Abstract
Nonalcoholic steatohepatitis (NASH) has similar clinical pathological changes to alcoholic hepatitis. It shows increased incidence and young trend year by year. Polyene phosphatidyl choline (PPC) is widely used in clinic for liver disease treatment. The effect and mechanism of PPC on NASH have not been fully elucidated. Thirty healthy male Wistar rats were randomly equally divided into control, NASH group, and PPC group. NASH model was established by high fat diet. PPC was intraperitoneal injected to NASH rat from the second week at 80 mg/kg·d for three weeks. Body weight, liver weight index, ALT, AST, TG, and TC were tested. TNF-α and IL-1β levels were detected by ELISA. NF-κB mRNA and protein expression in liver tissue were determined by real time PCR and Western blot. SOD activity and ROS content were measured by colorimetry. NASH rat presented significantly elevated body weight and liver weight index, increased ROS content, declined SOD activity, enhanced liver function and inflammatory factors expression, and upregulated NF-κB mRNA and protein levels compared with control (P < 0.05). PPC intervention obviously reduced body weight and liver weight index, declined ROS content, amplified SOD activity, decreased liver function, weakened inflammatory factor TNF-α and IL-1β expression, and downregulated NF-κB mRNA and protein levels compared with NASH group (P < 0.05). PPC can play a treatment effect on NASH through regulating oxidative balance, inhibiting inflammatory factors and NF-κB signaling pathway.
Keywords: Polyene phosphatidyl choline, nonalcoholic steatohepatitis, TNF-α, NF-κB, IL-1β
Introduction
Nonalcoholic steatohepatitis (NASH) presents similar clinicopathological changes to alcoholic hepatitis without history of heavy drinking. Its pathological features include fat storage, parenchyma hepatic cells fatty change, liver fatty infiltration, hepatic cells necrosis, ballooning degeneration, and inflammatory cells infiltration [1,2]. NASH is the most common cause of chronic liver disease. Severe NASH can appear different degrees of liver fibrosis and Mallory corpuscle, even develop to liver cirrhosis and hepatocellular carcinoma (HCC) [3,4]. Epidemiological investigation demonstrated that the incidence of NASH was more than 20% worldwide. More obesity and metabolic syndrome appeared following life rhythm speeding up, high-fat and high-calorie diet, eating habits changing, and long-term sitting, which caused NASH incidence elevation and young trend [5,6]. NASH has become a global public health problem. The pathogenesis of NASH is complex, involving heredity, physics, chemistry, and many other factors. The pathogenesis of NASH is related to a variety of metabolic disorder factors, such as adipocyte factor, oxygen stress, intestinal endotoxin disease, and insulin resistance [7,8]. Currently, it is considered that NASH is associated with second strike theory. The first strike is insulin resistance, leading to benign lipid deposition in the liver cell. The second strike is oxygen stress and lipid peroxidation, which is the key to disease progression [9]. In spite of multiple treatment choice for hepatitis, there is still lack of drugs with good effect on NASH so far [10].
Polyene phosphatidyl choline (PPC) is extracted from soy by the unique craft, which is rich in polyunsaturated fatty acid, including linoleic acid, linolenic acid, and oleic acid [11]. Polyunsaturated fatty acid, also known as essential fatty acid, cannot be autonomously synthetized in the human body and must be supplied by food [12]. Therefore, PPC plays a role in numerous aspects, such as anti-inflammation, antioxidation, and immune regulation function [13,14]. In clinic, PPC is widely used in various types of liver disease treatment [15]. However, the treatment effect of PPC and related mechanism on NASH has not been fully elucidated. This article intends to establish rat NASH model to investigate the effect and mechanism of PPC intervention on NASH.
Materials and methods
Experimental animals
Thirty healthy male Wistar rats at 5 weeks old and 120±20 g were purchased from animal center in Zhengzhou University and fed in SPF laboratory. Feeding conditions include constant temperature (21±1°C), constant humidity (50-70%), and 12 h diurnal cycle.
Rats were used for all experiments,and all procedures were approved by the Animal Ethics Committee of Henan Provincial People’s Hospital.
