Abstract
Osteosarcoma (OS) is the most common primary malignant bone tumor. Parafibromin-inactivating mutations have been reported in various malignancies. In this study, the effects and relevant mechanisms of ectopic parafibromin expression were identified in the extracellular environment, cytoplasm and nucleus of OS cells. Our results indicate that parafibromin located in the nucleus can induce apoptosis and G1 phase arrest in OS cells. Parafibromin was found to suppress the MEK/ERK and PI3K/AKT signaling pathways, leading to activation of caspase 3 and caspase 9. Overall, these studies demonstrate the anti-tumor activity of parafibromin in the OS cell line, and provide insight into relevant mechanisms that may lead to novel treatments for OS.
Keywords: Parafibromin, osteosarcoma cell, proliferation, apoptosis, cell cycle
Introduction
Osteosarcoma, the most common primary malignant bone tumor, accounts for 5% of childhood cancers and represents the fifth most frequent tumor in young adults [1]. Better knowledge of oncogenic processes in osteosarcoma could lead to the development of new therapeutic approaches [2].
Parafibromin is encoded by the hyperparathyroidism 2 (HRPT2) gene located on chromosome 1q31, whose germline mutation leads to hereditary hyperparathyroidism-jaw tumor (HPT-JT) syndrome [3]. Parafibromin-inactivating mutations have been reported in various malignancies, such as parathyroid cancer [4], major renal cell tumor [5], lung cancer [6]. Previous studies have demonstrated that parafibromin overexpression could inhibit colony formation and proliferation, and induce cell cycle arrest in oral squamous cell carcinoma cell [7], colon carcinoma cell [8], and cervical cancer cell [9].
In our study, the effects and relevant mechanisms of ectopic parafibromin expression was examined in the extracellular environment, cytoplasm and nucleus of osteosarcoma cells.
Methods
Cell culture
The human osteosarcoma cell lines, MG-63 and HOS, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37°C with 5% CO2.
Transfection
Parafibromin expression vector was constructed by PCR amplification of human CDC73 natural ORF mammalian expression plasmid (HG11656-UT, Sino Biological Inc, Beijing, China) utilizing the primers (Sangon Biotech, Shanghai, China): 5’-TTCGAATTCATGGCGGACGTGCTTAGCGTCC-3’ (forward) and 5’-TACCGTCGACATAATTCAGAATCTCAAGTGC-3’ (reverse), and cloning into the mammalian expression vector pEGFP-C2 (Clontech Laboratories, Inc., Shanghai, China). Cells were transfected with pEGFP-C2-parafibromin plasmid at 70% confluence 20 to 24 h after seeding on dishes using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. As the methods of Hahn et al. [10], site directed mutagenesis was carried out on pEGFP-C2-parafibromin to convert basic amino acids within each signal to neutral amino acids using the QuickChange II site directed mutagenesis kit (Stratagene, CA, USA).
RT-PCR
Cellular RNA was isolated using TRIzol reagent (Invitrogen). cDNA was then synthesized from 1 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. PCR amplification of cellular cDNA was performed in 15 μl mixtures. RT-PCR conditions were: one cycle at 94°C for 5 min, followed by 35 cycles of 94°C for 30 sec, 72°C for 45 sec, 58°C for 45 sec, and final extension at 72°C for 10 min. Finally, products were resolved by 1% agarose gel electrophoresis, and visualized by ethidium bromide staining and a UV imaging system (UVP, Upland, CA, USA).
Fluorescence
Parafibromin expression was indicated by using EGFP. Cells were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Beyotime, Shanghai, China). Photographic images were taken using an Olympus CX71 fluorescence microscope (Olympus, Tokyo, Japan).
Colony formation assay
Cells (300 cells/well) were seeded in twenty four-well plates. After 24 h, cells were treated with various concentrations of recombinant parafibromin protein (CSB-MP750783HU, CUSABIO® Biotech Inc., Wuhan, China) (0, 100, 200, 300, 400, or 500 ng/ml). Three days later, colonies were stained with 0.05% crystal violet (Beyotime) and counted in 4 to 5 random fields for each of the duplicate samples by using a microscope at 100 × magnification.
