Abstract
Aspergillus is widely distributed in the Earth’s biosphere. It has strong adaptive capacity, and lives as saprophytic or parasitic life. This study aims to investigate the role of E-cadherin for adhesion of Aspergillus fumigatus blastospores in a human epithelial cell line (A549) and search the correlated molecule in aspergillus. A. fumigatus blastospores were incubated with the total protein of A549 to investigate the binding of E-cadherin and blastospores followed by an affinity purification procedure. After establishing the adhesion model, the adhesion of A. fumigatus blastospores by A549 cells was evaluated by down-regulating E-cadherin of A549 cells with small interfering RNA (siRNA). FVB mice constructed with E-cadherin down-regulation were infected with aspergillus fumigatus. Preliminary exploration of E-cadherin interacting protein on the surface of aspergillus fumigates by immunoprecipitation and mass spectrometry analysis. E-cadherin was adhered to the surface of A. fumigatus blastospore. Adhesion of the blastospores was reduced by blocking or down-regulating E-cadherin in A549 cells. E-cadherin showed limited significance in the process of mice against aspergillus fumigates. Mass spectrometry (MS) analysis indicated the following proteins AFUA_8G07080, AfA24A6.130c, XP_747789 can bind to E-cadherin. In conclusion, E-cadherin is a receptor for adhesion of A. fumigatus blastospores in epithelial cells. This may open a new approach to treat this fungal infection.
Keywords: E-cadherin, receptor, A549, adherence, aspergillus fumigates
Introduction
As an ancient organism, aspergillus is widely distributed in the Earth’s biosphere. It has strong adaptive capacity, and lives as saprophytic or parasitic life. It is the natural decomposers, but also an important pathogen to human. Aspergillus can be the cause of many human diseases, from allergic bronchopulmonary aspergillosis to acute invasive pulmonary aspergillosis, and the clinical manifestations and prognosis vary greatly. Among these diseases, the most serious is invasive aspergillosis, the mortality of which remains very high despite of anti-fungal treatment, which prompts people to explore the pathogenesis of aspergillus.
The pathogenesis of aspergillus usually is that aspergillus spores floating in the air can be inhaled into the host terminal bronchus and alveoli easily as the diameters of spores are less than 5 μm. The inhaled aspergillus spores will face the host’s alveolar epithelial cells inevitably. However, the alveolar epithelial cells not only work as a simple cell barrier, but also participate in the interaction between host and aspergillus as indicated by current studies [1,2]. When aspergillus spores are exposed to epithelial cells, they can adhere to the epithelial cells, then can generate pseudopod and wrap up the aspergillus spores. The spores are phagocytized into the cells, and finally germinate, proliferate, and spread to other parts of the body and cause invasive aspergillosis. From this, it can be seen that adherence of conidia to epithelial cells may be the initial and key step for the pathogensis of apsergillus. This process involves aspergillus and cell surface molecules, epithelial cytoskeleton, cell membranes and so on. But there is no adequate research to identify which the specific molecule is. There are studies about the roles of Dectin-1, TLR and other pattern recognition receptors in the process of phagocytes phagocytizing aspergillus spores, but there is no clear understanding about the roles of them in the human alveolar epithelial cell phagocytosis of aspergillus spores. We have demonstrated in previous experiments, the most common pathogen in aspergillus, i.e. aspergillus fumigates, can adhere to E-cadherin of human alveolar epithelial cells [3]. However, it is unclear what role of E-cadherin is involved in the process of invasion of aspergillus fumigatus spores in vivo and the molecular mechanism in this procedure. So we intend to explore the role of E-cadherin in the adhesion of epithelial cells to aspergillus fumigatus spores through related experiments.
Materials and methods
Fungal strains and preparation of conidia
Conidia were obtained from A. fumigatus strain. The Fungi were cultured on 2% malt agar slants. Conidia were harvested after 7 days at 30°C by rinsing the slants with phosphate-buffered saline (PBS) supplemented with 0.1% Tween 20. Conidia were obtained after filtration through a 8-layer gauze (Millipore, Molsheim, France) to remove mycelium and suspended in cell culture medium (DMEM) without serum. The conidia were then passed through 8 layers of sterile gauze to remove hyphal fragments and enumerated on a hemacytometer. The conidia were incubated at 37°C in DMEM for the indicated times. Fungal cells were washed twice and stored at 4°C for use within 48 h.