Main materials and instruments
Surgical instruments were bought from medical instrument factory in Suzhou. Surgical microscope was from Zhenjiang optical instrument co., LTD. PPC injection was from Chengdu Tiantaishan pharmaceutical co., LTD. Trizol reagent was got from Invitrogen. RNA extraction kit and reverse transcription kit were from Axygen. SOD activity detection kit was purchased from Nanjing Jiancheng bioengineering institute. PVDF membrane was from Pall Life Sciences. Western blot related chemical reagents were bought from Beyotime. ECL reagent was from Amersham Biosciences. Rabbit anti rat NF-κB monoclonal antibody and HRP-tagged IgG secondary antibody were from Cell Signaling. Microplate reader was from BD Company. DNA amplifier was from PE Gene Amp PCR System 2400. Automatic biochemical analyzer was from Beckman. Other common reagents were purchased from Sangon.
Methods
Experimental animal grouping
Thirty healthy male Wistar rats were randomly equally divided into control, NASH group, and PPC group.
NASH model preparation and PPC treatment
The rats were fed with mixed food containing 84% basal feed, 10% lard, 5% egg yolk powder, and 1% cholesterol disinfected by cobalt 60 irradiation for five weeks after 1 week’s adaptive feeding. The rats in control were only gave normal basal feed. PPC was intraperitoneal injected to NASH rat from the second week at 80 mg/kg·d for three weeks.
Sample collecting
After modeling, the blood was extracted from aorta abdominalis and let stand at room temperature for 30 min. The serum was collected and store at -20°C after centrifuged at 4°C and 3600 rpm for 10 min. The rats were euthanatized and the liver tissue was preserved at -80°C. Liver weight index was calculated.
Liver function index detection
Serum ALT, AST, TG, TC, HDL-C, and LDL-C levels were tested by automatic biochemical analyzer.
ELISA
Serum was collected to detect inflammatory factors TNF-α and IL-6 expression according to the manual. 50 μl diluted standard substance was added to 96-well plate to prepare standard curve. 50 μl sample was added to the well with three replicates. After washed for 5 times, 50 μl enzyme-labelled reagent was added and the plate was incubated at 37°C for 30 min. Next, the plate was added with 50 μl color development agent A and B at 37°C for 10 min. At last, the plate was read at 450 nm within 15 min after adding 50 μl stop buffer. The linear regression equation of standard curve was calculated by OD value and used to test sample OD value.
SOD activity detection
SOD activity in liver tissue was tested according to the kit instruction. Protein was extracted and water bathed at 95°C for 40 min. Next, the protein was cooled and centrifuged at 4000 rpm for 10 min. Ethanol phase in the tissue homogenate was extracted using ethanol-chloroform mixture (v/v 5:3) to detect LDH and total SOD activity.
ROS content detection
Protein was extracted and water bathed at 95°C for 40 min. Next, the protein was cooled and centrifuged at 4000 rpm for 10 min. Then the tissue homogenate was incubated in 2’, 7’ - dichlorofluorescein diacetate (DCF-DA) for 15 min and centrifuged at 10,000 rpm for 15 min. The sediment was incubated in PBS at 37°C for 60 min. ROS content was determined by spectrophotometer.
Real time PCR
Total RNA was extracted from liver tissue using Trizol. Primer sequences were synthesized (Table 1). Real-time PCR was applied to detect the target genes. The reaction consists one cycle of 52°C for 1 min, followed by 35 cycles of 90°C for 30 s, 58°C for 50 s, and 72°C for 35 s. GAPDH was used as reference, and the results were calculated 2-△Ct method.
Table 1.
Primer sequence
| Gene | Forward 5’-3’ | Reverse 5’-3’ |
|---|---|---|
| GADPH | AGTGCCAGCCTCGTCTCATAG | CGTTGAACTTGCCGTGGGTAG |
| NF-κB | CTCATCTAAGCGGAACAATGG | GCACATTCTCTCCGTAGCG |
Western blot
The cells in each group were cracked on ice for 15-30 min and ultrasonicated for 4×5 s to extract protein. After centrifuged at 10,000 g and 4°C for 15 min, the protein was moved to a new Ep tube and store at -20°C. The protein was separated by 10% SDS-PAGE electrophoresis and transferred to PVDF membrane. After blocked by 5% skim milk for 2 h, the membrane was incubated in NF-κB primary antibody at 1:1000 and 4°C overnight. Then the membrane was incubated with secondary antibody at 1:2000 for 30 min and washed by PBST. At last, the membrane was treated with chemiluminescent agent for 1 min and imaged on X-ray. Protein image processing system and Quantity one software were used for data analysis. All experiments were repeated for four times.
