Abstract
Long non-coding RNAs (lncRNAs) have been identified as oncogenes or tumor suppressors that are involved in tumorigenesis and chemotherapy resistance. HOTTIP is located at the 5’ tip of the HOXA locus and coordinates the activation of multiple 5’ HOXA genes, which plays an important role in multiple cancers. However, its biological role in the development of the chemoresistance phenotype of osteosarcoma (OS) is still unknown. In this study, we explored the roles of lncRNA HOTTIP in the initiation and chemoresistance of OS. We found that HOTTIP was increased in OS and up-regulated expression of HOTTIP could promoted OS cell proliferation and cell cycle progression by activating the Wnt/β-catenin pathway. Down-regulated expression of HOTTIP inhibited cell proliferation and arrested cell cycle at G1 phase by inhibition of Wnt/β-catenin pathway. Furthermore, our data showed that increased expression of HOTTIP was correlated with chemoresistance in OS. In vitro, HOTTIP induced cellular resistance to cisplatin by activating the Wnt/β-catenin pathway, which could be reversed by treatment with the Wnt/β-catenin inhibitor. Taken together, these findings indicated that HOTTIP play a pivotal role in OS cell initiation and chemoresistance via activating Wnt/β-catenin signaling pathway, which suggested potential use of HOTTIP for the treatment of osteosarcoma.
Keywords: Osteosarcoma, HOTTIP, proliferation, Wnt/β-catenin, chemoresistance
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumor in children and adolescents [1]. In the past, the most common treatment for osteosarcoma was amputation [2]. Although the 5-year survival rate for patients with osteosarcoma has significantly improved, many patients are insensitive to available chemotherapeutics and have poor prognoses [3]. Therefore, a better understanding of the underlying molecular mechanisms of chemoresistance is urgently required for improved osteosarcoma treatment.
Recently, studies using a combination of various genome-wide approaches, such as the ENCODE project, have shown that the majority of the mammalian genome is transcribed, but only approximately 1.2% of these transcripts represent protein-coding genes [4]. Among these non-protein-coding transcripts, long non-coding RNAs (lncRNAs), which are greater than 200 nt in length, are characterized by the diversity and complexity of their sequences and action mechanisms [5]. Recent studies showed that lncRNA play an important role in diverse biological processes, including embryonic development, cell growth and tumorigenesis, by regulating gene expression at the transcriptional, post-transcriptional and transcriptional levels [6,7].
The HOXA transcript at the distal tip (HOTTIP) lncRNA, located at the 5’ end of the HOXA cluster, was recently functionally characterized [8]. The activity of HOTTIP is a result of its interaction with the WDR5/MLL complex, which enhances histone H3 lysine 4 trimethylation to activate the expression of multiple 5’ HOXA genes [9]. Recent studies showed that HOTTIP play a major role in tumor progression. For example, Quagliata et al. found that HOTTIP was a negative prognostic factor in hepatocellular carcinoma patients, and increased HOTTIP expression was associated with enhanced hepatocellular carcinoma metastasis [10]. Zhang et al. found lncRNA HOTTIP was highly expressed in tongue squamous cell carcinoma and positively correlated with T stage, clinical stage, distant metastasis and unfavorable prognosis [11]. Li et al. revealed that lncRNA HOTTIP could promote progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer [12]. In a recent study, HOTTIP was reported to be significantly up-regulated in human osteosarcoma tissues, which correlated with advanced clinical stage, distant metastasis and unfavorable prognosis [13]. Considering its multiple functions, we hypothesized that HOTTIP might be involved in the initiation and chemoresistance of osteosarcoma. Thus, in this study, we explored the underlying mechanisms of HOTTIP in the regulation of proliferation and chemoresistance of osteosarcoma cells.