A549 cell strains expressed different levels of E-cadherin adhere to aspergillus fumigatus spores
Construction of different A549 cell strains with enhanced and inhibited expression of E-cadherin
TRIzol reagent, T4DNA ligase, restriction enzyme, RPMI-1640 and DMEM cell culture medium, calf serum (Gibco); plasmid extraction and purification kit, DOTAP transfection reagent (Boehringer Mannhe-in, BRM), T4 ligase (Roche), methyl thiazolyl tetrazolium (MTT, Fluka), anti- E-cadherin antibody (Millipore), GeneHogs chemical competent bacteria, 293T cells, and shRNA lentiviral vector (Figure 1) were purchased from Shanghai ImmunoGEN Co., Ltd. With the technology of gene transfection, the cDNA and siRNA of human E-cadherin were cloned into a lentiviral vector. When the target cells in good condition grew to 70%~80%, they were digested and passaged by trypsin, inoculated and grown to the cell density of about 50%, and then shRNA lentivirus crude liquid was added to infect the target cells. After 72 h of the infection, fluorescence microscopy and qPCR were performed to detect interference efficiency. Stable positive strains were obtained after 2 weeks of puromycin screening, and Western-blot assay was conducted.
Figure 1.
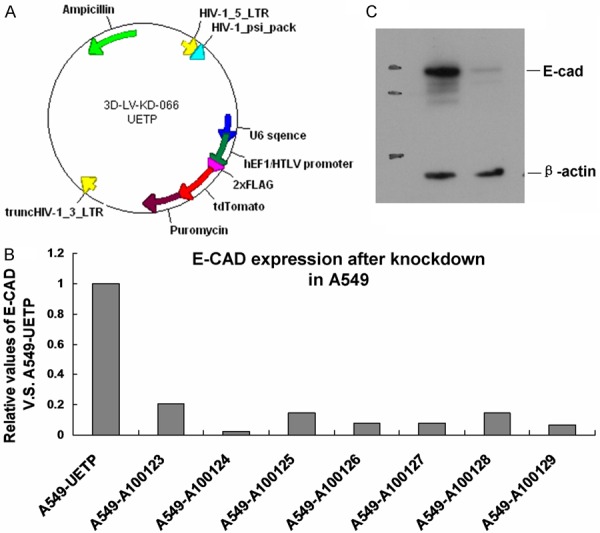
E-cadherin mRNA and protein expression. A. The map of shRNA lentiviral empty vector. B. mRNA level of E-cadherin in siRNA group. After verification, the inhibitory effects of seven shRNA were all over 80%, and finally A100124 was selected. C. The expression map of E-cadherin in transfection strains detected by WB, suggested that the cell strains with E-cadherin downregulation was constructed successfully.
Adherence assay
The immunofluorescence assays were performed to determine the A. fumigatus adherence of A549 cells. The number of organisms adhered by A549 cells was determined using fluorescence assay. The freshly harvested conidia were labeled with FITC (fluorescein isothiocyanate, Sigma) overnight with shaking at 4°C following the method of Sturtevant et al. [4] A549 cells were grown to confluency on Tc-coatedglass coverslips (Cosmobrand company) in a 24-well tissue culture plate. The cells were rinsed once with serum-free culture medium and infected with 106 FITC-cells of A. fumigatus in DMEM medium for 1 h at 37°C. Next, the cells were rinsed twice with PBS to remove any unbound spores. Then the cells were fixed with 3% paraformaldehyde for 15 min at room temperature. The coverslips were examined by laser confocal microscope, and at least 100 cells were examined. The results were expressed as the number of cell-associated organisms per 100 cells. All experiments were performed at least three times.