Pharmacological agents
IGF-1 is a PI3K/mTOR agonist described previously [11], and was purchased from R&D Systems. Cells transfected with pEGFP-C2-parafibromin plasmid were incubated with IGF-1 (100 ng/ml) for 24 hours.
Apoptosis
According to the manufacturer’s instructions (KeyGEN, Nanjing, China), cells were resuspended in 200 μl binding buffer containing Annexin V-FITC (0.5 μg/ml), and incubated at room temperature in the dark. After 20 min, 400 μl binding buffer was added and samples were immediately analyzed on a FACSCalibur flow cytometer (Becton Dickinson Medical Devices, Shanghai, China).
Cell cycle
Cells were harvested, and fixed with 70% ethanol at 4°C. After centrifugation (1500 × g for 5 min), supernatants were discarded, cellular DNA was stained with 10 μM propidium iodide (KeyGEN), and samples were analyzed by a FACSCalibur flow cytometer (Becton Dickinson).
Western blot
Protein extracts were resolved on SDS-PAGE, followed by electrotransfer to nitrocellulose membranes (Bio-Rad, Philadelphia, PA, USA). Following a blocking step in 5% milk in TBST, membranes were incubated with primary and secondary antibodies. Cell signaling-related proteins were probed using the following antibodies: parafibromin, Akt, phospho-Akt, PI3K, phospho-PI3K, MEK, phospho-MEK, ERK, and phospho-ERK antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). β-actin (Santa Cruz) was used as an internal control. The reaction was followed by probing with peroxidase-coupled secondary antibodies, including anti-mouse IgG, anti-rabbit IgG, or anti-goat IgG antibodies at dilutions ranging from 1:1000 to 1:2000 (ZS-BIO, Beijing, China), and binding results were visualized by enhanced chemiluminescence (Santa Cruz).
Statistical analysis
Differences between groups within experiments were examined by ANOVA test. Data are presented as the mean ± standard deviation (SD). Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 17.0; SPSS, Inc.) and significance was established at p < 0.05.
Results
Parafibromin-expressing OS cells
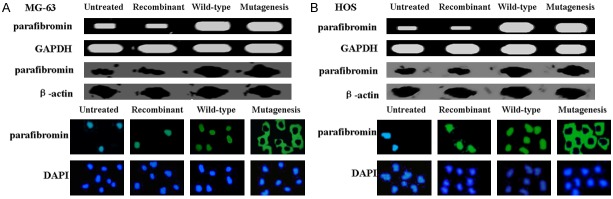
We investigated the consequence of exogenous parafibromin expression in OS cells. MG-63 and HOS cells were transfected with pEGFP-C2-parafibromin (wild-type) or pEGFP-C2-parafibromin mutagenesis (mutagenesis), and parafibromin expression was measured by using RT-PCR and western blot. The results of RT-PCR and western blot analysis confirmed exogenous expression of parafibromin expression in OS cells after transfection (Figure 1). Fluorescence analysis showed wild-type parafibromin was located in the nucleus of OS cells and mutational parafibromin was located in cytoplasm of OS cells (Figure 1).
Figure 1.
Detection of parafibromin following transfection. Parafibromin mRNA levels in MG-63 cells (A) and HOS cells (B) detected using RT-PCR. Detection of parafibromin protein in MG-63 cells (A) and HOS cells (B) in western blot assays. Location of parafibromin in MG-63 cells (A) and HOS cells (B) detected using fluorescence. Untreated: OS cells without treatment; Recombinant: OS cells treated with recombinant parafibromin; Wild-type: OS cells were transfected with pEGFP-C2-parafibromin plasmid; Mutagenesis: site directed mutagenesis was carried out on pEGFP-C2-parafibromin.