Statistical analysis
All the statistical analysis was performed on SPSS16.0 software. The data was presented as x̅±s. One-way ANOVA was used for mean comparison. P < 0.05 was considered as statistically significance.
Results
Liver weight and liver weight index comparison
After modeling, the rats were euthanatized and the liver tissue was collected. Liver wet weight was measured and liver weight index was calculated. The results showed that body weight and liver weight index in NASH group increased significantly compared with control (P < 0.05). PPC treatment obviously declined body weight and liver weight index compared with NASH group (P < 0.05) (Table 2).
Table 2.
Liver weight and liver weight index comparison
| Group | Weight (g) | Liver weight index (%) |
|---|---|---|
| Control | 401.19±11.53 | 3.21±0.06 |
| NASH | 482.87±14.12* | 4.56±0.11* |
| PPC | 418.79±9.54# | 3.63±0.09# |
P < 0.05, compared with control;
P < 0.05, compared with NASH group.
Liver function index comparison
Liver function injury was determined by analyzing AST, ALT, TC, and TG levels. It was revealed that ALT, AST, TG, and TC markedly enhanced in NASH group compared with control (P < 0.05). After PPC treatment, ALT, AST, TG, and TC significantly decreased compared with NASH group (P < 0.05) (Table 3).
Table 3.
Liver function detection
| Group | ALT (U/L) | AST (U/L) | TG (mmol/L) | TC (mmol/L) |
|---|---|---|---|---|
| Control | 61.23±11.06 | 101.25±12.09 | 1.08±0.23 | 1.01±0.17 |
| NASH | 148.15±17.33* | 214.76±11.41* | 2.43±0.31* | 2.81±0.48* |
| PPC | 78.22±11.86# | 123.63±21.59# | 1.17±0.51# | 1.21±0.62# |
P < 0.05, compared with control;
P < 0.05, compared with NASH group.
PPC impact on serum inflammatory cytokines expression in NASH rat
ELISA was applied to test PPC impact on serum inflammatory cytokines expression in NASH rat. It was demonstrated that TNF-α and IL-1β secretion obviously elevated in NASH group compared with normal control (P < 0.05). PPC can apparently suppress TNF-α and IL-1β secretion compared with NASH group (P < 0.05) (Figures 1 and 2).
Figure 1.
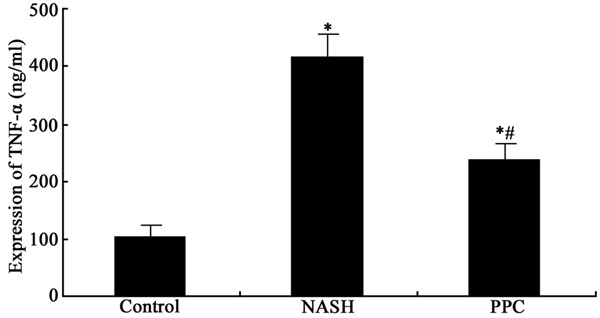
PPC impact on TNF-α secretion in NASH rat. *P < 0.05, compared with control; #P < 0.05, compared with NASH group.
Figure 2.
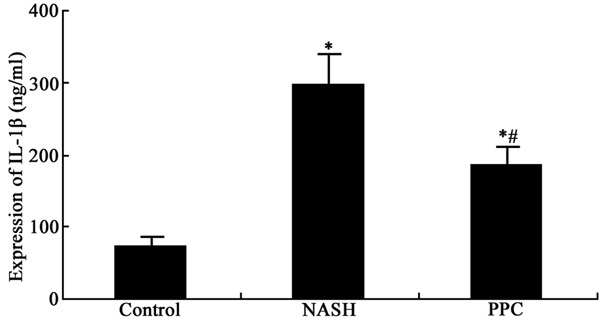
PPC impact on IL-1β secretion in NASH rat. *P < 0.05, compared with control; #P < 0.05, compared with NASH group.
