Abstract
Chemotherapy is a common type of preoperative neoadjuvant treatment and postoperative adjuvant or palliative therapy for many different types of malignancies. Certain chemotherapeutic agents can induce bizarre epithelial atypia that mimics malignancy. Unfamiliarity with these changes could potentially cause confusion with a neoplastic or infectious process. The endometrium is one of the few sites where chemotherapy-induced epithelial atypia has not been appreciated. We identified four patients with marked cytologic atypia of the endometrial glandular epithelium from the surgical pathology files of Severance Hospital. The histopathologic features, immunostaining results and medical records of these patients were reviewed. All patients underwent hysteroscopic examination with endometrial curettage for investigation of vaginal bleeding. They had previously undergone chemotherapy for uterine cervical cancer (n=1), rectal cancer (n=2) and myelodysplastic syndrome (n=1). The chemotherapy regimens included alkylating agents (busulfan, cyclophosphamide, ifosfamide, cisplatin, and oxaliplatin), pyrimidine antagonists (capecitabine, decitabine, and 5-fluorouracil), taxanes (paclitaxel), and topoisomerase inhibitors (irinotecan and etoposide). On histopathological examination, the atypical epithelial changes included marked nuclear enlargement and pleomorphism, a degenerative-looking chromatin pattern, abundant microvacuolated cytoplasm, and preservation of the nuclear/cytoplasmic ratio. This study demonstrates that certain chemotherapeutic agents may cause bizarre, reactive atypia of the endometrial glandular epithelium. These changes should not be interpreted as neoplastic or infectious in nature. An awareness of prior exposure to cytotoxic agents and a familiarity with the nature and distribution of these bizarre alterations is essential to avoid misinterpretation of the morphologic features and prevent unnecessary treatment.
Keywords: Endometrium, epithelial atypia, chemotherapy, malignancy, cytomegalovirus
Introduction
Chemotherapy uses medicine as a definitive treatment or as an adjuvant therapy to destroy and weaken malignant cells in the body, and it is known for its propensity to induce cytologic atypia in epithelial cells [1], including bizarre atypical epithelial alterations [2]. Although the lungs and urinary bladder have drawn the most attention as targets of cytotoxic effects [3,4], such atypical changes can be widespread throughout the body. Chemotherapy-induced epithelial atypia has been noted in many organs and tissues such as the sinonasal tract [2], esophagus [5], stomach [5-7], small intestine [5,8], uterine cervix [4,9] and breast [10,11]. Epithelial alterations associated with chemotherapeutic agents include marked nuclear enlargement, pleomorphism and hyperchromasia, and intracytoplasmic and/or intranuclear inclusion-like vacuoles, potentially causing pathologists to consider a diagnosis of a malignancy or viral-induced cytopathic effect.
We recently encountered some cases in which chemotherapy-induced epithelial atypia was initially thought by several observers to be a malignancy, but was later related to prior chemotherapy once an appropriate history and associated medical conditions were considered. We herein demonstrate chemotherapy-induced epithelial atypia observed in the endometrium of patients with epithelial or hematologic malignancies. These cases highlight the endometrium as yet another possible target of the cytotoxic effects of chemotherapeutic agents. To our knowledge, the endometrium is one of the few sites where chemotherapy-induced epithelial atypia has not been documented.
Patients and methods
Study group
Four patients with atypical epithelial alterations in their endometrial curettage specimens were identified from the surgical pathology files of the Severance Hospital, Yonsei University College of Medicine between 2006 and 2015. All patients underwent hysteroscopic examination with endometrial curettage for presumed non-neoplastic disease of the endometrium. This study was reviewed and approved by the Institutional Review Board at Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea (2015-1413-001).
