Abstract
The cardiovascular safety of DPP4 inhibitors as a class, especially in regards to heart failure, has been questioned after the publication of first trials (SAVOR-TIMI 53 and EXAMINE) assessing the cardiovascular risks of DPP4 inhibitors alogliptin and sitagliptin in 2013. Although there were no increased risks in composite cardiovascular outcomes, the SAVOR-TIMI 53 trial reported a 27% increase in hospitalization for heart failure in diabetic patients who received the DPP4 inhibitor saxagliptin. There has been substantial increase in knowledge on the heart failure effect of DPP4 inhibition since 2013. This review will summarize the role of the DPP4/incretin axis in heart failure and discuss the findings from recent large scale clinical trials assessing the effects of DPP4 inhibitors on heart failure.
Keywords: DPP4, incretin, heart failure, cardiovascular outcomes
Introduction
Dipeptidyl peptidase-4 (DPP4) inhibitors are a novel class of oral anti-diabetic agents. The first two large clinical trials assessing the cardiovascular safety of DPP4 inhibitors (EXAMINE and SAVOR-TIMI 53) suggest DPP4 inhibitors are safe from a cardiovascular perspective. Both alogliptin and saxagliptin neither increase nor decrease the risk of cardiovascular disease [1,2]. However, the SAVOR-TIMI 53 trial reported a 27% increase in hospitalization for heart failure in diabetic patients who received saxagliptin, raising concern of DPP4 inhibitors in heart failure. Outcomes with respect to heart failure were not mentioned in the initial report of the EXAMINE trial by White et al., although 28% of patients had congestive heart failure at baseline. Two other trials assessing cardiovascular outcomes of incretin-based drugs (sitagliptin and lixisenatide) were recently completed [3,4]. In addition, the heart failure data of EXAMINE trials was also reported last year. In this review, we will summarize the role of the DPP4/incretin axis in heart failure and discuss the findings from recent large scale clinical trials assessing the effects of DPP4 inhibitors on heart failure.
Diabetes as a risk factor for heart failure
Type 2 diabetes has been associated with increased risk for heart failure [5-7]. Almost 50% of patients with type 2 diabetes develop heart failure, and those with both diabetes and established heart failure have more severe outcomes [8,9]. This is largely due to type 2 diabetes’ ability to accelerate coronary artery disease. Diabetic patients are more likely to develop coronary artery atherosclerosis at an early stage and affect distal coronary segments [5,10]. Patients with diabetes develop fewer collateral vessels in response to ischemia [11], and this can be attributed to impaired production or responsiveness to angiogenic growth factors [12]. In addition, hypertension, dilated cardiomyopathy and extracellular fluid volume expansion are also important pathogenic factors for the development of heart failure in individuals with diabetes [13-15].
Anti-diabetic drugs and heart failure
Diabetes has been widely regarded as a major risk factor for cardiovascular disease, with doubled cardiovascular risk in patients with diabetes [16-18]. Interestingly, a large scale clinical trial in 2008 suggested intensive glycemic control increase, rather than decreased cardiovascular mortality in patients with diabetes (Hazard ratio: 1.35; 95% CI: 1.04-1.76; p = 0.02) [19]. The Food and Drug Administration (FDA) therefore recommends manufacturers to assess cardiovascular safety for all new anti-diabetic drugs.
The cardiovascular side effects of anti-diabetic drugs are believed to be an important reason for the increased heart failure risk in intensive glycemic control patients. Most of the currently available oral anti-diabetic drugs have more or less shown adverse cardiovascular side effects. In addition to DPP4 inhibitors, commonly used oral anit-diabetes agent categories include biguanides, thiazolidinediones, and sulfonylureas.
Metformin is the most commonly used biguanide for the treatment of diabetes. It decreases hepatic glucose production, improves glucose uptake and utilization, and improves insulin sensitivity [20]. Metformin has been contraindicated in diabetic patients with heart failure because it may increase the risk of lactic acidosis [21-23]. However, later studies demonstrated that metformin is safe and may be associated with lower morbidity and mortality in diabetic patients with established heart failure when compared to other diabetic therapy, although no placebo-controlled large scale trials on heart failure are available [24,25].