PPC impact on NF-κB mRNA expression in the liver tissue of NASH rat
Real time PCR was performed to determine PPC impact on NF-κB mRNA expression in the liver tissue of NASH rat. The results showed that NF-κB mRNA overexpressed markedly in the liver tissue of NASH rat compared with control (P < 0.05). PPC therapy obviously inhibited NF-κB mRNA expression compared with NASH group (P < 0.05) (Figure 3).
Figure 3.
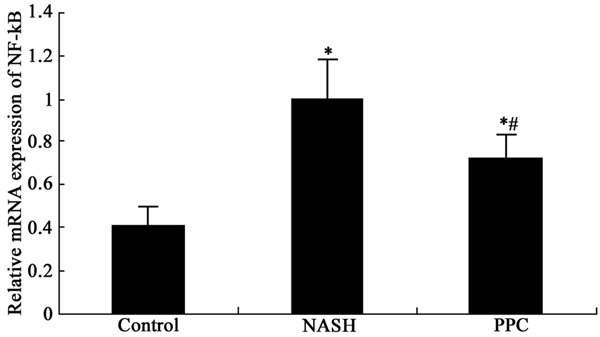
PPC impact on NF-κB mRNA expression in the liver tissue of NASH rat. *P < 0.05, compared with control; #P < 0.05, compared with NASH group.
PPC impact on NF-κB protein level in the liver tissue of NASH rat
Western blot was used to detect PPC impact on NF-κB protein level in the liver tissue of NASH rat. The results showed that NF-κB protein upregulated markedly in the liver tissue of NASH rat compared with control (P < 0.05). PPC therapy significantly restrained NF-κB protein level compared with NASH group (P < 0.05) (Figures 4 and 5).
Figure 4.
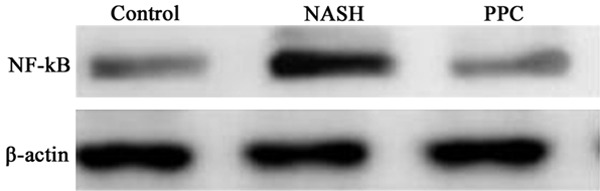
PPC impact on NF-κB protein level in the liver tissue of NASH rat.
Figure 5.
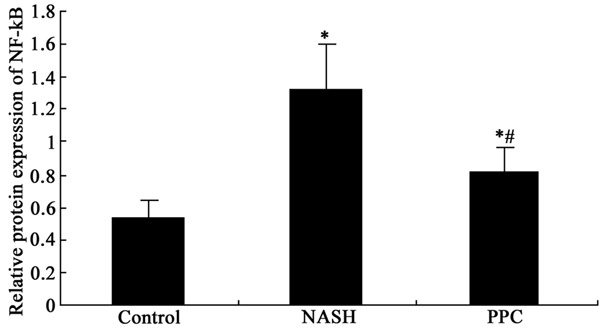
PPC impact on NF-κB protein level in the liver tissue of NASH rat analysis. *P < 0.05, compared with control; #P < 0.05, compared with NASH group.
PPC impact on oxidative stress in the liver tissue of NASH rat
ROS production and SOD content in the liver tissue were tested. The results revealed that SOD production obviously increased, while SOD content reduced in NASH group compared with control (P < 0.05). PPC apparently suppress ROS production and enhanced SOD content compared with NASH group (P < 0.05) (Table 4).
Table 4.
PPC impact on oxidative stress in the liver tissue of NASH rat
| Index | Control | NASH | PPC |
|---|---|---|---|
| ROS | 51.10±8.21 | 201.67±31.22* | 123.18±21.22*,# |
| SOD | 132.35±25.18 | 47.01±15.31* | 92.21±15.41*,# |
P < 0.05, compared with control;
P < 0.05, compared with NASH group.