Construction of mice with E-cadherin down-regulation
Since E-cadherin gene defect has embryonic lethality, and yet there was no mature pulmonary epithelial cell specific promoter, so we chosed to construct mice with E-cadherin downregulation. We selected FVB mice as genetic background. We constructed shRNA expression vectors (without eukaryotic resistance), and identified lentiviral vector by sequencing, and finally chose the expression vector {U6-shRNA-loop-anti shRNA (UBC-eGFP)}. Suitable shRNA sequence was screened. The best interference sequence was linearized and purified, and linearized DNA that can be used for DNA microinjection was obtained. The linearized DNAs were injected into 200 fertilized ova, and the microinjected fertilized ova were sent back to the oviducts of 3-4 surrogate female mice. PCR assay was conducted.
Model of mice infected with aspergillus fumigatus
In FVB mice (purchased) and FVB mice with E-cadherin gene downregulation respectively, 12 mice in each group, cyclophosphamide 200 μg/weight (g) was intraperitoneally injected at Day 0, and conidia of aspergillus fumigatus were instilled through nasal cavity after anesthesia with chloral hydrate from Day 1. Cyclophosphamide 250 mg/kg was injected in mice intraperitoneally at Day 3 and Day 1 before infection, and neutrophils in peripheral blood reduced significantly. From the day of infection to Day 4 after infection, the neutrophil count was consistently lower than 100/μl. Compared with normal group, the difference of neutropenia in IPA and PBS groups has statistical significance (P<0.05), and the neutropenia met the standard of immunodeficiency. The mice underwent tracheotomy and were injected with spores (5×106/mouse). The mice were observed with polypnea, uneven rhythm, and bubbling sound in the air passage, which indirectly confirmed that the spore suspension was injected into the air passage successfully. The survival time of mice, fungal load in tissues and the expression levels of inflammatory factors in mouse serum after infection were observed.
Preliminary exploration of E-cadherin interacting protein on the surface of aspergillus fumigatus
Purified E-cadherin protein was purchased from Beijing Sino Biological Inc. A DNA sequence encoding the extracellular domain of human E-Cad precusor (NP_004351.1) (Met 1 - Ile 707) was fused with a C-terminal polyhistidine tag. The recombinant pro form of human E-Cad consists of 696 amino acids and has a calculated molecular mass of 77 kDa. Aspergillus fumigatus spores and 1 ml of pre-cooling 1× lysis buffer were prepared. The known protein of E-cadherin was added and bound with some proteins of aspergillus fumigatus spores for 2-4 h at 4°C, and finally 1 μg purified antibody was added to a new centrifuge tube. The antigen-antibody mixture was slowly shaken overnight at 4°C, or for 2 h at room temperature. 100 μl agarose beads of Protein A were added to capture the antigen-antibody complex. The antigen-antibody mixture was slowly shaken overnight at 4°C, or for 2 h at room temperature. The protein supernatant was obtained by immunoprecipitation, and analyzed by electrophoresis. Enzymolysis, mass spectrometry analysis, and peptide fingerprint analysis were performed.
The basic parameters of target protein predicted by bioinformatics
The target protein was preliminarily analyzed through the computer. The target sequence was entered into the pubmed, and the basic parameters, structure and other things of protein were analyzed by protein analysis software (www.expasy.org/tools).
Data statistics and analysis
The experimental data were showned as x±SD, and processed factor analysis of variance. Then detected the significance of differences by post hoc Tukey, if P<0.05, it means that the difference has statistical significance.
Results
E-cadherin involved in adhesion to and phagocytosis of aspergillus fumigatus spores by A549 cell
A549 cell strains with E-cadherin downregulation were constructed and the effects of adhesion and phagocytosis were observed with the technology of gene transfection, the cDNA and siRNA of human E-cadherin gene were cloned into a lentiviral vector, and infected A549 cells after being successfully packaged as virus. The transfection was proved successfully by PCR and Western-blot method, and A549 cells with stable low expression of E-cadherin were obtained. A549 cell strains with low expression of E-cadherin were constructed successfully.
The ability of A549 cells with E-cadherin down-regulation to adhere to and phagocytize aspergillus fumigatus spores significantly decreased (Figure 2, P<0.05).