Thiazolidinediones, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by activating nuclear peroxisome proliferator activated receptor γ (PPAR-γ). However, they have been associated with both fluid retention and increased risk for heart failure [26,27]. The use of rosiglitazone has been reported to cause diabetic macular edema-related vision lost in several cases [28,29] and it increases the risk of fractures in women [30]. In a study involving 30 diabetic patients who used pioglitazone or rosiglitazone and had both lower extremity edema and macular edema, Ryan et al. reported that thiazolidinedione use may be the cause of fluid retention in certain patients and that drug cessation could result in rapid resolution of both peripheral and macular edema [31]. Moreover, in 2007 Nissen and Wolski [32] found that rosiglitazone was associated with an increased risk of myocardial infarction and a borderline increased risk of cardiovascular death, although this finding could not be confirmed in an interim analysis of the RECORD study (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of glycaemia in Diabetes; a company-sponsored clinical trial evaluating cardiovascular outcomes of rosiglitazone) at that time [33]. Analysis of other recent studies by the European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP) also suggests an increased risk of cardiovascular diseases in rosiglitazone users [34-37].
Sulfonylureas such as glipizide, glyburide, and glimepiride have been widely used for hypoglycemic management of type 2 diabetes. Clinical evidence has shown that sulfonylurea treatment increases the risks of heart failure [38,39]. Compared to metformin, the 2nd generation sulphonylureas (glipizide, gliquidone, glimepiride, glibenclamide, and gliclazide) increased the risk of developing congestive heart failure by 18% (hazard ratio, 95% CI: HR 1.18, 1.04-1.34) in a retrospective cohort study involving 91,521 diabetic patients in the UK. All-cause mortality also increased in patients treated with either 1st or 2nd generation sulphonylureas [38]. In another study following 4,902 diabetic women for a mean duration of 11 years, Li et al. reported that sulfonylurea increased the risks of coronary heart disease and a longer duration of sulfonylurea use was associated with a higher risk of coronary heart disease [40]. Furthermore, metformin/sulfonylurea combination therapy increased the risk of coronary heart disease by 3.27-fold compared with users of metformin monotherapy (relative risk, 95% CI: 3.27, 1.31-8.17) [40]. Patients treated with high-dose sulfonylurea also showed higher risks for heart failure than those with low-dose sulfonylurea [39]. These results suggest sulfonylureas dose-dependently and time-dependently increase the risk of heart failure.
DPP4 and incretin hormones in diabetes
DPP4 is an enzyme that cleaves N-terminal dipeptides from proteins with alanine, proline or serine at the penultimate position. It is widely expressed in a variety of cell types including T cells, macrophages, dendritic cells, adipocytes, hepatocytes, endothelial cells and epithelial cells [41,42]. The substrates of DPP4 include various regulatory peptides (eg. glucagon-like peptide-1 [GLP-1], GLP-2, gastric inhibitory polypeptide [GIP], etc), chemokines/cytokines (eg. stromal cell-derived factor 1 [SDF-1], eotaxin, RANTES, GM-CSF, interleukin-3 [IL-3], etc), and neuropeptides (eg. neuropeptide Y, peptide YY, etc). Cleavage of N-terminal dipeptides by DPP4 changes the bioactivity of its substrates. In addition to its enzymatic activity, DPP4 also interacts with many ligands. For example, it provides co-stimulatory signals to T cells via interacting with adenosine deaminase (ADA) and it also serves as the entry protein for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) by interacting with spike protein located on the envelope of the virus [43-46].