Discussion
As a common liver inflammatory disease, NASH is featured as high incidence, chronic deferment, and refractory [16]. The pathogenesis of NASH is closely associated with obesity, hyperlipidemia, and insulin resistance [17]. NASH is generally developed from fatty liver, which is mainly caused by fat synthesis increase and fatty degeneration. It leads to insulin resistance and becomes the first strike together with intrahepatic lipid transport decrease. Later, hepatic endotoxin level elevation promotes free fatty acid metabolism in the liver, weakens antioxidant activity, and increases mitochondrial decoupling protein activity. Mitochondrial dysfunction accelerates oxidative stress production. Proinflammatory factor can change simple steatosis to steatohepatitis, and even liver cirrhosis, which is the second strike. Thus, oxidative stress and inflammatory factor are the main causing factors of NASH [18,19].
Our results confirmed that NASH can elevate body weight and liver weight index, injure liver function, and enhance NF-κB expression. NF-κB expression can activate corresponding target genes, including immune receptor, adhesion molecule, inflammatory cytokines, and C-reactive protein, thus regulate immune response and amplify inflammation [20]. NASH can increase ROS content and decline SOD activity. ROS overproduction can lead to oxidative injury, while SOD is an important antioxidase to eliminate free radical. SOD plays a critical role for oxidation balance, and its activity indirectly reflects free radical elimination ability [21]. PPC is reported to play an important role in anti-inflammation and oxidative balance, whereas its effect in eliminating free radical has not been confirmed [13,14]. This study verified that PPC intervention declined ROS content and amplified SOD activity, thus recovering oxidative balance, downregulating inflammatory factors TNF-α and IL-1β expression, and reducing NF-κB level. It can alleviate liver injury and promote liver function restoration.
In conclusion, PPC plays a therapeutic role on NASH through regulating oxidative balance, inhibiting inflammatory factors, and suppressing NF-κB signaling pathway.
Acknowledgements
Molecular mechanism of the NAFLD progress inhibition and correlation research on the FibroScan and its pathological staging on liver (142300410403); Research on molecular mechanism of NASH inflammation progress inhibition (15A320012).
Disclosure of conflict of interest
None.
References
- 1.Rinella ME, Lominadze Z, Loomba R, Charlton M, Neuschwander-Tetri BA, Caldwell SH, Kowdley K, Harrison SA. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol. 2016;9:4–12. doi: 10.1177/1756283X15611581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khlaiphuengsin A, Kiatbumrung R, Payungporn S, Pinjaroen N, Tangkijvanich P. Association of PNPLA3 Polymorphism with Hepatocellular Carcinoma Development and Prognosis in Viral and Non-Viral Chronic Liver Diseases. Asian Pac J Cancer Prev. 2015;16:8377–8382. doi: 10.7314/apjcp.2015.16.18.8377. [DOI] [PubMed] [Google Scholar]
- 3.Teufel U, Peccerella T, Engelmann G, Bruckner T, Flechtenmacher C, Millonig G, Stickel F, Hoffmann GF, Schirmacher P, Mueller S, Bartsch H, Seitz HK. Detection of carcinogenic etheno-DNA adducts in children and adolescents with non-alcoholic steatohepatitis (NASH) Hepatobiliary Surg Nutr. 2015;4:426–435. doi: 10.3978/j.issn.2304-3881.2015.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruszewska E, Gudowska M, Wojtowicz E, Cylwik B, Szmitkowski M, Chrostek L. The Higher Prevalence of Non-Alcoholic versus Alcoholic Steatohepatitis in Alcoholics. Clin Lab. 2015;61:1769–1774. doi: 10.7754/clin.lab.2015.150434. [DOI] [PubMed] [Google Scholar]