Histopathological evaluation
For each case, tissue had been fixed in 10% neutral-buffered formalin, embedded in paraffin and stained with hematoxylin and eosin. A representative tissue block of each case was selected for immunohistochemical staining. Routinely processed tissue sections from the endometrial curettage specimens were evaluated for a variety of histopathological findings associated with chemotherapy-induced cytotoxic effects. These included the location and distribution of the atypical changes, nuclear size and the degree of pleomorphism, hyperchromaticity and chromatin quality, appearance of nucleoli, nuclear/cytoplasmic ratio, presence and location of mitoses and atypical mitotic figures, and the quality of the cytoplasmic content. Clinical information was obtained from the medical records or by communication with the referring pathologist or the patients’ physicians in order to determine their oncologic history, treatment regimen, associated medical diseases and the condition prompting endometrial curettage.
Immunohistochemical evaluation
Formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated with a xylene and alcohol solution. Immunohistochemical staining used the Ventana Benchmark XT automated staining system (Ventana Medical Systems, Inc., Tucson, AZ, USA) or Dako Omnis (Dako, Agilent Technologies, Inc., Carpinteria, CA, USA) according to the manufacturer’s instructions. Antigen retrieval used Cell Conditioning Solution (CC1; Ventana Medical Systems, Inc.) or EnVision FLEX Target Retrieval Solution, High pH (Dako, Agilent Technologies, Inc.). Sections were incubated with primary antibodies (Table 1). After chromogenic visualization using ultraView Universal DAB Detection Kit (Ventana Medical Systems, Inc.) or EnVision FLEX/HRP (Dako, Agilent Technologies, Inc.), slides were counterstained with hematoxylin and coverslipped. Appropriate positive and negative controls were stained concurrently to validate staining.
Table 1.
Antibodies used for immunohistochemical staining
| Antibody | Source | Clone | Dilution |
|---|---|---|---|
| Ki-67 | Dako, Agilent Technologies, Inc., Carpinteria, CA, USA | MIB-1 | 1:150 |
| p16 | Ventana Medical Systems, Inc., Tucson, AZ, USA | E6H4 | Prediluted |
| p53 | NovoCastra Laboratories, Ltd., Newcastle upon Tyne, UK | DO-7 | 1:300 |
| CMV | Dako, Agilent Technologies, Inc., Carpinteria, CA, USA | CCH2+DDG9 | 1:100 |
| HSV | Dako, Agilent Technologies, Inc., Carpinteria, CA, USA | Polyclonal | RTU |
CMV: cytomegalovirus; HSV: herpes simplex virus.
Epstein-Barr virus-encoded RNA in situ hybridization
The tissue sections obtained from the endometrial curettage specimens were used for Epstein-Barr virus (EBV)-encoded RNA in situ hybridization (EBER-ISH). The sections were deparaffinized with xylene, pretreated with proteinase K for 20 min, and incubated with fluorescein isothiocyanate (FITC)-conjugated EBER oligonucleotide probes (Novocastra Laboratories) at 55°C for 2 h. The sections were rinsed in water and incubated with horseradish peroxidase-conjugated anti-FITC antibody for 15 min before adding the chromogen to produce an alcohol-insoluble dark nuclear stain in EBV-positive cells. We used EBV-absent lymphoid tissues processed using the hybridization mixture without EBER oligonucleotides as negative controls.
Results
Clinical findings
Table 2 summarizes the clinical features of four patients whose endometrial curettage specimens exhibited severe epithelial atypia. The age of the patients ranged from 39 to 63 years. In all patients, endometrial curettage was performed to investigate vaginal bleeding. The contributing pathologist was initially unaware of a history of prior chemotherapy in either case. However, all patients had malignancies: one (Patient 1) had uterine cervical cancer, two (Patients 2 and 3) had rectal cancer and one (Patient 4) had myelodysplastic syndrome (MDS). Patient 1, with a history of chemotherapy for FIGO stage IIB uterine cervical cancer, had developed septic shock and neutropenia. Patients 2 and 3, with histories of neoadjuvant chemotherapy for stage III rectal cancer, underwent low anterior resection of the colon. Patient 3 also received postoperative palliative chemotherapy. Patient 4, with a history of conditioning treatment with alkylating agents prior to allogeneic peripheral blood stem cell transplantation for MDS, developed acute cutaneous graft-versus-host disease and cytomegalovirus (CMV) viremia. Moreover, she had an intrauterine device inserted.