Incretin hormones, such as Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are short peptide hormones secreted by the gut in response to nutrients. GLP-1(7-36), the active form of GLP-1, is secreted by L cells located in the ileum and colon, while GIP is secreted by intestinal K cells which are located in the proximal small intestine. These incretin hormones are released into circulation within minutes of meal ingestion and promote insulin secretion by activating their receptors located on the pancreatic β cells. Incretin-receptor activation leads to glucose-induced insulin secretion and stimulates β-cell proliferation. GLP-1 also induces glucose-dependent inhibition of glucagon, promotes satiety, and delays gastric emptying, which are responsible for the weight loss observed from persistent use of GLP-1 analogs. GIP also promotes adipose tissue energy storage and augments bone formation by stimulating osteoblast proliferation. Both GLP-1(7-36) and GIP(1-42), the active forms of GLP-1 and GIP respectively, are rapidly degraded by DPP4, resulting in very short half-lives (minutes long) in vivo [47,48]. DPP4 inhibitors and DPP4 resistant GLP-1R agonists are increasingly used in clinic as anti-diabetic drugs due to their weight neutral/weight loss effect, and good safety and tolerability profiles [42,49]. DPP4 inhibitors are a novel class of oral anti-diabetic drugs. Since the first DPP4 inhibitor, sitagliptin, was approved by FDA in 2006, 8 more DPP4 inhibitors have entered the market for the treatment of diabetes: three approved by the FDA (saxagliptin, linagliptin, and alogliptin), one approved by the EU (vildagliptin), and four approved in Japan (anagliptin, teneligliptin, trelagliptin, and omarigliptin). DPP4 inhibitors have modest effects on glycemic control, resulting in a 0.2-0.8% reduction in glycated hemoglobin (HbA1c) [50-52].
Clinical trials on DPP4 inhibitors and heart failure
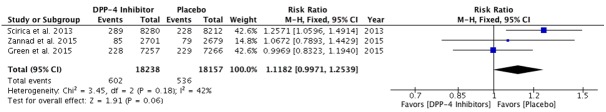
DPP4 inhibitors have been increasingly used in clinic due to a low incidence of hypoglycemia, and weight neutrality. Although animal studies suggest a beneficial effect of DPP4 inhibition on cardiovascular disease, their cardiovascular safety, especially in regards to heart failure of DPP4 inhibitors as a class, has been questioned after the completion of the first of two large clinical trials (EXAMINE and SAVOR-TIMI 53) assessing the cardiovascular risks of DPP4 inhibitors [1,2]. While both trials showed no increased risks in composite cardiovascular outcomes (Table 1), the SAVOR-TIMI 53 trial reported a 27% increase in hospitalization for heart failure, without excess heart failure related mortality, among diabetic patients who received saxagliptin as compared with those who received placebo (3.5% vs. 2.8%; hazard ratio, 95% CI: 1.27, 1.07-1.51; P = 0.007). In a later analysis, findings suggested that previous history of heart failure, eGFR<60 ml/m, elevated BNP and albumin/creatinine ratio were the strongest predictors of heart failure hospitalization [53]. Outcomes with respect to heart failure were not mentioned in the initial report of EXAMINE trial by White et al., although 28% of patients had congestive heart failure at baseline. There have been two other large scale trials, one on DPP4 inhibitor sitagliptin (TECOS) and one on GLP-1R agonist lixisenatide (ELIXA), which reported the cardiovascular outcomes of these two drugs including effects on heart failure hospitalization in 2015 [3,4]. There was no excess heart failure risk noted with sitagliptin or lixisenatide. In TECOS, the hospitalization rate for heart failure was identical with sitagliptin and placebo (3.1 vs. 3.1%; HR, 1.00, 0.83-1.20; P = 0.98). Randomized controlled clinical trial evidence for lixisenatide also showed similar heart failure hospitalization rates compared to placebo (HR = 0.96; 95% CI, 0.75-1.23). In addition to these two trials completed this year, the EXAMINE group also published heart failure hospitalization data earlier this year [54]. First occurrences of heart failure hospitalization for alogliptin and placebo groups were 3.1 and 2.9% respectively (HR 1.07, 0.79-1.46, p = 0.68). Further investigation is still needed to conclude if there is an excess heart failure risk in DPP4 inhibition therapy as there is a marginal effect based on currently available trials (overall risk 1.12, 0.99-1.25; P = 0.06, Figure 1). However, it must be noted that the weight of SAVOR-TIMI 53 trial is over 40% in this analysis. The heart failure effects of gliptins and GLP-1R agonists need further confirmation in ongoing cardiovascular outcome trials, including two trials on linagliptin and several trials on incretin therapies.
Table 1.