- 5.Honda Y, Imajo K, Kato T, Kessoku T, Ogawa Y, Tomeno W, Kato S, Mawatari H, Fujita K, Yoneda M, Saito S, Nakajima A. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS One. 2016;11:e0146337. doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orime K, Shirakawa J, Togashi Y, Tajima K, Inoue H, Nagashima Y, Terauchi Y. Lipid-lowering agents inhibit hepatic steatosis in a non-alcoholic steatohepatitis-derived hepatocellular carcinoma mouse model. Eur J Pharmacol. 2016;772:22–32. doi: 10.1016/j.ejphar.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 7.Ramadori P, Drescher H, Erschfeld S, Schumacher F, Berger C, Fragoulis A, Schenkel J, Kensler TW, Wruck CJ, Trautwein C, Kroy DC, Streetz KL. Hepatocyte-specific Keap1 deletion reduces liver steatosis but not inflammation during non-alcoholic steatohepatitis development. Free Radic Biol Med. 2016;91:114–126. doi: 10.1016/j.freeradbiomed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Li JS, Chen ZY, Jiang JP, He BH. Regulation of pure total flavonoids from Citrus on TH17/Treg balance in mice with NASH. Zhongguo Zhong Yao Za Zhi. 2015;40:2644–2648. [PubMed] [Google Scholar]
- 9.Nosach OV, Ovsyannikova LM, Chumak AA, Alekhina SM, Sarkisova EO, Hasanova OV, Pleskach OY, Nezhovorova GA, Zelinska AV, Kadyuk OM. Peculiarity of prooxidant-antioxidant balance indicators in patients with nonalcoholic fatty liver disease who have been exposed to ionizing radiation due to the Chornobyl NPP accident. Probl Radiac Med Radiobiol. 2015;20:420–431. [PubMed] [Google Scholar]
- 10.Ai L, Xu Q, Wu C, Wang X, Chen Z, Su D, Jiang X, Xu A, Lin Q, Fan Z. A20 Attenuates FFAs-induced Lipid Accumulation in Nonalcoholic Steatohepatitis. Int J Biol Sci. 2015;11:1436–1446. doi: 10.7150/ijbs.13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang Z, Chen YP, Ma KF, Ye YF, Zheng L, Yang YD, Li YM, Jin X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: a systematic review. BMC Gastroenterol. 2013;13:140. doi: 10.1186/1471-230X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Wang Y, Qian H, Zhao Y, Liu B, Fu C. Polyprenols from Taxus chinensis var. mairei prevent the development of CCl(4)-induced liver fibrosis in rats. J Ethnopharmacol. 2012;142:151–160. doi: 10.1016/j.jep.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Duan RL, Sun X, Liu J, Gong T, Zhang ZR. Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacol Sin. 2011;32:108–115. doi: 10.1038/aps.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan XF, Deng YQ, Ye L, Li YD, Chen J, Lu WW, Li JP. Effect of Xuezhikang Capsule on serum tumor necrosis factor-alpha and interleukin-6 in patients with nonalcoholic fatty liver disease and hyperlipidemia. Chin J Integr Med. 2010;16:119–123. doi: 10.1007/s11655-010-0119-7. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhang XJ, Lan Y, Xu L, Zhang XZ, Wang HH. Treatment of non-alcoholic fatty liver disease by Qianggan Capsule. Chin J Integr Med. 2010;16:23–27. doi: 10.1007/s11655-010-0023-1. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Mizuguchi T, Tanimizu N, Ichinohe N, Ooe H, Kawamoto M, Meguro M, Hirata K, Mitaka T. Preoperative hepatocyte transplantation improves the survival of rats with nonalcoholic steatohepatitis-related cirrhosis after partial hepatectomy. Cell Transplant. 2014;23:1243–1254. doi: 10.3727/096368913X668645. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Ren Q, Wu T, Guo Y, Liang Y, Liu S. Ezetimibe prevents the development of nonalcoholic fatty liver disease induced by highfat diet in C57BL/6J mice. Mol Med Rep. 2014;10:2917–2923. doi: 10.3892/mmr.2014.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Gong M, French BA, Li J, Tillman B, French SW. Mallory-Denk Body (MDB) formation modulates Ufmylation expression epigenetically in alcoholic hepatitis (AH) and non-alcoholic steatohepatitis (NASH) Exp Mol Pathol. 2014;97:477–483. doi: 10.1016/j.yexmp.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Takahashi S, Fang ZZ, Matsubara T, Krausz KW, Qu A, Gonzalez FJ. Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2014;1841:1596–1607. doi: 10.1016/j.bbalip.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Ren H, Han J, Wang W, Zheng Q, Wang D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3beta. Oxid Med Cell Longev. 2015;2015:481405. doi: 10.1155/2015/481405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Chen X, Wang H, Yan Q. Catalpol protects mice against renal ischemia/reperfusion injury via suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin Exp Med. 2015;8:2038–2044. [PMC free article] [PubMed] [Google Scholar]