The distinct effects of parafibromin in extracellular environment, cytoplasm and nucleus of OS cells
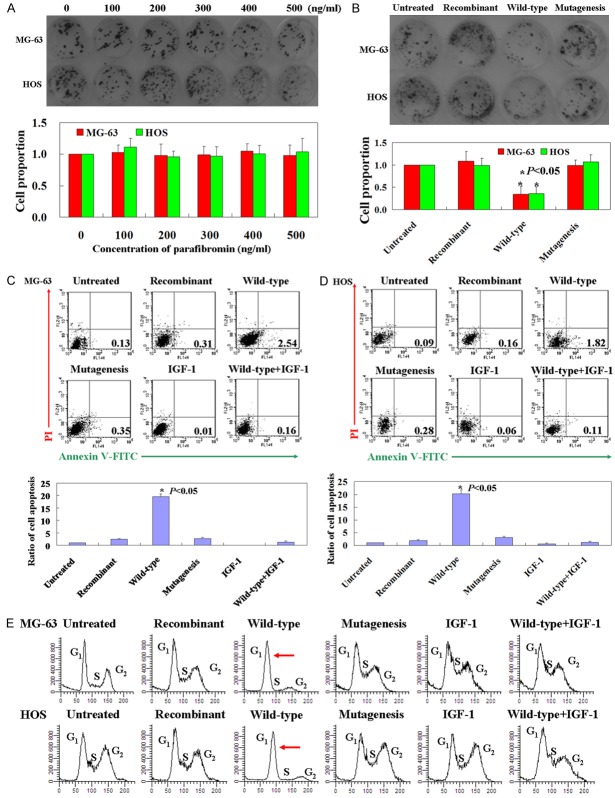
Recombinant parafibromin and mutational parafibromin showed no anti-tumor effect on OS cells by using colony formation assay (Figure 2A and 2B, p > 0.05). However, wild-type parafibromin could inhibit proliferation of both MG-63 and HOS cells (Figure 2B, p < 0.05). Annexin V-FITC and PI double staining was used to detect apoptotic cells. The apoptotic ratio was 18-21 times higher in cells expressing wild-type parafibromin protein than that of recombinant parafibromin or mutational parafibromin treated cells (Figure 2C and 2D, p < 0.05). Cell cycle was examined by using PI staining. Wild-type parafibromin protein could increase the cell number in G1 phase, while no changes were found in recombinant parafibromin or mutational parafibromin treated cells (Figure 2E). IGF-1 is a PI3K/mTOR agonist commonly used to active the P13K/AKT signaling pathway [11]. In this study, we found that IGF-1 could reverse the anti-tumor effect of wild-type parafibromin in OS cells (Figure 2).
Figure 2.
Anti-tumor roles of parafibromin in vitro. A. Cell proliferation of OS cells treated with different concentrations of recombinant parafibromin was detected by using colony formation assay. B. Cell proliferation of OS cells treated with recombinant parafibromin, wild-type parafibromin and mutational parafibromin. C, D. Apoptotic ratio of cells were determined from Annexin-V/PI double-staining assays. The histogram indicates statistically significant results (p < 0.05). E. Cell cycle changes were determined by staining with PI. The red arrow indicates G1 phase arrest. Untreated: OS cells without treatment; Recombinant: OS cells treated with recombinant parafibromin; Wild-type: OS cells were transfected with pEGFP-C2-parafibromin plasmid; Mutagenesis: site directed mutagenesis was carried out on pEGFP-C2-parafibromin. IGF-1 was used as a positive control.