Table 2.
Summary of clinical features
| Patient | Age | Malignancy (stage) | Chemotherapy regimens (purpose if available) | Associated medical problems | Gynecologic symptoms | Interval from chemotherapy to curettage | Hysteroscopic findings | Current status | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | Uterine cervical cancer (FIGO IIB) | Paclitaxel, cisplatin, ifosfamide | Septic shock, neutropenia | Vaginal bleeding | 4 months | Atrophic endometrium | NED | 54 months |
| 2 | 39 | Rectal cancer (cT4N+M0/ypT0N0M0) | Capecitabine (NAC) | None | Vaginal bleeding | 2 months | Atrophic endometrium | NED | 12 months |
| 3 | 42 | Rectal cancer (cT3N+M0/ypT3N1aM0) | Capecitabine (NAC); FOLFOX, FOLFIRI, etoposide, ifosfamide (palliative) | None | Vaginal bleeding | 10 months | Atrophic endometrium | NED | 17 months |
| 4 | 52 | Myelodysplastic syndrome (RAEB-2) | Decitabine; cyclophosphamide, busulfan (conditioning) | PBSCT, CMV viremia, IUD in situ | Vaginal bleeding | 4 months | Atrophic endometrium | NED | 4 months |
NED: no evidence of endometrial disease; NAC: neoadjuvant chemotherapy; FOLFOX: leucovorin, 5-fluorouracil and oxaliplatin; FOLFIRI: leucovorin, 5-fluorouracil and irinotecan; RAEB: refractory anemia with excess blasts; PBSCT: allogeneic peripheral blood stem cell transplantation; CMV: cytomegalovirus; IUD: intrauterine device.
There was significant variation in the chemotherapeutic treatment agents among individual patients. Nevertheless, three of four patients had been exposed to single or multiple alkylating agents (busulfan, cyclophosphamide, ifosfamide, cisplatin or oxaliplatin), and three patients had been exposed to single pyrimidine antagonists (capecitabine, 5-fluorouracil or decitabine). In addition, one patient with rectal cancer (Patient 3) received postoperative palliative treatment with combination regimens including topoisomerase inhibitors (irinotecan and etoposide). The time interval from chemotherapy to endometrial curettage ranged from 2 to 10 months.
Hysteroscopic examination revealed an atrophic endometrium that was not easily indented by pressure and looked thin and relatively homogeneous. In two patients, a small amount of fluid was identified within the endometrial cavity. However, no evidence of cystic changes, polyps, irregular thickening, or mass-like lesions was identified. Ultrasonography findings were similar to the hysteroscopic ones in all cases. No patient had endometrial thickening, a mass-like lesion, cavitary distortion, abnormal echogenicity, or adnexal abnormalities. The endometrial thickness was less than 5 mm in all patients.
Clinical follow-up information was available in all patients. The follow-up period ranged from 4 to 54 months. All patients were still alive at the end of the follow-up period. Patient 3 developed vaginal bleeding once again 7 months after the curettage, but a repeat curettage was not performed. The remaining three patients reported no gynecologic symptoms during the post-curettage period.