Figure 2.
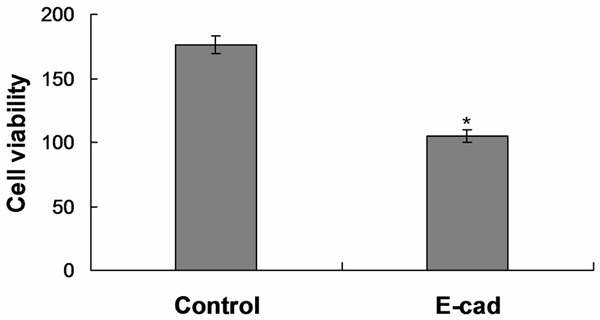
The ability of A549 cells with E-cadherin down-regulation to adhere to and phagocytize spores significantly decreased.
E-cadherin shows limited significance in the process of mice against aspergillus fumigatus
The mice with E-cadherin downregulation were constructed, and aspergillus fumigatus infection model was established. Compared with wild type mice, the survival curves, inflammatory indicators, and fungal load were not significantly different in the mice with down-regulation.
On Day 3 and Day 1 before infection, the mice were intraperitoneally injected with cyclophosphamide 250 mg/kg, the peripheral blood neutrophils reduced significantly. From the same day of infection to Day 4, the number of neutrophils was consistently lower than 100/μl. Compared with the normal group, the difference of neutropenia in IPA and PBS groups had statistical significance (P<0.05), and the neutropenia reached the standard of immunodeficiency, as shown in Table 1.
Table 1.
The number of peripheral blood neutrophils in mice after intraperitoneal injection of cyclophosphamide (neutrophil count/μl)
| Group | n | d0 | d1 | d2 | d3 | d4 |
|---|---|---|---|---|---|---|
| Normal Group | 5 | 658±93 | ||||
| E-cad- Group* | 3 | 76±19 | 73±13 | 73±12 | 63±13 | 78±9 |
| Control# | 3 | 85±52 | 65±14 | 76±25 | 85±7 | 86±35 |
Compared E-cad- group with normal group, P<0.05;
Compared control group with normal group, P<0.05.
No difference between survival curves of aspergillus fumigatus infected mice with reduced E-cad and FVB wild-type
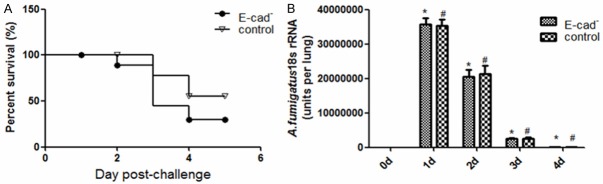
The general conditions of mice after infection. The mice underwent tracheotomy and were injected with PBS/aspergillus fumigatus spores of 5×106/mouse, and the total volume is 25 μl. The mice were observed with polypnea, uneven rhythm, and water-bubbling sound in the air passage, which indirectly confirmed that the spore suspension was injected into the air passages successfully. After recovery of mice from anesthesia, their activities reduced, and their acts were obviously slower. They ate less, lost weight, and liked to gather into groups. The mortality of the two mice groups deaths observed after inoculating aspergillus fumigatus were showed in Figure 3A. The differences between control group and E-cad group had no statistical significance (P>0.05).
Figure 3.
Survival curves and fungal 18S rRNA levels after infection with aspergillus fumigatus. A. After infection with aspergillus fumigatus, the survival curves of mice with reduced E-cad and FVB wild-type mice showed no statistical significance. B. rRNA level of fungal 18S detected by RT-PCR, *compared with Day 0, P<0.05; #compared with Day 0, P<0.05.
rRNA level of fungal 18S decreases following with the treatment time
The whole lung tissues of three mice alive in IPA group were collected every 24 h, their RNA was extracted. rRNA of aspergillus fumigatus 18S was measured by real-time PCR, and the content of aspergillus fumigatus spores in lung was quantified with a standard curve. As shown in Figure 3B, in E-cad- and control groups, compared with Day 0, the content of fungus in lung increased at Day 1, Day 2, Day 3, and Day 4, and the differences had statistical significance (P<0.05). Among them, at Day 1 after infection, the content of aspergillus fumigatus spores was the highest, and the content decreased gradually over time. The content in lung tissue decreased to the positive critical value at Day 4, but there was no statistical significance of differences (P>0.05) between E-cad and control groups.