Materials and methods
Patient samples and cell lines
A total of 21 pairs of OS tissues and adjacent non-tumor tissues were available from patients who underwent surgery at The First Affiliated Hospital of Zhengzhou University and Shanghai First People’s Hospital. The diagnosis of osteosarcoma was confirmed pathologically. All the tissue samples were collected, immediately snap frozen in liquid nitrogen, and stored at -80°C until use. The study was approved by the Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University. Informed consent was obtained from all patients.
Human OS cell lines (SaOS2, MG63 and U2OS) and the human osteoblastic cell line (hFOB1.19) were purchased from the American Tissue Culture Collection. hFOB1.19 cells were cultured in Ham’s F12/DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco, FBS), Human OS cells were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin (Invitrogen). Cells were incubated at 37°C with 5% CO2 in a humidified chamber.
Plasmid construction and cell transfection
The HOTTIP sequence was subcloned into the pcDNA3.1 vector (Invitrogen). HOTTIP Ectopic expression was achieved through pcDNA3.1-HOTTIP transfection using lipofectamine 2000 (Invitrogen), with an empty pCDNA3.1 vector used as a control. The si-HOTTIP and si-NC were purchased from Genepharm (Shanghai, China). The si-HOTTIP and si-NC was transfected into OS cells using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were collected 48 h after transfection. The expression levels of HOTTIP were calculated by qRT-PCR.
Cell proliferation assay
Cell proliferation was analyzed by using cell counting assay Kit-8 (CCK-8) (DOJINDO) according to the manufacture’s protocol. Cells were incubated in 10% CCK-8 diluted in normal culture media at 37°C until the visual color conversion occurred. Proliferation rates were determined at 0, 24, 48 and 72 h after transfection. The absorbance of each well was measured with a microplate reader set at 450 nM.
Colony formation assay
To assess colony formation, 100 cells/well were plated into 6-well plates and routinely cultured for 2 weeks. Then, cells were fixed with prechilled 10% methanol and stained with 0.5% crystal violet. The colony numbers (containing more than 50 cells) were counted under an optical microscope. This experiment was replicated in triplicate and at least three times.
Cell cycle analysis
After 48 h transfection, the OS cells were collected and washed with phosphate buffered saline (PBS). The washed cells were re-suspended in PBS and fixed in 75% ethanol. Then, the fixed cells were stained with propidium iodide (PI) supplemented with RNaseA (Sigma) for cell cycle analysis with a FACScan flow cytometer (BD Biosciences). Data were collected and analyzed with the ModFit software (BD Biosciences).
RNA extraction and qRT-PCR analyses
Total RNA was isolated from tissues using TRIzol reagent (Invitrogen), and cDNA was synthesized with PrimeScript reverse transcriptase (TaKaRa) and oligo (dT) following the manufacturer’s instructions. Real-time PCR was performed using SYBR Premix Ex TaqTM II kit (TaKaRa). The conditions of real-time PCR were as follows: 94°C for 10 s, 94°C for 5 s, 52°C for 30 s to anneal, 72°C for 15 s followed by 40 cycles. The primers are as follows: HOTTIP, forward primer: 5’-CCTAAAGCCACGCTTCTTTG-3’; reverse primer: 5’-TGCAGGCTGGAGATCCTACT-3’; GAPDH, forward primer 5’-GTCAACGGATTTGGTCTGTATT-3’ and reverse primer: 5’-AGTCTTCTGGGTGGCAGTGAT-3’. The relative expression levels of the gene of interest were calculated using the 2-ΔΔCt method.
Western blotting analysis
Cultured cells were lysed in RIPA buffer with 1% PMSF. Protein was loaded and separated by SDS-PAGE gel and transferred onto PVDF membrane. The blots were probed with primary antibodies at 4°C Covernight and subsequently incubated with HRP-conjugated secondary antibodies. Signals were visualized using ECL Substrates (Pierce). GAPDH was used as an endogenous control.