Histopathological findings
Under the light microscope, endometrial curettage specimens demonstrated severely enlarged nuclei showing hyperchromasia and pleomorphism with irregular and misshapen outlines, while the nuclear/cytoplasmic ratio was not increased (Figure 1A). The enlarged nuclei were three to seven times their normal size (Figure 1B). Despite prominent hyperchromasia, the chromatin pattern showed homogeneous basophilic staining without coarse clumping. The distribution of the atypical cells was variable. They were present in the endometrial glandular epithelium in all cases, whereas the endometrial stromal cells or endothelial cells did not exhibit significant cytologic atypia. Some of the endocervical glands also contained atypical cells with irregularly contoured, hyperchromatic nuclei (Figure 1C). In some areas, the epithelial atypia involved the endometrial glands in a discontinuous, patchy fashion (Figure 1D). In other areas, the atypical cells were distributed more densely and occupied the full thickness of the epithelium (Figure 1E). In addition to the nuclear alterations, epithelial atypia consisted of occasional conspicuous nucleoli, either single or multiple, a normal or slightly low nuclear/cytoplasmic ratio, and abundant, basophilic cytoplasm with microvesiculation. Some of the atypical cells contained intracytoplasmic (Figure 1F) and intranuclear (Figure 1G, 1H) inclusion-like vacuoles, raising the suspicion of a viral-induced cytopathic effect. In particular, CMV endometritis associated with CMV viremia was strongly suspected in Patient 4. However, an inflammatory infiltration of lymphocytes and plasma cells, a characteristic feature of CMV endometritis, was not identified. No evidence of an invasive tumor was identified. No mitotic figures or abnormal ones were discovered despite a thorough search and serial sectioning. Necrosis was not observed.
Figure 1.
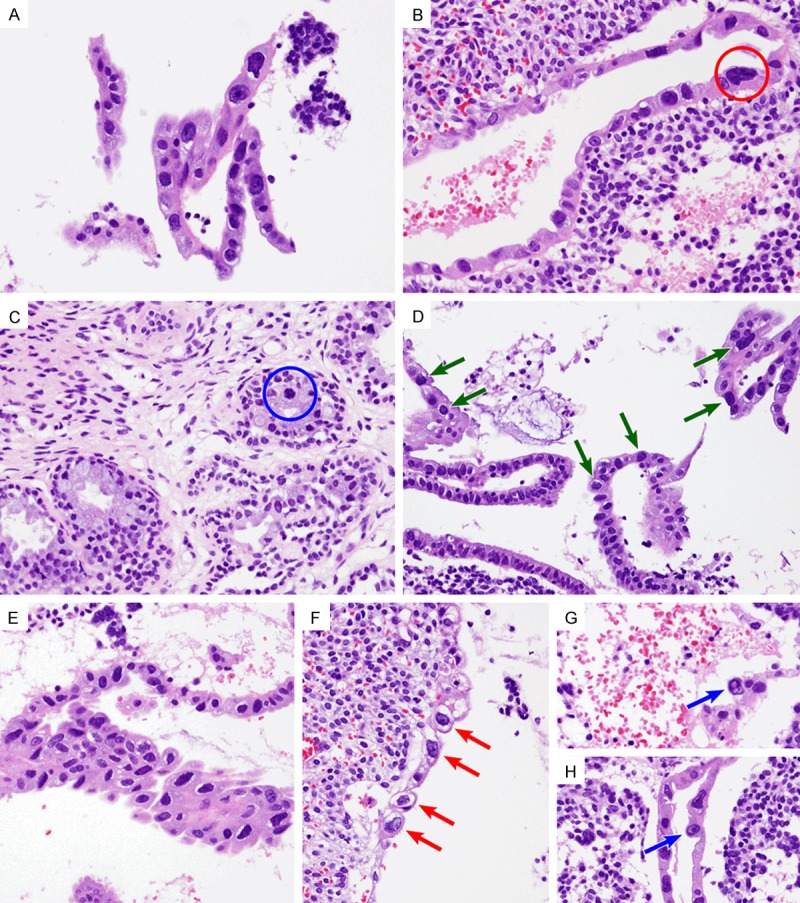
Histopathological findings. A. Detached strips of endometrial glandular epithelium revealed atypical cells showing abundant, amphophilic, partly vacuolized cytoplasm and enlarged nuclei with irregular outlines and hyperchromasia. The nuclear/cytoplasmic ratio was not increased, because the quantity of cytoplasm was also increased. A few conspicuous nucleoli were identified. B. A bizarre nucleus (red circle) was approximately five times larger compared to its normal counterpart. C. An endocervical gland had a few large, hyperchromatic nuclei (blue circle). D and E. Variable distribution of the atypical cells was observed. D. In some areas, atypical cells showing variable degrees of nuclear pleomorphism were distributed in a patchy fashion (green arrows). E. In other areas, pseudostratified atypical cells with a degenerative-looking chromatic pattern showed architectural disarray. F-H. Intracytoplasmic vacuolar change (red arrows) and/or intranuclear microvesiculation (blue arrows) raise the suspicion of viral-induced cytopathic effect.