No difference between TNF-α and IL-6 expression in E-cadherin and control group
The expression level of inflammatory factors in infected mice The serum of mice on Day 3 after infection was collected, and the expression level of TNF-α and IL-6 were detected by Elisa. As shown in Figure 4, compared with control group, the expression level of both TNF-α and IL-6 in E-cad- group showed no statistical significance (P>0.05).
Figure 4.
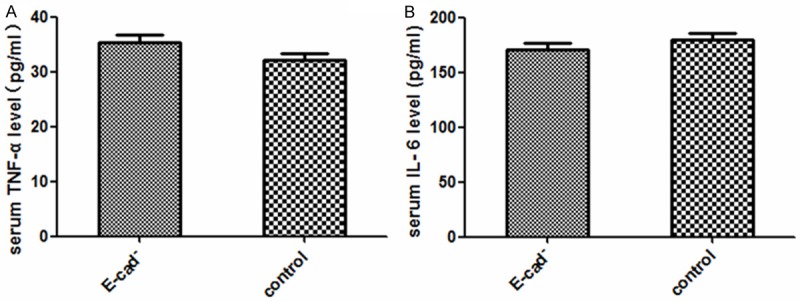
Observation of the TNF-α and IL-6 protein. A. Compare with control group, the expression level of TNF-α in serum of E-cad- group showed no statistical significance (P>0.05); B. Compared with control group, the expression level of IL-6 in serum of E-cad- group had no statistical significance (P>0.05).
The binding protein in aspergillus fumigates combined with human epithelial cell E-cadherin
The protein of E-cadherin was obtained through construction of plasmid, separation, and purification. Meanwhile aspergillus fumigatus was dissociated, and E-cadherin protein was added. The protein combined with E-cadherin was separated through co-immunoprecipitation. Mass spectrometry (MS) analysis indicated the following proteins AFUA_8G07080, AfA24A6.130c, XP_747789 can bind to E-cadherin. Protein sequences were obtained by Mass spectrometry analysis. The AFUA_8G07080, AfA24A6.130c and XP_747789 were listed in Tables 2, 3 and 4, respectively.
Table 2.
AFUA_8G07080 (Protein sequence coverage: 43%)
| MRGLLLAGAL | ALPASVFAHP | AHQSYGLNRR | TVDLNAFRLK | SLAKYVNATE |
| TVIEAPSSFA | PFKPQSYVEV | ATQHVKMIAP | DATFRVVDDH | YVGDNGVAHV |
| HFRQTANGLD | IDNADFNVNV | GKDGKVFSYG | NSFYTGQIPS | SAALTKRDFS |
| DPVTALKGTT | NTLQLPITVD | SASSESTEEK | ESYVFKGVSG | TVSDPKAKLV |
| YFVKDDGTLA | LAWRVETDID | SNWLLTYIDA | KSGEEIHGVV | DYVAEADYQV |
| YAWGINDPTE | GERTVIKDPW | DSVASEFTWI | SDGSTNYTTS | RGNNGIAQSN |
| PSGGSSYLNN | YRPSSSSLSF | KYPYSVSSSP | PSSYIDASII | QLFYTANIYH |
| DLLYTLGFTE | KAGNFEYNTN | GQGGLGNDYV | ILNAQDGSGT | NNANFATPPD |
| GQPGRMRMYV | WTESTPYRDG | SFEAGIVIHE | YTHGLSNRLT | GGPANSNCLN |
| ALESGGMGEG | WSDFMATAIR | LKPGDKRSTD | YTMGEWASNR | AGGIRQYPYS |
| TSLSTNPLTY | TSVNSLNAVH | AIGTVWASML | YEVLWNLIDK | HGKNDAPKPT |
| LRDGVPTDGK | YLAMKLVMDG | MALQPCNPNF | VQARDAILDA | DTALTGGENQ |
| CEIWTAFAKR | GLGAGAKYSS | RNRVGSTEVP | SGVC |
Matched peptides shown in bold.