Completed cardiovascular outcome trials for DPP4 inhibitors and GLP-1R agonist
| Study | Drug (Sponsor) | Phase | Study Design | Duration (weeks) | Intervention Arms Dosage (N)+ | Hazard Ratio, 95% CI, (p value) |
|---|---|---|---|---|---|---|
| DPP4 Inhibitors | ||||||
| SAVOR-TIMI 53 | Saxagliptin (AstraZeneca) | 4 | RCT/DB | 104 | Saxagliptin 5 mg or 2.5 mg (8280) | 1.00, 0.89-1.12, (0.99) |
| Placebo (8212) | ||||||
| EXAMINE | Alogliptin (Takeda) | 3 | RCT/DB | 160 | Alogliptin 25/12.5/6.25 mg (2701) | 0.96, 1.16*, (0.32) |
| Placebo (2679) | ||||||
| TECOS | Sitagliptin (Merck Sharp & Dohme Corp.) | 3 | RCT/DB | 156 | Sitagliptin 100 mg or 50 mg (7332) | 0.98, 0.88-1.09, (NS) |
| Placebo (7339) | ||||||
| GLP-1 Receptor Agonists | ||||||
| ELIXA | Lixisenatide (Sanofi) | 3 | RCT/DB | 108 | Lixisenatide 10-20 μg (3034) | 1.02, 0.89-1.17, (NS) |
| Placebo (3034) | ||||||
DB, double blind; NS, not significant; RCT, randomized control trial;
the upper boundary of the one-sided repeated confidence interval, at an alpha level of 0.01.
Figure 1.
Effects in trials of incretin therapies on heart failure hospitalization. The risk ratios are given for DPP4 inhibitors versus placebo. The diamond indicates the overall risk and 95% CI for heart failure hospitalization.
Possible cardiovascular mechanisms of DPP4 inhibition in heart failure
GLP-1R is widely expressed in cardiovascular system such as endothelium, vascular smooth muscle, and cardiac atrium [55,56]. GLP-1R activation on endothelial cells has been shown to be able to increase cAMP, followed by the activation of Protein kinase A (PKA) and endothelial nitric oxide synthase (eNOS) [57]. The activation of eNOS subsequently results in the release of nitric oxide (NO) and vessel relaxation. Studies in humans also confirmed the vasodilatory effect of GLP-1 [58,59]. GLP-1 analogs are also able to reduce blood pressure by increasing urinary sodium excretion [60], promoting Atrial natriuretic peptide (ANP) release from atrium [61], and relaxing vascular smooth muscle cells [62]. The activation of GLP-1R in the central nervous system induces satiety and thus reduces body weight and cardiovascular risk [63]. In addition to enhancing GLP-1 effect, DPP4 inhibitors also increases SDF-1, a chemoattractant for many types of hematopoietic cells including cardiac stem cells, endothelial progenitor cells, and mesenchymal stem cells [64]. Preservation of SDF-1 by DPP4 inhibition enhances chemotaxis and repopulation ability of hematopoietic progenitor cells and stem cells, increasing the neovascularization of injured tissues [65-69].
However, GLP-1R activation has also been shown to increase heart rate and blood pressure by activating sympathetic nervous system [70]. A recent study also showed DPP4 inhibitor MK-0626 impaired cardiac function accompanied by modest cardiac hypertrophy, while genetic DPP4 deficiency improved cardiac function after transverse aortic constriction surgery [71], suggesting drug related unspecific effects may play a role in cardiac function. Therefore, DPP4 inhibition modulates cardiovascular function by mechanisms involving multiple organs (Figure 2).
Figure 2.