Statistical analysis
Statistical analysis was carried out using SPSS version 18.0. Data is expressed as the mean ± SD from at least three separate experiments. Experimental results were assessed using chi-square test, t test or ANOVA as appropriate. P value less than 0.05 was considered to be statistically significant.
Results
HOTTIP were upregulated in both OS tissues and cell lines
Expressions of HOTTIP in 21 paired tissue samples from OS patients and cell lines were explored. Compared with the expression levels of the adjacent non-tumor tissues, a significant increase of HOTTIP level was observed in OS tissues (Figure 1A; P<0.05). Three human OS cell lines were also investigate. The OS cell lines showed a remarkably high expression of HOTTIP compared to the human osteoblastic cell line (hFOB1.19) (Figure 1B; P<0.05). Those data indicated that HOTTIP play a major role in OS progression.
Figure 1.
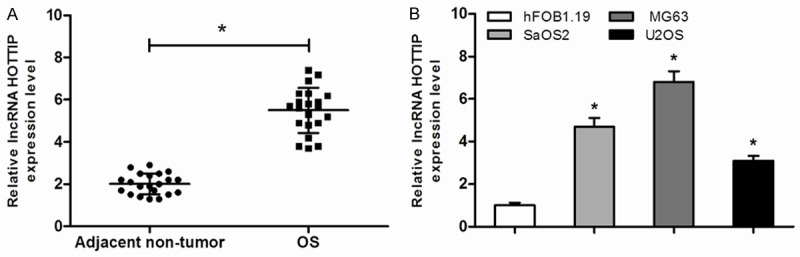
Expression of HOTTIP were increased in OS tissues and cell lines. A. Relative HOTTIP levels in OS tissues and adjacent non-tumor tissues. B. Relative HOTTIP levels in OS cell lines (SaOS2, MG63 and U2OS) and human osteoblastic cell line (hFOB1.19), *P<0.05.
Overexpression of HOTTIP promoted cell proliferation via activation of the Wnt/β-catenin pathway
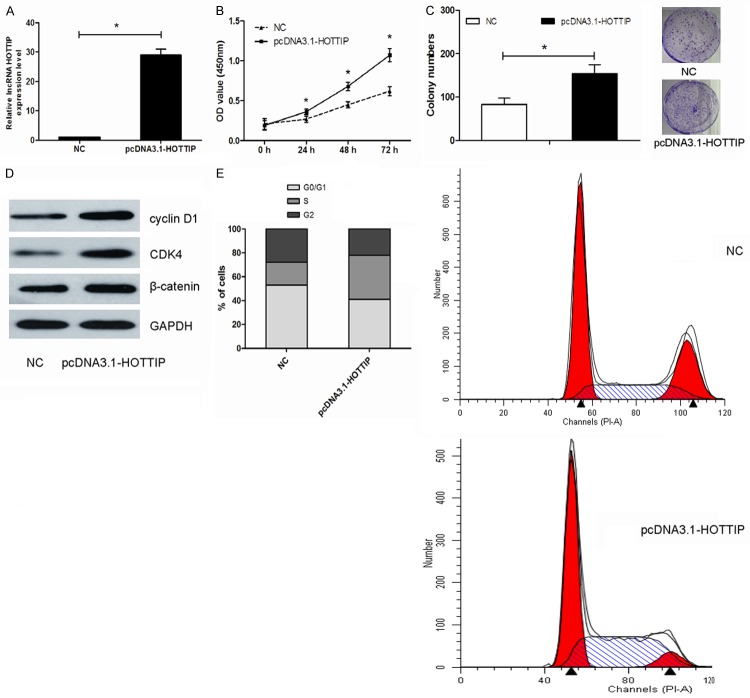
To assess the biological effects of HOTTIP in OS, we increased HOTTIP expression by transfecting pcDNA3.1-HOTTIP into MG63 cells, using control pcDNA3.1 as the negative control. After 48 h of transfection, qRT-PCR analyses revealed that HOTTIP relative levels were significantly up-regulated compared with those cells transfected with the empty vector (Figure 2A; P<0.05). The proliferation and colony formation of pcDNA3.1-HOTTIP transfected MG63 cells were determined. Compared with cells transfected with the empty vector, the proliferation and colony formation ability of pcDNA3.1-HOTTIP transfected cells was significantly increased (Figure 2B and 2C; P<0.05, respectively). Next, we examined the levels of cyclin D1, CDK4 and β-catenin in the HOTTIP-overexpressed MG63 cells. Our data showed that elevation of HOTTIP notably increased the expression levels of cyclin D1, CDK4 and β-catenin (Figure 2D; P<0.05). Cell cycle analysis indicated that upregulated HOTTIP expression in MG63 cells significantly promoted cells into S phase (Figure 2E; P<0.05).
