Abstract
Purpose: The present study was aimed to evaluate the clinical significance of miR-100 and miR-203 in epithelial ovarian cancer (EOC) patients. Methods: The expression levels of miR-100/203 in EOC tissue and adjacent non-cancerous samples were determined by real-time RT-PCR. Associations between miRNAs expressions and various clinicopathological characteristics were analyzed. Survival rate was determined with Kaplan-Meier and statistically analyzed with the log-rank method between groups. Survival data were evaluated through multivariate. Cox regression analysis. Findings: Our findings showed that miR-100 was significantly down-regulated in EOC tissue specimens than in adjacent non-cancerous tissues. The expression level of miR-203 was significantly higher in EOC tissues compared to adjacent non-cancerous tissues. Decreased expression of miR-100 was strongly associated with high FIGO stage (P=0.012). The high expression of miR-203 was significantly correlated with advanced FIGO stage (p=0.006), advanced histological grade (p=0.03). Kaplan-Meier analysis and log-rank test have suggested that EOC patients with down-regulated miR-100 expression and up-regulated miR-203 expression have shorter overall survival when compared with patients with other expression groups (log-rank test P<0.001). Multivariate Cox proportional hazards model indicated that the status of miR-100 and miR-203 expression levels were independent predictor of overall survival in patients with EOC. Conclusion: Decreased expression and increased expression of miR-100 and miR-203 may be correlated with progression and poor prognosis of EOC.
Keywords: Ovarian cancer, mir-203/Mir-100, oncology, prognosis, PCR
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy and the fifth cause of cancer-related death among women [1,2]. Five-year survival for EOC is dependent on the clinical stage and the most patients are diagnosed at advanced stages where the prognosis of EOC patients is very poor [3,4]. Current data show that the clinical outcome in patients with EOC may be strongly high in early diagnosis. Therefore, effective diagnostic and prognostic biomarkers are required for EOC. It has been indicated that miRNAs may have crucial roles in various biological processes such as differentiation, proliferation, and apoptosis [5-8].
Recent evidences have suggested that miRNAs may have important roles in invasion and metastasis of cancer cells. In the context of EOC, abnormal expression of miRNAs has been previously observed [9-14]. Yeh et al. [12] indicated that miR-138 can act as inhibitor of invasion and metastasis in ovarian cancer cell by targeting SOX4 and HIF-1a. Wang et al., [13], reported that miR-182 can promote cell growth, invasion by targeting programmed cell death 4 (PDCD4) in patients with ovarian carcinomas. Down-regulation of miR-150 has been reported in most primary EOC tissues. Moreover, down-regulation of miR-150 has been confirmed in pre-surgical plasma samples in patients with EOC compared with healthy controls [9,10]. In the context of nasopharyngeal cancer, under expressed miR-100 lead to PLK1 up-regulation, which is involved in progression of NPC [15] Peng et al. [16] suggested that decreased expression of miR-100 can act as a tumor suppressor gene by targeting PLK1 in EOCs. These results highlight that our understanding of the biological processes and miRNA-mediated mechanisms may be helpful for improvement of the diagnosis and treatment for various kinds of human cancers. Abnormal expression of miR-203 has been reported in different kinds of malignant diseases. Iorio et al. [17] reported that miR-203 was up-regulated in ovarian cancer than those normal tissues.
Nevertheless, the clinical significance of these miRNAs in EOC may be helpful. Therefore, the present study was conducted to evaluate the clinical significance of miR-100 and miR-203 in EOC.
Materials and methods
Ethic statement
All protocols in the present study were conducted in accordance with the Declaration of Helsinki Guidelines. All procedures and treatments were reviewed and approved by the Ethics Committees. All participating patients signed the consent forms.
Tissue samples collection
A total of 55 EOC tissue specimens and adjacent non-cancerous tissues from primary EOC patient were collected between 2007 and 2013 in Tehran province hospitals, Iran. The patient’s ages were between 30 to 72 years with a mean age of 51 years. FIGO stage was performed according to International Federation of Gynecology and Obstetrics (FIGO). Moreover, histological subtype and tumor grade were determined using the World Health Organization (WHO) criteria (Figures 1 and 2). Tissues were transported to the Pathology Laboratory, and storedat -80°C. The diagnosis and the histological grading were approved by pathologists. Written informed consent was collected from all patients. The median follow-up time was obtained to be 40 months (range 7-90 months).We defined overall survival (OS) based on the elapsed time from the end of treatment to the death.