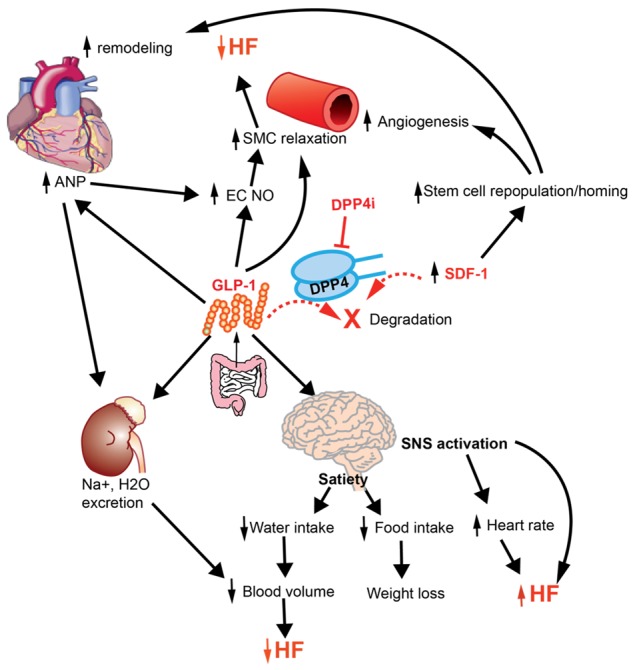
Cardiovascular Effects of DPP4 inhibition. DPP4 inhibition modulates cardiovascular function by preserving GLP-1 and SDF-1. ANP, Atrial natriuretic peptide; EC, endothelial cell; GLP-1, glucagon-like peptide-1; HF, heart failure; NO, nitric oxide; SDF-1, stromal cell-derived factor 1; SMC, smooth muscle cell; SNS, sympathetic nervous system.
Conclusions
The cardiovascular safety, especially in regards to heart failure, of DPP4 inhibitors has gained much attention since 2013. The heart failure assessments on three out of the four FDA-approved DPP4 inhibitors showed saxaglitpin, but not alogliptin and sitagliptin, may increase the risks of heart failure. These results suggest this might not be a class effect of all DPP4 inhibitors. Furthermore, meta-analysis of these 3 trials indicates a marginal effect on hospitalization rate for heart failure. Further investigations are required to come to a conclusion on whether DPP4 inhibition may result in increased risk of heart failure.
Acknowledgements
This work was supported by grants from NIH (K01 DK105108), AHA (15SDG25700381 and 13POST17210033), Mid-Atlantic Nutrition Obesity Research Center (NORC) under NIH award number P30DK072488, and Boehringer Ingelheim (IIS2015-10485).
Disclosure of conflict of interest
J.Z. has received funding support from Boehringer Ingelheim. The other authors declare no conflict of interests.
References
- 1.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 2.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F, Zannad F EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 4.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB. Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta Diabetol. 2003;40(Suppl 2):S358–361. doi: 10.1007/s00592-003-0120-0. [DOI] [PubMed] [Google Scholar]
- 7.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 9.Leung AA, Eurich DT, Lamb DA, Majumdar SR, Johnson JA, Blackburn DF, McAlister FA. Risk of heart failure in patients with recent-onset type 2 diabetes: population-based cohort study. J Card Fail. 2009;15:152–157. doi: 10.1016/j.cardfail.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 11.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 12.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 13.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 14.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 15.Fioretto P, Muollo B, Faronato PP, Opocher G, Trevisan R, Tiengo A, Mantero F, Remuzzi G, Crepaldi G, Nosadini R. Relationships among natriuresis, atrial natriuretic peptide and insulin in insulin-dependent diabetes. Kidney Int. 1992;41:813–821. doi: 10.1038/ki.1992.125. [DOI] [PubMed] [Google Scholar]
- 16.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB Sr, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D’Agostino R, Liau CS, Mas JL, Rother J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 18.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail. 2011;4:53–58. doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338:265–266. doi: 10.1056/NEJM199801223380415. [DOI] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:e129. doi: 10.2337/dc07-1686. [DOI] [PubMed] [Google Scholar]
- 24.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 25.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 26.Benbow A, Stewart M, Yeoman G. Thiazolidinediones for type 2 diabetes. All glitazones may exacerbate heart failure. BMJ. 2001;322:236. [PubMed] [Google Scholar]
- 27.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 28.Colucciello M. Vision loss due to macular edema induced by rosiglitazone treatment of diabetes mellitus. Arch Ophthalmol. 2005;123:1273–1275. doi: 10.1001/archopht.123.9.1273. [DOI] [PubMed] [Google Scholar]
- 29.Oshitari T, Asaumi N, Watanabe M, Kumagai K, Mitamura Y. Severe macular edema induced by pioglitazone in a patient with diabetic retinopathy: a case study. Vasc Health Risk Manag. 2008;4:1137–1140. doi: 10.2147/vhrm.s3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, Group AS. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 31.Ryan EH Jr, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, Williams DF. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–570. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 33.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 34.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 36.Ruiter R, Visser LE, van Herk-Sukel MP, Geelhoed-Duijvestijn PH, de Bie S, Straus SM, Mol PG, Romio SA, Herings RM, Stricker BH. Prescribing of rosiglitazone and pioglitazone following safety signals: analysis of trends in dispensing patterns in the Netherlands from 1998 to 2008. Drug Saf. 2012;35:471–480. doi: 10.2165/11596950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Nissen SE. The rise and fall of rosiglitazone. Eur Heart J. 2010;31:773–776. doi: 10.1093/eurheartj/ehq016. [DOI] [PubMed] [Google Scholar]