Figure 2.
HOTTIP overexpression promoted cell growth and cell cycle by activating Wnt/ β-catenin pathway. (A) qRT-PCR revealed that HOTTIP was efficiently overexpression by transfected with pcDNA3.1-HOTTIP in MG63 cells. (B, C) Overexpression of HOTTIP significantly promoted cell proliferation (B) and enhanced colony formation (C) compared to the negative control (NC). (D) Overexpression of HOTTIP significantly increased the expression levels of cyclin D1, CDK4 and β-catenin in MG63 cells. (E). Overexpression of HOTTIP led an increase in the number of cells in the S phase and a decrease in the percentage of cells in the G0/G1 phase, *P<0.05.
Knockdown of HOTTIP inhibited cell proliferation via inhibiting of the Wnt/β-catenin pathway
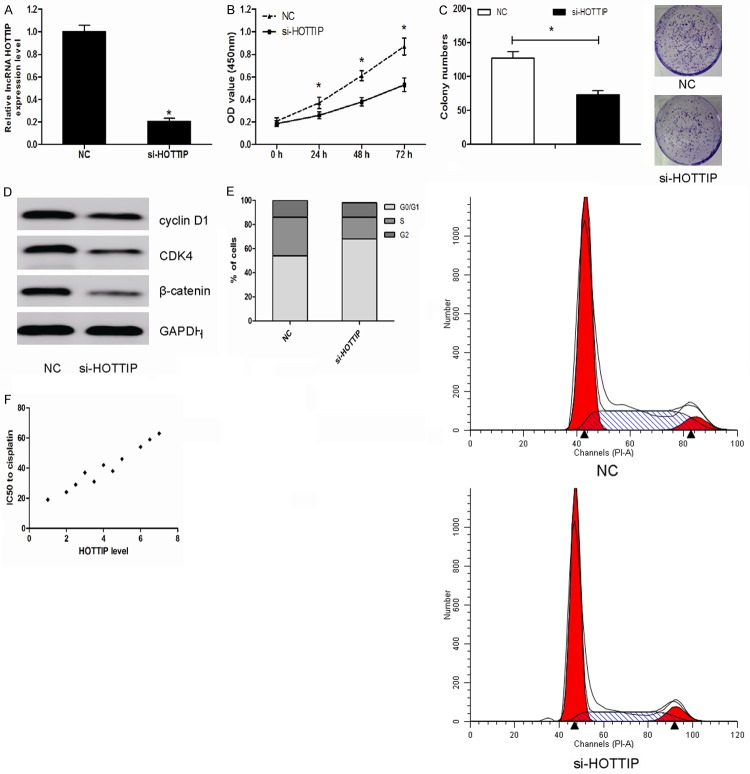
We then downregulated the expression of HOTTIP by transfecting si-HOTTIP into MG63 cells. After 48 h of transfection, our results showed that HOTTIP expression were significantly down-regulated (Figure 3A; P<0.05). MTT assays were utilized to detect the impact of HOTTIP knockdown on proliferation of the MG63 cells. We found that siRNA transfection mediated HOTTIP knockdown significantly inhibited MG63 cell proliferation (Figure 3B; P<0.05). Furthermore, colony formation assays revealed that decreased expression of HOTTIP significantly reduced colony formation ability of OS cells (Figure 3C; P<0.05). Western blotting analysis revealed that downregulation of HOTTIP decreased the expression levels of cyclin D1, CDK4 and β-catenin (Figure 3D; P<0.05). Cell cycle analysis found that downregulated HOTTIP expression in MG63 cells significantly induced G1 phase arrest (Figure 3E; P<0.05). Then, we treated the OS cells with different doses of cisplatin. As shown, higher HOTTIP expression could predict cellular resistance to cisplatin (Figure 3F; P<0.05), suggesting that HOTTIP might play an important role of HOTTIP in patients’ responses to chemotherapy.
