [DOI] [PubMed] [Google Scholar]
- 2.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, Peragut JC, Gouin FM. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–212. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 3.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon-Dominguez U, Izzetoglu M, Leon-Carrion J, Solís-Marcos I, Garcia-Torrado FJ, Forastero-Rodríguez A, Mellado-Miras P, Villegas-Duque D, Lopez-Romero JL, Onaral B, Izzetoglu K. Molecular concentration of deoxyHb in human prefrontal cortex predicts the emergence and suppression of consciousness. NeuroImage. 2014;85:616–25. doi: 10.1016/j.neuroimage.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Shirasaka T, Yonaha T, Onizuka S, Tsuneyoshi I. Effects of orexin-A on propofol anesthesia in rats. J Anesth. 2011;25:65–71. doi: 10.1007/s00540-010-1071-6. [DOI] [PubMed] [Google Scholar]
- 6.Shyr MH, Tsai TH, Yang CH, Chen HM, Ng HF, Tan PP. Propofol anesthesia increases dopamine and serotonin activities at the somatosensory cortex in rats: a microdialysis study. Anesth Analg. 1997;84:1344–1348. doi: 10.1097/00000539-199706000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Gao M, Shen JX, Shi WX, Oster AM, Gutkin BS. Cortical control of VTA function and influence on nicotine reward. Biochem Pharmacol. 2013;86:1173–1180. doi: 10.1016/j.bcp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Willis J, Zhu W, Perez-Downes J, Tan S, Xu C, Seubert C, Gravenstein N, Martynyuk A. Propofol-induced electroencephalographic seizures in neonatal rats: the role of corticosteroids and gamma-aminobutyric acid type A receptor-mediated excitation. Anesth Analg. 2014;120:433–439. doi: 10.1213/ANE.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehead KJ, Rose S, Jenner P. Involvement of intrinsic cholinergic and GABAergic innervation in the effect of NMDA on striatal dopamine effluxand metabolism as assessed by microdialysis studies in freely moving rats. Eur J Neurosci. 2001;14:851–60. doi: 10.1046/j.0953-816x.2001.01702.x. [DOI] [PubMed] [Google Scholar]
- 11.Shirasaka T, Yonaha T, Onizuka S, Tsuneyoshi I. Effects of orexin-A on propofol anesthesia in rats. J Anesth. 2011;25:65–71. doi: 10.1007/s00540-010-1071-6. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos D, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [Google Scholar]
- 13.Bjorefeldt A, Wasling P, Zetterberg H, Hanse E. Neuromodulation of fast-spiking and non-fast-spiking hippocampal CA1 interneurons by human cerebrospinal fluid. J Physiol. 2015;594:937–52. doi: 10.1113/JP271553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller CP, Pum ME, Amato D, Schüttler J, Huston JP, Silva MA. The in vivo neurochemistry of the brain during general anesthesia. J Neurochem. 2011;119:419–446. doi: 10.1111/j.1471-4159.2011.07445.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner EJ, Goudreau JL, Moore KE, Lookingland KJ. GABAergic regulation of tuberoinfundibular dopaminergic neurons in the male rat. Brain Res. 1994;659:194–200. doi: 10.1016/0006-8993(94)90878-8. [DOI] [PubMed] [Google Scholar]
- 16.Goudreau JL, Wagner EJ, Lookingland KJ, Moore KE. gamma-Aminobutyric acid receptor-mediated regulation of periventricular hypophysial dopaminergic neurons: possible role in mediating stress- and 5-hydroxytryptamine-induced decreases in neuronal activity. J Pharmacol Exp Ther. 1994;271:1000–6. [PubMed] [Google Scholar]
- 17.Locklear MN, Cohen AB, Jone A, Kritzer MF. Sex Differences Distinguish Intracortical Glutamate Receptor-Mediated Regulation of Extracellular Dopamine Levels in the Prefrontal Cortex of Adult Rats. Cereb Cortex. 2016;26:599–610. doi: 10.1093/cercor/bhu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–68. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Yang B, Wang BF, Lai MJ, Zhang FQ, Yang XW, Zhou WH, Lian QQ. Differential involvement of GABAA and GABAB receptors in propofol self-administration in rats. Acta Pharmacol Sin. 2011;32:1460–5. doi: 10.1038/aps.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi T, Wang Y, Sato K, Okumura F. In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br J Anaesth. 1998;80:644–8. doi: 10.1093/bja/80.5.644. [DOI] [PubMed] [Google Scholar]