Immunohistochemical findings
In all patients, immunohistochemical staining for herpes simplex virus (HSV) and CMV, p53, p16 and Ki-67 was performed. The atypical cells were negative for HSV (Figure 2A) and CMV (Figure 2B). p53 nuclear staining was patchy and weak, indicating a wild-type TP53 gene (Figure 2C). p16 expression was absent (Figure 2D). Ki-67 stained less than 5% of the nuclei of the atypical cells (Figure 2E). We also performed EBER-ISH to investigate whether EBV infected the atypical cells; no atypical cells exhibited EBV positivity (Figure 2F).
Figure 2.
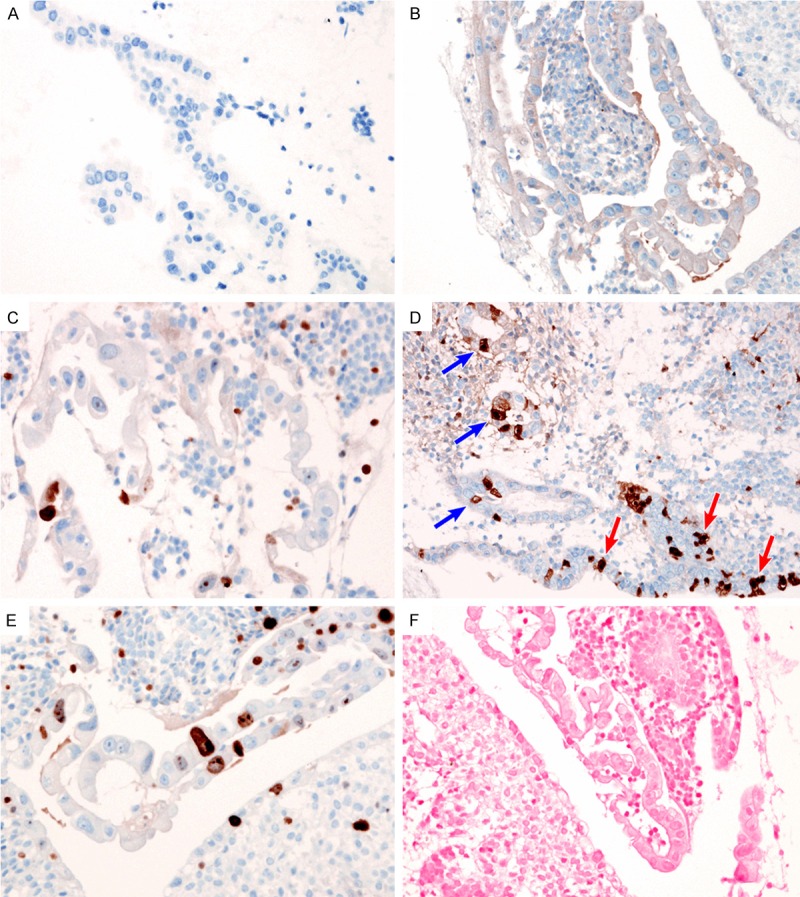
Immunohistochemical findings. Lack of (A) HSV and (B) CMV immunoreactivity excluded the possibility of a viral-induced cytopathic effect. (C) Patchy nuclear staining for p53 indicated the absence of the TP53 mutation. (D) The patchy expression pattern of p16 expression in atypical cells (blue arrows) was the same as that in normal endometrial glandular epithelium (red arrows). (E) Ki-67 proliferation index was low (less than 5%) in the atypical epithelial cells. (F) EBER-ISH did not reveal EBV infection in the atypical epithelial cells.