Figure 1.
Photomicrographs of the histologic types of epithelial ovarian cancer, stained with hematoxylin and eosin. A. High-grade serous carcinoma. B. Low-grade serous carcinoma.
Figure 2.
Photomicrographs of the histologic types of epithelial ovarian cancer, stained with hematoxylin and eosin. A. Clear cell carcinoma contains clear cells. B. Undifferentiated carcinoma with high-grade malignant cells. C. Well-differentiated ovarian mucinous carcinoma. D. Moderate differentiated adenocarcinoma of ovarian.
Quantitative real-time PCR
MiRNA expression level in EOC tissue specimens and adjacent non-cancerous tissues was evaluated using RT-PCR. We used TRIzol reagent (Invitrogen, Carlsbad, California, USA) to extracted total RNA according to manufacturer’s protocol. The TaqMan microRNA assay and TaqMan universal PCR master mix were used to determine the expression levels of miRNAs, Moreover, the relative amount of miRNAs was normalized with respect to U6 RNA. Relative expression levels of miRNAs were analyzed with the comparative cycle threshold (CT).
Statistical analysis
All variables were evaluated using the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The expression levels of miRNAs compared between EOC tissue specimens and adjacent non-cancerous tissues by the Student’s t-tests. Furthermore, association between miRNAs expression and the clinicopathological characteristics were also evaluated by the chi-square test. Moreover, we plotted survival curves using the Kaplan-Meier method and compared by the log-rank test. The survival data were evaluated by univariate and multivariate Cox regression analyses. The P<0.05 was considered to be significant.
Results
The expression levels of miR-100/203 in collected tissues were determined by real-time RT-PCR (Table 1). Our findings showed that miR-100 was significantly downregulated in EOC tissue specimens than in adjacent non-cancerous tissues (mean ± SD: 4.1 ± 1.23 vs. 9.72± 2.87; p<0.001; Figure 3). On the other hand, the expression level of miR-203 was significantly upregulated in EOC tissues compared to adjacent non-cancerous tissues (mean ± SD: 10.26 ± 3.10 vs. 4.51± 1.27; p<0.001; Figure 3). According to the median value of relative miRNAs expression, the patients were categorized into low and high expression groups. Decreased expression of miR-100 was significantly associated with high FIGO stage (P=0.012). No significant correlation was determined between miR-100 expression and other clinicopathological factors (Table 2). On the other hand, our result revealed that upregulation of miR-203 was remarkably correlated with advanced FIGO stage (p= 0.006), higher histological grade (p=0.03), but no significant correlation with other clinicopathological factors (Table 2).
Table 1.
Sequence of the primers used in this study
| MiRNAs | Primer sequences |
|---|---|
| MiR-100 | RT primer: 5’ GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAG-3’ |
| Forward primer: 5’-GCGGC AACCCGTAGATCCGAA-3’ | |
| Reverse primer: 5’-GTGCAGGGTCCGAGGT-3’. | |
| U6 small nuclear RNA; forward primer: 5’-CGCTTCGGCAGCACATATAC-3’ | |
| Reverse primer: 5’-TTCACGAATTTGCGTGTCAT-3’. | |
| MiR-203 | RT primer: 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTAGTGGT-3’ |
| Forward primer: 50-ACA CTC CAG CTG GCG TGA AAT GTT TAG GAC CA-3 | |
| Reverse primer: 5’-CTC AAC TGG TGT CGT GGA-3’ |
Figure 3.
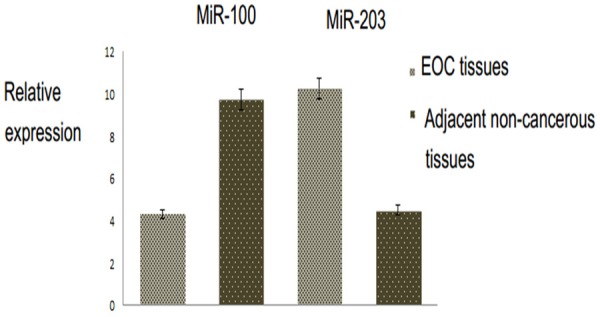
MiR-100 and miR-203 expressions in EOC tissue specimens and adjacent non-cancerous tissues were respectively detected by real-time quantitative RT-PCR assay.