Wild-type parafibromin suppresses the P13K/AKT and MEK/ERK signaling pathways in OS cells
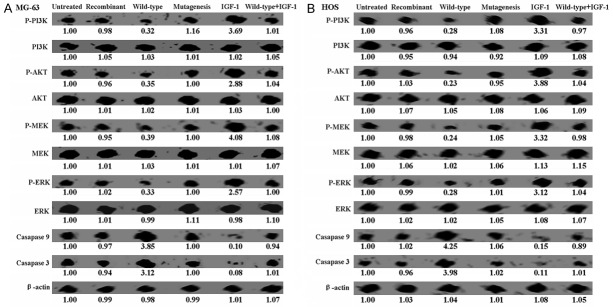
Western blot was performed to identify the mechanism of apoptosis induced by wild-type parafibromin. While total levels of P13K, AKT, MEK, and ERK showed no changes, the levels of phospho-P13K, phospho-AKT, phospho-MEK, and phospho-ERK were observed to be significantly lower in wild-type parafibromin expressing cells (Figure 3). Following the inhibition of the MEK/ERK and PI3K/AKT pathways, activation of caspase 3 and caspase 9 was observed in wild-type parafibromin expressing cells (Figure 3). To test the hypothesis that wild-type parafibromin can induce apoptosis in OS cells by suppressing the P13K/AKT and MEK/ERK signaling pathways, IGF-1 was used to treat OS cells as a control. IGF-1 could up-regulated the levels of phospho-P13K, phospho-AKT, phospho-MEK, and phospho-ERK in parafibromin transfected OS cells (Figure 3). In combination, these results suggest that wild-type parafibromin induced apoptosis in OS cells via the P13K/AKT and MEK/ERK signaling pathways.
Figure 3.
Western blot assays of cell signaling proteins. While total levels of P13K, AKT, MEK, and ERK showed no changes, the levels of phospho-P13K, phospho-AKT, phospho-MEK, and phospho-ERK were observed to be significantly lower in wild-type parafibromin expressing cells. Untreated: OS cells without treatment; Recombinant: OS cells treated with recombinant parafibromin; Wild-type: OS cells were transfected with pEGFP-C2-parafibromin plasmid; Mutagenesis: site directed mutagenesis was carried out on pEGFP-C2-parafibromin. IGF-1 was used as a positive control.
Discussion
Downregulated expression of parafibromin protein promoted the pathogenesis, proliferation, differentiation, and mobility of many cancers [12-14]. Lin et al. [15] reported that overexpression of wild-type parafibromin induced apoptosis in transfected HeLa cells. In our study, we also found that parafibromin could induce apoptosis and G1 arrest in OS cells. These data indicated that parafibromin is a suppressor in cancer cells.
The main finding of this study is that distinct anti-tumor roles of parafibromin in the extracellular environment, cytoplasm and nucleus of OS cells. We confirmed that recombinant parafibromin showed no effects on OS cells. It means that parafibromin in extracellular environment could not play anti-tumor roles in OS cells. Lack of receptor of parafibromin in OS cell surface may be the main reason for losing its function. Hahn et al. [10] reported that parafibromin has three distinct nucleolar localization signals (NoLS) at amino acid residues 76-92 (NoLS 1), 192-194 (NoLS 2), and 393-409 (NoLS 3) in addition to its known nuclear localization (NLS) signal at 125-139. In this study, we constructed mutational parafibromin which is located in the cytoplasm of OS cells by using site directed mutagenesis. Interestingly, we found that mutational parafibromin showed no anti-tumor roles in OS cells. Parafibromin could play its anti-tumor roles only in the nucleus of OS cells. Furthermore, we confirmed the mechanisms of parafibromin in OS cells. Inhibition of MEK/ERK and PI3K/AKT pathways could induce genes that play major roles in cell cycle, apoptosis, and angiogenesis [16]. In our study, we found that parafibromin could induce dephosphorylation of MEK, ERK, PI3K and AKT. To test the hypothesis that parafibromin can induce apoptosis in OS cells by suppressing the P13K/AKT and MEK/ERK signaling pathways, IGF-1 was used to treat OS cells as a control. IGF-1 is an agonist commonly used to active the PI3K/mTOR signaling pathway [11]. In this study, IGF-1 was found to induce the proliferation of OS cells, and to inhibit apoptosis in OS cells. More importantly, the mechanism of parafibromin in the OS cells was determined using IGF-1 as a positive control.