Figure 3.
HOTTIP knockdown suppressed cell growth and cell cycle by inhibiting Wnt/β-catenin pathway. (A) qRT-PCR suggested that HOTTIP was efficiently downregulation by transfected with si-HOTTIP in MG63 cells. (B, C) Decreased expression of HOTTIP significantly inhibited cell proliferation (B) and reduced colony formation (C) compared to the negative control (NC). (D) Decreased expression of HOTTIP significantly decreased the expression levels of cyclin D1, CDK4 and β-catenin in MG63 cells. (E) Reduced expression of HOTTIP led a decrease in the number of cells in the S phase and an increase in the percentage of cells in the G0/G1 phase. (F) In MG63 cells, higher HOTTIP expression predicted cellular resistance to cisplatin, *P<0.05.
HOTTIP induced the chemoresistance to cisplatin through activation of the Wnt/β-catenin pathway
To explored the effects of HOTTIP on chemosensitivity in OS cells. Separate overexpression and ablation experiments were performed using either pcDNA3.1-HOTTIP or si-HOTTIP. Figure 4A shows that MG63 cells with HOTTIP overexpression became much more resistant to cisplatin (IC50=41.3 μM) as compared with the negative control (IC50=13.7 μM). This resistance was partially reversed by treatment with the Wnt/β-catenin inhibitor, XAV939 (IC50=25.4 μM). Consistently, in the presence of cisplatin (10 μM), HOTTIP overexpression promoted a greater fraction of cells into S phase, which could be partially rescued by the combination of HOTTIP overexpression and Wnt/β-catenin inhibition (XAV939) (Figure 4B, P<0.05). Furthermore, the alterations of cyclin D1, CDK4 and β-catenin presented similar trends (Figure 4C). As expected, HOTTIP knockdown results in reduced resistance to cisplatin (IC50=7.1 μM) as compared with the negative control (IC50=16.3 μM). Furthermore, this sensitivity was neutralized by treatment with the Wnt/β-catenin agonist, LiCl (IC50=12.5 μM)(Figure 4D, P<0.05). These data suggested that HOTTIP could increase cisplatin resistance of OS cells through activation of the Wnt/β-catenin pathway.
Figure 4.