- 38.Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAlister FA, Eurich DT, Majumdar SR, Johnson JA. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur J Heart Fail. 2008;10:703–708. doi: 10.1016/j.ejheart.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care. 2014;37:3106–3113. doi: 10.2337/dc14-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matheeussen V, Baerts L, De Meyer G, De Keulenaer G, Van der Veken P, Augustyns K, Dubois V, Scharpe S, De Meester I. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem. 2011;392:189–198. doi: 10.1515/BC.2011.002. [DOI] [PubMed] [Google Scholar]
- 42.Zhong J, Maiseyeu A, Davis SN, Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015;116:1491–1504. doi: 10.1161/CIRCRESAHA.116.305665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, Zhang B, Shi Y, Yan J, Gao GF. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. doi: 10.1016/j.atherosclerosis.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Scheen AJ. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med. 2013;125:7–20. doi: 10.3810/pgm.2013.05.2659. [DOI] [PubMed] [Google Scholar]
- 47.Zhong J, Gong Q, Goud A, Srinivasamaharaj S, Rajagopalan S. Recent Advances in Dipeptidyl-Peptidase-4 Inhibition Therapy: Lessons from the Bench and Clinical Trials. J Diabetes Res. 2015;2015:606031. doi: 10.1155/2015/606031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohrborn D, Wronkowitz N, Eckel J. DPP4 in Diabetes. Front Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 51.Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE. Efficacy and tolerability of vildagliptin in drug-naive patients with type 2 diabetes and mild hyperglycaemia*. Diabetes Obes Metab. 2008;10:675–682. doi: 10.1111/j.1463-1326.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 52.Aschner P, Katzeff HL, Guo H, Sunga S, Williams-Herman D, Kaufman KD, Goldstein BJ Sitagliptin Study 049 Group. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:252–261. doi: 10.1111/j.1463-1326.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 53.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL SAVOR-TIMI 53 Steering Committee and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 54.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 55.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravassa S, Zudaire A, Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94:316–323. doi: 10.1093/cvr/cvs123. [DOI] [PubMed] [Google Scholar]
- 57.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 59.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 60.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38:132–139. doi: 10.2337/dc14-1958. [DOI] [PubMed] [Google Scholar]
- 61.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 62.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 63.van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1–16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 64.Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015;6:477. doi: 10.3389/fimmu.2015.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18:867–873. doi: 10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigeta T, Aoyama M, Bando YK, Monji A, Mitsui T, Takatsu M, Cheng XW, Okumura T, Hirashiki A, Nagata K, Murohara T. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. doi: 10.1161/CIRCULATIONAHA.112.096479. [DOI] [PubMed] [Google Scholar]
- 67.Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 68.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Leré-Déan C, Contreres JO, Sulpice E, Levy BI, Plouët J, Tobelem G, Le Ricousse-Roussanne S. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 70.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- 71.Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KW, Abdullah T, Baggio LL, Drucker DJ. Inhibition of Dipeptidyl Peptidase-4 Impairs Ventricular Function and Promotes Cardiac Fibrosis in High Fat-Fed Diabetic Mice. Diabetes. 2016;65:742–754. doi: 10.2337/db15-1224. [DOI] [PubMed] [Google Scholar]