Table 3.
AfA24A6.130c (Protein sequence coverage: 64%)
| MTGGTVKSDP | GYPLALDAEF | VGPRMRLYVT | MSFAETESYQ | LTCNQQLLYM |
| NYTGVVTMGP | AEQAVFSGNA | EDGATPFGNS | FTHFTFEVRS | HPAYYLTFQN |
| LTGLQTGDER | YKEFENRVFV | GQGRFRVEKA | RSSRVRATLA | INLIYSKTPS |
| SNVEPLEDSR | LHAASVSAVK | ALPLPGDVRN | HPLLHLQSQT | SYAPTNIVAS |
| LGHTLTATQA | VKNVIVLTWH | PNGKARSSHL | SSEPRGQIVD | EPKGANIGTR |
| ARAMRQLIRS | TGHQLQGASL | FRHAPHACRP | RHIEKRGSSL | RGTTVSLTLS |
| LPAAFNSVLR | WGG |
Matched peptides shown in bold.
Table 4.
XP_747789 (Protein sequence coverage: 66%)
| MGRTVDQEVH | AAFVEFRAKE | DDKCLSVQCI | YCQQIRAKNT | SRQKQHLLEC |
| PGLRGQNQTA | QTAPNGIGGA | NGYSATPNGA | AATAPGAGPG | GPGTAALPTP |
| NGPMMTNGVN | PHATSMQTPL | QNMQGRAALP | TPGPPTGPAS | ATSSHQPTRA |
| TPKSKTKAST | SNLPAPPLDD | VHAAFVEFRA | KEEDKCLSVQ | CIYCQQVRAK |
| NTSRQRQHLL | ECPTYLSVMK | DSIPANNLLH | TFPEGDIARS | LQIPAPTLEL |
| DFRMSIKMNP | KVVVGPSIWG | QRDWVTFIGG | QWAGRWGKGI | VLPGGQDSQI |
| VTKESVTHMR | SNYVLQTADD | PPAFIIVKTE | GWLTGAKDVL | DKVNDPNIAD |
| TINPNTYKYR | LNLSMETGDE | RYAFLNTLMW | VASGCRRGHE | VIFDAFRVN |
Matched peptides shown in bold.
Alternative protein parameters and possible functions were analyzed by protein software
The alternative protein parameters and the possible functions have investigated in this study. The results indicated that the protein AFUA_8G07080 may be a protease zymogen secreted to the extracellular space (Table 5). Table 5 also indicated that the protein AfA24A6.130c and XP_747789 may be the outer membrane protein.
Table 5.
Alternative protein parameters of proteins
| ID | Score | Expect | Nominal mass | Piindex | Function |
|---|---|---|---|---|---|
| AFUA_8G07080 | 101 | 1.4e-06 | 68666 | 5.20 | A protease zymogen secreted to the extracellular space |
| AfA24A6.130c | 156 | 5.2e-10 | 34302 | 9.87 | This sequence had no clear similar sequence, but its N-terminal sequence (187 residues) was similar to sequences of other aspergillus. This similar fragment is an outer membrane protein |
| XP_747789 | 221 | 1.6e-16 | 43247 | 8.79 | This sequence may contain two domains. The N terminal is transcription activation domain (Transcription activator BRG1) and is similar to human source. The C terminal is UPF0311 protein which is an outer membrane protein |
Discussion
The adhesion of pathogen to the body, especially the adhesion to structural cells such as epithelial cells and other cells is the first step to invade into the body. The molecules involved in the adhesion of pathogen to the body’s cells may become promising therapeutic targets, such as M protein of influenza virus. For aspergillus, the aspergillus spores which have failed to adhere to or invaded into epithelial cells can be eventually packaged by pulmonary surface active protein or directly phagocytized by macrophages, and therefore lose the opportunity of growth and reproduction within the body. As an important adhesion molecule with the largest number, E-cadherin not only mediates adhesion between epithelial cells, but also participates in the adhesion between microorganisms and host cells [5,6]. Our study also confirmed the important role of E-cadherin in the process of aspergillus fumigatus’s adhesion to alveolar epithelial cells. However, even after blocking E-cadherin, there are still fumigatus spores adhering to alveolar epithelial cells, suggesting that E-cadherin only plays part role in aspergillus fumigatus’s adhesion to alveolar epithelial cells. As an evolutionarily advanced microorganism, the aspergillus may not completely dependent on E-cadherin pathway in the process of adhesion to epithelial cells.