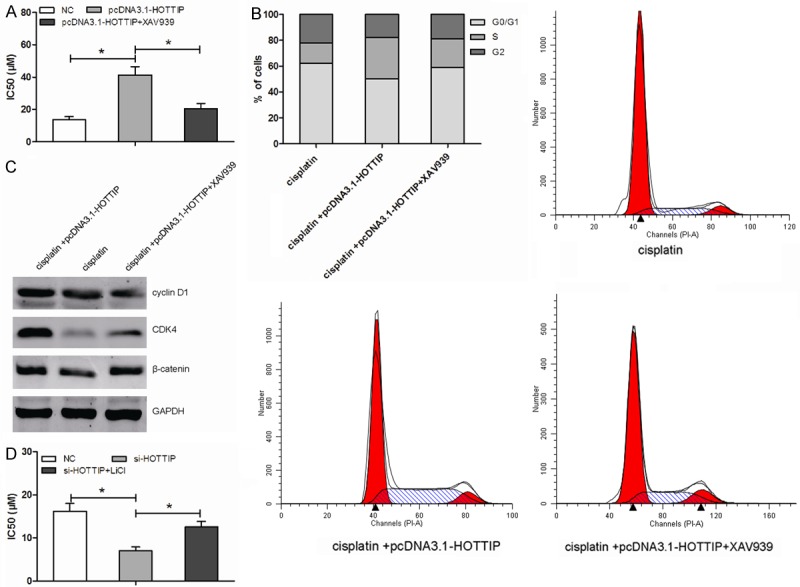
HOTTIP induced the chemoresistance to cisplatin through activation of the Wnt/β-catenin pathway. A. MG63 cells with HOTTIP overexpression were more chemoresistance to cisplatin (IC50=32.6 μM for pcDNA3.1-HOTTIP group and IC50=11.6 μM for negative control group, P<0.05), which was reversed by treatment with XAV939 (IC50=17.3 μM, P<0.05). B, C. Increased expression of HOTTIP promoted MG63 cells into S phase, which could be partially rescued by the combination of HOTTIP overexpression and Wnt/β-catenin inhibition (XAV939). The alterations of cyclin D1, CDK4 and β-catenin presented similar trends. D. Compared with the negative control (IC50=16.3 μM), MG63 cells with HOTTIP downregulation enhanced the chemosensitivity to cisplatin (IC50=7.1 μM), the phenomenon could be neutralized by treatment with LiCl (IC50=12.5 μM), *P<0.05.
Discussion
Osteosarcoma, mainly arising from the metaphysis of the long bones, is the most common pediatric bone malignancy in the world [14]. Though advances of modern treatments such as surgery, chemotherapy and the combination of surgery and chemotherapy are improved, the prognosis of osteosarcoma patients remains poor [15]. Because, the chemoresistance that develops in osteosarcoma cells is a significant obstacle to the success of chemotherapy [16]. Therefore, it is very important to identify predictive markers of therapeutic response and develop effective chemotherapeutic drugs.
Recent studies showed that lncRNAs play important roles in diverse biological processes, including cell growth, tumorigenesis and chemoresistance [17]. For example, Zhang et al. indicated that silencing of lncRNA HULC could enhance chemotherapy induced apoptosis in human gastric cancer [18]. Liu et al. showed that lncRNA MEG3 overexpression might contribute to increased cisplatin chemosensitivity via the p53 and Bcl-xl induced mitochondria apoptosis pathway in lung adenocarcinoma patients [19]. Liu et al. showed that lncRNA HOTAIR contributed to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21 (WAF1/CIP1) expression [20].
In the present study, we explored the role of HOTTIP in the regulation of proliferation and chemoresistance of osteosarcoma. Our results showed that HOTTIP was frequently up-regulated in OS tissues and cell lines. Similar to Li findings, HOTTIP was up-regulated and associated with poor prognosis in OS patients [13]. In addition, our findings revealed that overexpression of HOTTIP could increase cell proliferation and colony formation by promoting cell cycle progression. Whereas decreased expression of HOTTIP could inhibit cell proliferation and reduce colony formation by arresting the cell cycle. Furthermore, we found that HOTTIP could up-regulated expression of cell cycle-related proteins (cyclin D1 and CDK4) and β-catenin (a key regulator of the Wnt/β-catenin pathway). Likewise, decreased expression of HOTTIP deceased the expression of cyclin D1, CDK4 and β-catenin. Previous studies revealed that activation of Wnt/β-catenin signaling could up-regulated the expression of cyclin D1, which consequently activates its ligands such as CDK4 and CDK6 and accelerates cell cycle progression [21]. Therefore, we hypothesize that HOTTIP might manipulate the cell cycle through the Wnt/β-catenin pathway.