Discussion
A variety of benign epithelial proliferations and metaplastic changes within the endometrium may cause pathologists to consider a diagnosis of malignancy. Common conditions, including syncytial papillary change, eosinophilic cell change, hobnail cell metaplasia, clear cell change, and Arias-Stella reaction, result in a differential diagnosis of carcinoma. In this study, we add another lesion to the list: chemotherapy-induced epithelial atypia. We found some specific references to this phenomenon occurring in diverse organs and tissues, but there was no available data on the effects of chemotherapeutic agents on the endometrium. Initially, our cases were mistaken for premalignant neoplastic processes or definitive malignancies. Unlike dysplasia or carcinoma, however, the chemotherapy-induced epithelial atypia was not associated with significantly increased mitotic activity or atypical mitoses. Even though there was bizarre nuclear atypia with marked cellular enlargement and pleomorphism exceeding those seen in malignancy, preservation of nuclear/cytoplasmic ratio, abundant vacuolated cytoplasm, a degenerative-looking chromatin pattern and a low proliferative index did not support the diagnosis of carcinoma. Pathologists should be aware of chemotherapy-related alterations in order to avoid an erroneous diagnosis of malignancy. These changes were probably caused by an arrest in nuclear division due to the metabolic effects of the cytotoxic drugs [12]. The benign nature of the atypical cells was supported by the lack of an increase in their nuclear DNA content [13].
The association of epithelial atypia and exposure to cytotoxic drugs is very compelling. However, at least in this study, it may be impossible to clarify that any single specific agent causes epithelial alterations in the endometrium. All except one patient had been exposed to more than one cytotoxic drug in complex combinations, and treatment regimens varied across patients. The chemotherapeutic agents used in our patients were alkylating agents, pyrimidine antagonists, taxanes and topoisomerase inhibitors. The alkylating agents are known as one of the most common and direct causes of non-neoplastic, reactive epithelial atypia in many organ systems such as the upper and lower respiratory tract, gastrointestinal tract, uterine cervix, urothelium and skin. Previous studies have demonstrated that the bizarre, pleomorphic cells observed in cytology and/or biopsy specimens obtained from alkylating agent-treated patients display anaplastic changes exceeding those of a carcinoma, and most importantly, the nuclear/cytoplasmic ratio is preserved and there is a microvacuolar and vesicular appearance of abundant, amphophilic or eosinophilic cytoplasm [2,7,9,12,14-18]. There is agreement in the literature that cyclophosphamide is the drug most strongly associated with epithelial atypia administered either alone or, most often, with busulfan. 5-fluorouracil, a pyridine analogue that acts as an antimetabolite to uracil [7,19], interferes with DNA synthesis by blocking the conversion of deoxyuridylic acid to thymidylic acid [19]. Because of the damaged DNA, one can speculate that regenerating cells may go through a phase of genotypic and phenotypic atypia in their pathway to repair [7]. Regarding the possible effect of paclitaxel on epithelial cells, skin biopsy specimens from patients receiving taxane therapy exhibit numerous apoptotic and mitotic figures including atypical forms [20]. Epithelial cells of the gastrointestinal tract mucosa display extensive apoptosis and numerous mitotic figures and ring mitoses, indicating mitotic arrest [21]. Accordingly, one can speculate that the histopathological findings we observed in the specimen obtained from Patient 1, who received combination chemotherapy with paclitaxel and two alkylating agents, may be attributable to the latter rather than the former. In addition, the presence of epithelial atypia in the specimen obtained from Patient 2, who received neoadjuvant chemotherapy with capecitabine for rectal cancer, raises the possibility that capecitabine may cause epithelial atypia by itself. The possible correlation between topoisomerase inhibitors and chemotherapy-induced epithelial atypia also exists. Further investigations are necessary in order to clarify the effects of capecitabine and/or topoisomerase inhibitors on non-neoplastic epithelial cells.