The improvement of large-scale and high-throughput sequencing technology has brought about rapid development of bioinformatics, which makes bioinformatics permeate into various life science studies. Under the impetus of bioinformatics, we can understand the structural basis of interaction between proteins (even pathogen) and host quickly, and presume the role of new protein [7-10]. Through mass spectrometry method, we found there were three kinds of protein in aspergillus fumigatus that could bind to E-cadherin. However, as shown by the protein software analysis, one of the proteins, AFUA_8GO7080, was secretory protein, and thus it is impossible to mediate the adhesion of aspergillus fumigatus fixed to epithelial cells effectively. Therefore, we just need to focus on the other two kinds of proteins in the subsequent confirmatory experiments.
Disclosure of conflict of interest
None.
References
- 1.Rutella S, Locatelli F. Strategies to harness immunity against infectious pathogens after heploidentical stem cell transplantation. Am J Transl Res. 2011;3:404–421. [PMC free article] [PubMed] [Google Scholar]
- 2.Alekseeva L, Huet D, Femenia F, Mouyna I, Abdelouahab M, Cagna A, Guerrier D, Tichanne-Seltzer V, Baeza-Sguiban A, Chermette R, Latge JP, Berkova N. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol. 2009;9:33. doi: 10.1186/1471-2180-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu XY, Shi Y, Zhang PP, Zhang F, Shen YY, Su X, Zhao BL. E-cadherin mediates adhesion and endocytosis of Aspergillus fumigatus blastospores in human epithelial cells. Chin Med J (Engl) 2012;125:617–621. [PubMed] [Google Scholar]
- 4.Sturtevant J, Latgé JP. Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J Infect Dis. 1992;166:580–586. doi: 10.1093/infdis/166.3.580. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Tatley F, Aldwell FE, Dunbier AK, Guilford PJ. N-terminal E-cadherin peptides act as decoy receptors for Listeria monocytogenes. Infect Immun. 2003;71:1580–1583. doi: 10.1128/IAI.71.3.1580-1583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderton JM, Rajam G, Romero-Steiner S, Summer S, Kowalczyk AP, Carlone GM, Sampson JS, Ades EW. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb Pathog. 2007;42:225–236. doi: 10.1016/j.micpath.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Tuncbag N, Gursoy A, Keskin O. Prediction of protein-protein interactions: unifying evolution and structure at protein interfaces. Phys Biol. 2011;8:035006. doi: 10.1088/1478-3975/8/3/035006. [DOI] [PubMed] [Google Scholar]
- 8.Wass MN, David A, Sternberg MJ. Challenges for the prediction of macromolecular interactions. Curr Opin Struct Biol. 2011;21:382–390. doi: 10.1016/j.sbi.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Goh WQ, Ow GS, Kuznetsov VA, Chong S, Lim YP. DLAT subunit of the pyruvate dehydrogenase complex is upregulated in gastric cancer-implications in cancer therapy. Am J Transl Res. 2015;7:1140–1151. [PMC free article] [PubMed] [Google Scholar]
- 10.Sudha G, Nussinov R, Srinivasan N. An overview of recent advances in tructural bioinformatics of protein-protein interactions and a guide to their principles. Prog Biophys Mol Biol. 2014;116:141–150. doi: 10.1016/j.pbiomolbio.2014.07.004. [DOI] [PubMed] [Google Scholar]