Chemoresistance is the main reason for poor survival of OS patients. Most of OS patients present chemoresistance and die of tumor relapse and widespread metastasis [22]. However, the underlying mechanisms of chemoresistance are still unclear. In the present study, our data showed that HOTTIP over-expression significantly decreases cell sensitive to cisplatin, whereas HOTTIP inhibition increased cell sensitive to cisplatin, indicating that HOTTIP play an important role in tumor response to chemotherapy. Considering the roles of Wnt/β-catenin signaling, we explored the association between the HOTTIP/Wnt/β-catenin axis and cellular chemosensitivity in OS. We found that the ability of HOTTIP to promote cell proliferation was notably suppressed by the Wnt/β-catenin inhibitor XAV939, whereas the suppressive effects induced by HOTTIP knockdown were neutralized by a potent Wnt/β-catenin agonist. Therefore, we concluded that HOTTIP induced chemoresistance in OS by activating the Wnt/β-catenin pathway.
Taken together, our present study demonstrates that HOTTIP, which is significantly overexpressed in OS, plays a significant role in OS proliferation, accelerate cell cycle progression, and induce chemoresistance via the Wnt/β-catenin pathway. Thus, these results indicate HOTTIP may be a potential therapeutic target for the treatment of OS patients.
Disclosure of conflict of interest
None.
References
- 1.Unni KK. Osteosarcoma of bone. J Orthop Sci. 1998;3:287–294. doi: 10.1007/s007760050055. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma[M] //Pediatric and adolescent osteosarcoma. Springer US; 2010. pp. 3–13. [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 7.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 8.Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie H, Zhu D, Xu C, Zhu H, Chen P, Li H, Liu X, Xia Y, Tang W. Long none coding RNA HOTTIP/HOXA13 act as synergistic role by decreasing cell migration and proliferation in Hirschsprung disease. Biochem Biophys Res Commun. 2015;463:569–574. doi: 10.1016/j.bbrc.2015.05.096. [DOI] [PubMed] [Google Scholar]
- 10.Quagliata L, Matter MS, Piscuoglio S, Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z, Boldanova T. Long noncoding RNA HOTTIP HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Zhao L, Wang YX, Xi M, Liu SL, Luo LL. Long non-coding RNA HOTTIP is correlated with progression and prognosis in tongue squamous cell carcinoma. Tumor Biology. 2015;36:8805–8809. doi: 10.1007/s13277-015-3645-2. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H, Wang Y, Zeng J, Song Y, Gao W. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Cao L, Hang D, Wang F, Wang Q. Long non-coding RNA HOTTIP is up-regulated and associated with poor prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2015;8:11414. [PMC free article] [PubMed] [Google Scholar]
- 14.Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar-Graney K, Shmookler B, Malawer MM. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65:1123–1132. [PubMed] [Google Scholar]
- 15.Spangler JG. Bone biology and physiology: implications for novel osteoblastic osteosarcoma treatments? Med Hypotheses. 2008;70:281–286. doi: 10.1016/j.mehy.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Walters DK, Steinmann P, Langsam B, Schmutz S, Born W, Fuchs B. Identification of potential chemoresistance genes in osteosarcoma. Anticancer Res. 2008;28:673–679. [PubMed] [Google Scholar]
- 17.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Song X, Wang X, Hu J, Jiang L. Silencing of LncRNA hulc enhances chemotherapy induced apoptosis in human gastric cancer. J Med Biochem. 2015;34:1–7. doi: 10.1515/jomb-2015-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P, Lu B, Liu G, Wang Z. The Long Noncoding RNA MEG3 Contributes to Cisplatin Resistance of Human Lung Adenocarcinoma. PLoS One. 2015;10:e0114586. doi: 10.1371/journal.pone.0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21 (WAF1/CIP1) expression. PLoS One. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W. Wnt/β-catenin signaling pathway as novel cancer drug targets. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 22.Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: a 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:323–332. doi: 10.1067/moe.2000.108274. [DOI] [PubMed] [Google Scholar]