The morphologic changes induced by chemotherapeutic agents can mimic a neoplastic process. Indeed, many studies have cautioned against misinterpreting chemotherapy-induced epithelial atypia as carcinoma [2,6,7,9,17,20,21]. Several factors may render the endometrium particularly susceptible to erroneous interpretation of chemotherapy-induced atypia. First, the endometrium has not drawn much attention as a target of such cytotoxic effects, and few pathologists anticipate such alterations at this site. Second, when these changes do come across the microscope of an unwary pathologist, first-time encounters are likely to occur inopportunely during an intraoperative frozen section. Frozen section artifacts tend to accentuate structural distortion and cytologic atypia while obscuring subtle differences between chemotherapy-induced atypia and true malignancies.
We noted that Patient 4 with MDS developed CMV viremia after receiving conditioning treatment with busulfan and cyclophosphamide for peripheral blood stem cell transplantation. In this patient, the epithelial atypia was initially misinterpreted as CMV endometritis. Concurrent CMV viremia might contribute to the wrong diagnosis. A thorough review of histologic slides confirmed that there was no definitive evidence of deeply amphophilic to basophilic intranuclear inclusions, which are characteristic of CMV infection. The absence of immunoreactivity for CMV and lymphoplasmacytic infiltration did not support the diagnosis of CMV endometritis. In addition, in this patient, it may be also possible that dislocation of an intrauterine device caused vaginal bleeding.
In conclusion, chemotherapy, alone or as part of a multimodal treatment for various malignancies, is popular. It is crucial for pathologists to be aware of the full spectrum and considerable variability of morphological changes induced by chemotherapeutic agents. Recognition of these histopathological changes, combined with the knowledge of the patient’s history of prior chemotherapy, allows the examining pathologist to strongly consider chemotherapy-induced epithelial atypia as the correct diagnosis in cases of bizarre epithelial atypia. Whenever there is a suspicion that the atypical changes may be attributable to chemotherapy, a history of this therapy should be specifically sought. An awareness of prior exposure to cytotoxic agents, and a familiarity with the nature and distribution of these changes is essential to avoid misinterpretation of the morphologic features and prevent unnecessary treatment. Statistics and sample size limitations should be discussed.
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2015 (6-2015-0072).
Disclosure of conflict of interest
None.
References
- 1.Baker PM, Young RH. Radiation-induced pseudocarcinomatous proliferations of the urinary bladder: a report of 4 cases. Hum Pathol. 2000;31:678–683. doi: 10.1053/hupa.2000.7894. [DOI] [PubMed] [Google Scholar]
- 2.Westra WH, Holmes GF, Eisele DW. Bizarre epithelial atypia of the sinonasal tract after chemotherapy. Am J Surg Pathol. 2001;25:652–656. doi: 10.1097/00000478-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Kirschner RH, Esterly JR. Pulmonary lesions associated with busulfan therapy of chronic myelogenous leukemia. Cancer. 1971;27:1074–1080. doi: 10.1002/1097-0142(197105)27:5<1074::aid-cncr2820270511>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Koss LG, Melamed MR, Mayer K. The effect of busulfan on human epithelia. Am J Clin Pathol. 1965;44:385–397. doi: 10.1093/ajcp/44.4.385. [DOI] [PubMed] [Google Scholar]