Table 2.
Correlation of miRNAs expression with clinicopathological features
| Variables | No. of cases | No. expression of miR-100 | No. expression of miR-203 | P value of miR-100 | P value of miR-203 | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| High=21 | Low=34 | High=30 | Low=25 | ||||
| Age | NS | NS | |||||
| <50 | 33 | 13 | 20 | 14 | 19 | ||
| ≥50 | 22 | 8 | 14 | 16 | 6 | ||
| Tumor size (cm) | NS | NS | |||||
| <2 | 35 | 15 | 20 | 16 | 19 | ||
| ≥2 | 20 | 6 | 14 | 14 | 6 | ||
| Histological type | NS | NS | |||||
| Serous | 24 | 10 | 14 | 13 | 11 | ||
| Endometrioid | 15 | 6 | 9 | 8 | 7 | ||
| Mucinous | 7 | 2 | 5 | 4 | 3 | ||
| Clear cell | 9 | 3 | 6 | 5 | 4 | ||
| Histological grade | NS | 0.03 | |||||
| G1 | 22 | 10 | 12 | 9 | 13 | ||
| G2 | 13 | 5 | 8 | 8 | 5 | ||
| G3 | 20 | 6 | 14 | 13 | 7 | ||
| FIGO stage | 0.012 | 0.006 | |||||
| I-II | 21 | 8 | 13 | 6 | 15 | ||
| III-IV | 34 | 13 | 21 | 24 | 10 | ||
| Lymph node involvement | 0.016 | 0.02 | |||||
| No | 23 | 12 | 11 | 10 | 13 | ||
| Yes | 32 | 9 | 23 | 20 | 12 | ||
| Serum CA125 level (U/l) | 0.001 | NS | |||||
| <35 U/ml | 20 | 13 | 7 | 12 | 8 | ||
| ≥C35 U/ml | 35 | 8 | 27 | 18 | 17 | ||
The relationship of miRNAs expression with prognosis
Kaplan-Meier analysis and log-rank test have suggested that EOC patients with down-regulated miR-100 expression and upregulated miR-203 expression have shorter overall survival when compared with patients with other expression groups (log-rank test P<0.001, Figure 4). Univariate and multivariate analyses were used to assess whether the miRNAs expression levels and clinicopathological parameters were independent prognostic factors of EOC patient outcomes.
Figure 4.
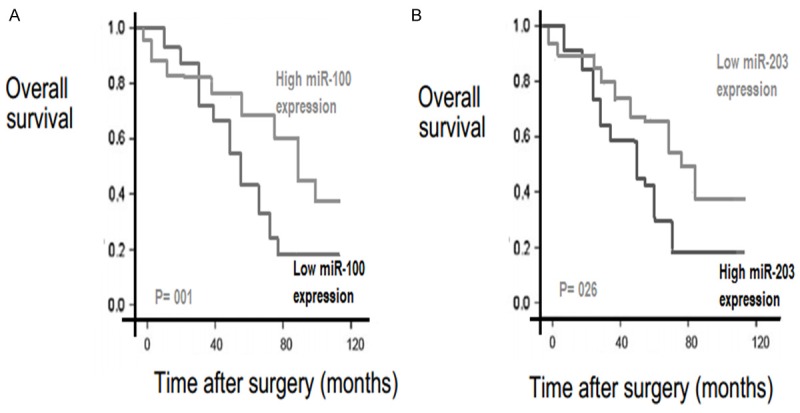
Kaplan-Meier curves for survival time in patients with epithelial ovarian cancer divided according to miRNAs expression levels.
Multivariate Cox proportional hazards model showed that the low expression of miR-100 and high expression of miR-203 and advanced FIGO stage were independent predictor of overall survival (Table 3).
Table 3.