Collectively, parafibromin could play its anti-tumor roles only in the nucleus of OS cells, and these involved suppression of the MEK/ERK and PI3K/AKT signaling pathways. Moreover, apoptosis was induced by activation of caspase 3 and caspase 9 following the inhibition of the MEK/ERK and PI3K/AKT pathways. These results provide valuable insight into potential novel treatments for OS.
Disclosure of conflict of interest
None.
References
- 1.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Ando K, Heymann MF, Stresing V, Mori K, Rédini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers (Basel) 2013;5:591–616. doi: 10.3390/cancers5020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, Agarwal SK, Sood R, Jones MP, Moses TY, Haven C, Petillo D, Leotlela PD, Harding B, Cameron D, Pannett AA, Höög A, Heath H 3rd, James-Newton LA, Robinson B, Zarbo RJ, Cavaco BM, Wassif W, Perrier ND, Rosen IB, Kristoffersson U, Turnpenny PD, Farnebo LO, Besser GM, Jackson CE, Morreau H, Trent JM, Thakker RV, Marx SJ, Teh BT, Larsson C, Hobbs MR. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 4.Truran PP, Johnson SJ, Bliss RD, Lennard TW, Aspinall SR. Parafibromin, galectin-3, PGP9.5, Ki67, and cyclin D1: using an immunohistochemical panel to aid in the diagnosis of parathyroid cancer. World J Surg. 2014;38:2845–2854. doi: 10.1007/s00268-014-2700-2. [DOI] [PubMed] [Google Scholar]
- 5.Cui C, Lal P, Master S, Ma Y, Baradet T, Bing Z. Expression of parafibromin in major renal cell tumors. Eur J Histochem. 2012;56:e39. doi: 10.4081/ejh.2012.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia P, Wang W, Xu XY, Wang JP, Takano Y, Zheng HC. Parafibromin expression in lung normal tissue and carcinoma: its comparison with clinicopathological parameters of carcinoma. Histol Histopathol. 2011;26:1039–1047. doi: 10.14670/HH-26.1039. [DOI] [PubMed] [Google Scholar]
- 7.Rather MI, Swamy S, Gopinath KS, Kumar A. Transcriptional repression of tumor suppressor CDC73, encoding an RNA polymerase II interactor, by Wilms tumor 1 protein (WT1) promotes cell proliferation: implication for cancer therapeutics. J Biol Chem. 2014;289:968–976. doi: 10.1074/jbc.M113.483255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo JH, Chung TM, Youn H, Yoo JY. Cyto-plasmic parafibromin/hCdc73 targets and destabilizes p53 mRNA to control p53-mediated apoptosis. Nat Commun. 2014;5:5433. doi: 10.1038/ncomms6433. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Zhang JH, Panicker LM, Simonds WF. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–17425. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn MA, Marsh DJ. Identification of a functional bipartite nuclear localization signal in the tumor suppressor parafibromin. Oncogene. 2005;24:6241–6248. doi: 10.1038/sj.onc.1208778. [DOI] [PubMed] [Google Scholar]
- 11.Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Yang XF, Huang KQ, Ren L, Gou WF, Shen DF, Zhao S, Sun HZ, Takano Y, Zheng HC. The clinicopathological significance and biological functions of parafibromin expression in head and neck squamous cell carcinomas. Tumour Biol. 2015;36:9487–9497. doi: 10.1007/s13277-015-3618-5. [DOI] [PubMed] [Google Scholar]
- 13.Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Downregulated parafibromin expression is a promising marker for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Virchows Arch. 2008;452:147–155. doi: 10.1007/s00428-007-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng HC, Wei ZL, Xu XY, Nie XC, Yang X, Takahashi H, Takano Y. Parafibromin expression is an independent prognostic factor for colorectal carcinomas. Hum Pathol. 2011;42:1089–1102. doi: 10.1016/j.humpath.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Zhang JH, Panicker LM, Simonds WF. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–17425. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–6866. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]