- 5.Slavin RE, Dias MA, Saral R. Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients. Cancer. 1978;42:1747–1759. doi: 10.1002/1097-0142(197810)42:4<1747::aid-cncr2820420413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Kwee WS, Wils JA, Schlangen J, Nuyens CM, Arends JW. Gastric epithelial atypia complicating hepatic arterial infusion chemotherapy. Histopathology. 1994;24:151–154. doi: 10.1111/j.1365-2559.1994.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 7.Brien TP, Farraye FA, Odze RD. Gastric dysplasia-like epithelial atypia associated with chemoradiotherapy for esophageal cancer: a clinicopathologic and immunohistochemical study of 15 cases. Mod Pathol. 2001;14:389–396. doi: 10.1038/modpathol.3880323. [DOI] [PubMed] [Google Scholar]
- 8.Schuger L, Peretz T, Goldin E, Durst AL, Okon E. Duodenal epithelial atypia. A specific complication of hepatic arterial infusion chemotherapy. Cancer. 1988;61:663–666. doi: 10.1002/1097-0142(19880215)61:4<663::aid-cncr2820610408>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Ekinci O, Yilmaz IB, Ataoglu O. Bizarre atypia of the cervical epithelium due to chemotherapy with busulfan and cyclophosphamide. Turk J Pathol. 2007;23:173–176. [Google Scholar]
- 10.Nelson BM, Andrews GA. Breast Cancer and Cytologic Dysplasia in Many Organs after Busulfan (Myleran) Am J Clin Pathol. 1964;42:37–44. doi: 10.1093/ajcp/42.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy S, Merino MJ, Swain SM, Lippman ME. The effects of hormonal and chemotherapy on tumoral and nonneoplastic breast tissue. Hum Pathol. 1990;21:192–198. doi: 10.1016/0046-8177(90)90128-r. [DOI] [PubMed] [Google Scholar]
- 12.Stella F, Battistelli S, Marcheggiani F, De Santis M, Giardini C, Baronciani D, Manenti F, Mattioli S, Troccoli R. Urothelial cell changes due to busulfan and cyclophosphamide treatment in bone marrow transplantation. Acta Cytol. 1990;34:885–890. [PubMed] [Google Scholar]
- 13.Borgmann V, al-Abadi H, Friedrichs R, Nagel R. Effect of different local and systemic therapy upon urinary bladder cytology. Urol Int. 1993;50:21–26. doi: 10.1159/000282442. [DOI] [PubMed] [Google Scholar]
- 14.Castano E, Rodriguez-Peralto JL, Lopez-Rios F, Gomez C, Zimmermann M, Iglesias Diez L. Keratinocyte dysplasia: an usual finding after transplantation or chemotherapy. J Cutan Pathol. 2002;29:579–584. doi: 10.1034/j.1600-0560.2002.291002.x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RG, Colquhoun M, Alloub M, Chetty U, Smart GE. Cervical intraepithelial neoplasia in patients with breast cancer: a cytological and colposcopic study. Br J Cancer. 1993;67:1082–1085. doi: 10.1038/bjc.1993.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ognenovski VM, Marder W, Somers EC, Johnston CM, Farrehi JG, Selvaggi SM, McCune WJ. Increased incidence of cervical intraepithelial neoplasia in women with systemic lupus erythematosus treated with intravenous cyclophosphamide. J Rheumatol. 2004;31:1763–1767. [PubMed] [Google Scholar]
- 17.Walker T, Mukerjee D, Levine TS. Bronchial epithelial atypia mimicking squamous cell carcinoma secondary to cyclophosphamide therapy. Cytopathology. 2002;13:330–332. [PubMed] [Google Scholar]
- 18.Slavin RE, Millan JC, Mullins GM. Pathology of high dose intermittent cyclophosphamide therapy. Hum Pathol. 1975;6:693–709. doi: 10.1016/s0046-8177(75)80078-5. [DOI] [PubMed] [Google Scholar]
- 19.Au JL, Rustum YM, Ledesma EJ, Mittelman A, Creaven PJ. Clinical pharmacological studies of concurrent infusion of 5-fluorouracil and thymidine in treatment of colorectal carcinomas. Cancer Res. 1982;42:2930–2937. [PubMed] [Google Scholar]
- 20.Plummer RS, Shea CR. Dermatopathologic effects of taxane therapy. J Am Acad Dermatol. 2011;65:592–596. doi: 10.1016/j.jaad.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Daniels JA, Gibson MK, Xu L, Sun S, Canto MI, Heath E, Wang J, Brock M, Montgomery E. Gastrointestinal tract epithelial changes associated with taxanes: marker of drug toxicity versus effect. Am J Surg Pathol. 2008;32:473–477. doi: 10.1097/PAS.0b013e3181582331. [DOI] [PubMed] [Google Scholar]