Univariate and multivariate analysis of prognostic parameters by Cox (miR-100)
| Clinicopathological Characteristics | Relative risk (RR) | Univariate log-rank test (P) | Cox multivariable analysis (P) |
|---|---|---|---|
| Age | 0.64 | 0.6 | 0.7 |
| Tumor diameter (cm) | 0.87 | 0.5 | 0.6 |
| Histological type | 0.56 | 0.7 | 0.9 |
| Histological grade | 1.06 | 0.3 | 0.6 |
| FIGO stage | 4.132 | 0.001 | 0.01 |
| Lymph node involvement | 3.282 | 0.027 | 0.031 |
| Serum CA125 level (U/l) | 1.49 | 0.1 | 0.5 |
| miR-100 expression (High/Low) | 5.83 | 0.009 | 0.001 |
| miR-203 expression (High/Low) | 6.17 | 0.003 | 0.001 |
Discussion
Previous studies highlight that our understanding of the biological processes and miRNA-mediated mechanisms may be helpful for improvement of the diagnosis and treatment for various kinds of human cancer. Nevertheless, understandings of the clinical importance of different kinds of miRNAs in EOC are necessary to find effectual markers that can predict the prognosis in patients with advanced EOC.
In the present study, the expression levels of miR-100/203 in EOC tissue specimens and adjacent non-cancerous tissues were determined by real-time RT-PCR. Our findings suggested that miR-100 was significantly down-regulated in EOC tissue specimens than those adjacent normal tissues.
In this study, decreased expression of miR-100 was found to be significantly associated with advanced FIGO stage. Kaplan-Meier analysis and log-rank test suggested that EOC patients with down-regulated miR-100 expression have shorter overall survival. Under expressed miR-100 was confirmed to lead to PLK1 up-regulation, which is involved in progression of nasopharyngeal cancer [15]. MiR-100 has been demonstrated to have both tumor suppressor and oncogenic roles depending on the cell type [18-22]. Furthermore decreased expression of miR-100 has been shown in prostate cancer [23], and in the early stages of hepatocarcinoma indicating that it is involved in carcinogenesis [22]. Peng et al. [16] suggested that miR-100 can act as a tumor suppressor by targeting PLK1 in EOCs. Moreover, they indicated that decreased expression of miR-100 was strongly related to high FIGO stage, higher serum CA125 level and lymph node involvement. Petrelli et al. [22] found that miR-100 expression in vivo was related to the stage of the maturation block underlying the subtypes of myeloid leukemia. They indicated that miR-100 has important role in the molecular etiology of AML, and showed the potential therapeutic effect of miR-100 in cancer.
Abnormal expression of miR-203 has been reported in different kinds of malignant diseases. Iorio et al. [17] reported that miR-203 was up-regulated in ovarian cancer than those normal tissues. Zhao et al. [24] found that the down-regulation of miR-203 may be related to lymph node metastasis in cervical cancer. Down-regulation of miR-203 has been revealed in bladder cancer [25]; the ectopic expression of miR-203 increased the apoptosis in bladder cancer cell lines and is involved in inhibition of cell proliferation. Viticchie’ et al. [26] also demonstrated that miR-203 decreased in clinical primary prostatic tumors and metastatic prostate cancer cell lines. On the other hand, up-regulation of miR-203 was demonstrated to be an independent predictor of poor prognosis in patients with pancreatic adenocarcinoma.
Consistent with these results of previous investigations, we found that the expression level of miR-203 was significantly higher in EOC tissues compared to adjacent non-cancerous tissues. Furthermore, high expression of miR-203 was strongly linked to advanced FIGO stage, higher histological grade. Wang et al., [27] suggested that high miR-203 expression was closely associated with advanced FIGO stage, higher histological grade, lymph node involvement, and positive recurrence.
Furthermore, they reported that high miR-203 expression was associated with shorter overall survival and shorter progression-free survival of EOC patients that these findings are in agreement with our study. MiR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in HCC, and activating multiple targets during hepatocarcinogenesis [28].
Kaplan-Meier analysis and log-rank test indicated that EOC patients with up- regulated miR-203 expression have shorter overall survival when compared with patients with other expression groups. Multivariate Cox proportional hazards model suggested that Multivariate Cox proportional hazards model showed that the low expression of miR-100 and high expression of miR-203 and advanced FIGO stage were independent predictor of overall survival. In this study, the molecular mechanisms of miR-203 were not studied in patients with EOC. Therefore, further studies are needed to prove the prognostic value of these miRNAs.
Conclusions
Our findings indicated that decreased expression and increased expression of miR-100 and miR-203 may be correlated with progression and poor prognosis of EOC.
Disclosure of conflict of interest
None.
References
- 1.Foley OW, Rauh-Hain JA, Del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology. 2013;27:288–294. [PubMed] [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer Statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Farazi TA, Spitzer JI, Morozov P, Tuschl T. MiRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Vang S, Wu HT, Fischer A, Miller DH, MacLaughlan S. Identification of ovarian cancer metastatic miRNAs. PLoS One. 2013;8:e58226. doi: 10.1371/journal.pone.0058226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapira I, Oswald M, Lovecchio J, Khalili H, Menzin A. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110:976–983. doi: 10.1038/bjc.2013.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin M, Yang Z, Ye W, Xu H, Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLoS One. 2014;9:e103965. doi: 10.1371/journal.pone.0103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1a. Int J Cancer. 2013;133:867–878. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 13.Wang YQ, Guo RD, Guo RM, Sheng W, Yin LR. MicroRNA-182 promotes cell growth, invasion, and chemoresistance by targeting programmed cell death 4 (PDCD4) in human ovarian carcinomas. J Cell Biochem. 2013;114:1464–1473. doi: 10.1002/jcb.24488. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Xiao Z, Wang K, Liu W, Hao Q. MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys Res Commun. 2013;441:693–700. doi: 10.1016/j.bbrc.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Alajez NM, Bastianutto C. Significance of Plk1 regulation by miR-100 in human nasopharyngeal cancer. Int J Cancer. 2010;126:2036–2048. doi: 10.1002/ijc.24880. [DOI] [PubMed] [Google Scholar]
- 16.Peng DX, Luo M, Qiu LW, He YL, Wang XF. Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol Rep. 2012;27:1238–44. doi: 10.3892/or.2012.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 18.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–1176. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 20.Henson BJ, Bhattacharjee S, O’Dee DM, Feingold E, Gollin SM. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromosomes Cancer. 2009;48:569–582. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng YS, Zhang H, Zhang XJ, Feng DD, Luo XQ, Zeng CW. MiR-100 regulates cell differentiation and survival by targeting RBSP3, a phosphatase-like tumor suppressor in acute myeloid leukemia. Oncogene. 2011;31:80–92. doi: 10.1038/onc.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrelli A, Perra A, Schernhuber K, Cargnelutti M, Salvi A, Migliore C. Sequential analysis of multistage hepatocarcinogenesis reveals that miR-100 and PLK1 dysregulation is an early event maintained along tumor progression. Oncogene. 2012;31:4517–4526. doi: 10.1038/onc.2011.631. [DOI] [PubMed] [Google Scholar]
- 23.Sun D, Lee YS, Malhotra A, Kim HK, Matecic M, Evans C. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71:1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao S, Yao DS, Chen JY, Ding N. Aberrant expression of miR-20a and miR-203 in cervical cancer. Asian Pac J Cancer Prev. 2013;14:2289–2293. doi: 10.7314/apjcp.2013.14.4.2289. [DOI] [PubMed] [Google Scholar]
- 25.Bo J, Yang G, Huo K, Jiang H, Zhang L, Liu D, Huang Y. MicroRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011;278:786–792. doi: 10.1111/j.1742-4658.2010.07997.x. [DOI] [PubMed] [Google Scholar]
- 26.Viticchie’ G, Lena AM, Latina A, Formosa A, Gregersen LH, Lund AH, Bernardini S. MiR-203 controls proliferation, migration and invasive potential of prostate cancer cell lines. Cell Cycle. 2011;10:1121–1131. doi: 10.4161/cc.10.7.15180. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Zhao X, Wang J, Wen Y, Zhang L, Wang D, Chen H. Upregulation of microRNA-203 is associated with advanced tumor progression and poor prognosis in epithelial ovarian cancer. Med Oncol. 2013;30:681. doi: 10.1007/s12032-013-0681-x. [DOI] [PubMed] [Google Scholar]
- 28.Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. MiR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]


