Abstract
Psychoactive pharmaceuticals have been found as teratogens at clinical dosage during pregnancy. These pharmaceuticals have also been detected in minute (ppb) concentrations in drinking water in the US, and are environmental contaminants that may be complicit in triggering neurological disorders in genetically susceptible individuals. Previous studies have determined that psychoactive pharmaceuticals (fluoxetine, venlafaxine and carbamazepine) at environmentally relevant concentrations enriched sets of genes regulating development and function of the nervous system in fathead minnows. Altered gene sets were also associated with potential neurological disorders, including autism spectrum disorders (ASD). Subsequent in vitro studies indicated that psychoactive pharmaceuticals altered ASD-associated synaptic protein expression and gene expression in human neuronal cells. However, it is unknown if environmentally relevant concentrations of these pharmaceuticals are able to cross biological barriers from mother to fetus, thus potentially posing risks to nervous system development. The main objective of this study was to test whether psychoactive pharmaceuticals (fluoxetine, venlafaxine, and carbamazepine) administered through the drinking water at environmental concentrations to pregnant mice could reach the brain of the developing embryo by crossing intestinal and placental barriers. We addressed this question by adding 2H-isotope labeled pharmaceuticals to the drinking water of female mice for 20 days (10 pre-and 10 post-conception days), and quantifying 2H-isotope enrichment signals in the dam liver and brain of developing embryos using isotope ratio mass spectrometry. Significant levels of 2H enrichment was detected in the brain of embryos and livers of carbamazepine-treated mice but not in those of control dams, or for fluoxetine or venlafaxine application. These results provide the first evidence that carbamazepine in drinking water and at typical environmental concentrations is transmitted from mother to embryo. Our results, combined with previous evidence that carbamazepine may be associated with ASD in infants, warrant the closer examination of psychoactive pharmaceuticals in drinking water and their potential association with neurodevelopmental disorders.
Keywords: Psychoactive pharmaceuticals, Environmental Concentrations, Drinking water, Autism Spectrum Disorders (ASD)
1. Introduction
Multiple studies have identified a strong genetic component to the manifestation of neurodevelopmental disorders such as autism spectrum disorders (ASD) [1–3] that nonetheless can account only for a subset of diagnosed cases. In addition, the presence of individual low risk contributing susceptibility genes, or common variants [3] are not sufficient causal agents without the interaction with other environmental, epigenetic, or stochastic factors to cause ASD [4–6]. These findings suggest that likely a majority of cases results from the presence of unknown environmental triggers in genetically susceptible individuals [1, 5–7]. Additional studies have provided evidence that maternal environmental exposures (especially in the first trimester of pregnancy) play a role in the etiology of ASD [1, 7]. These studies have added to a relatively smaller but expanding body of literature addressing the role of environmental contaminants in the etiology of neurodevelopmental disorders [1, 3].
In industrialized countries, humans are exposed to nearly 3000 synthetic compounds through daily use and the environment [1, 8]. For many of these compounds it remains poorly understood how their presence in water, air, and/or food affect human health [1, 8] and to what extent they may contribute to neurological disorders in susceptible individuals [6, 7]. Pharmaceuticals and personal care products (PPCPs) are some of the largest and most widely used classes of synthetic compounds. These products include commonly prescribed pharmaceuticals, phthalates in cosmetics, bis-phenol A (BPA) in plastics and other teratogenic chemicals [1]. Among PPCPs, our laboratory has been studying psychoactive pharmaceuticals (including fluoxetine, venlafaxine and carbamazepine) as potential neurological disorder-relevant contaminants that have been detected in the drinking water [8, 9].
Psychoactive pharmaceuticals (PPs) are widely prescribed [9] and, following excretion from patients, found in water discharged from waste-water treatment plants (WWTP) [8, 10]. The excreted products of PPs are metabolically active and have long half-lives [8, 10]. The pharmaceuticals reach surface waters after inefficient filtration at WWTP and thereafter reach drinking water through ground-water or other supply routes [8, 10].
We studied psychoactive pharmaceuticals such as fluoxetine, venlafaxine and carbamazepine, as they are among the most commonly prescribed antidepressants [9]. In addition, at clinical doses these drugs have demonstrated effects on the prevalence of autism spectrum disorders (ASDs) [9]. Clinical studies have reported that 1.8% – 2.8% of women consume antidepressants during their pregnancy [11] and prenatal exposure to these antidepressants is linked to increased risk of ASD in infants [12]. Furthermore, at clinical doses, anti-epileptic drugs (AEDs), such as carbamazepine, have well-studied teratogenic effects [13]. A group of researchers examined infants exposed to carbamazepine during prenatal development and found them to have lower IQ scores and impaired cognitive function compared to non-exposed infants [14].
To determine if these PPs alter neurophysiology at concentrations found at WWTP, our lab carried out preliminary studies by treating juvenile fathead minnow fish with pharmaceuticals (fluoxetine, venlafaxine and carbamazepine) at environmentally relevant concentrations, which were 6 – 10 times higher than concentrations measured in drinking water throughout the US [8, 9]. The increase was chosen to accommodate for the various byproducts and modified forms of each drug as they occur in the environment and to account for the cumulative effects of multiple drugs [8, 9]. Using DNA microarray-based expression profiling of exposed fish brains, we found that PP-treated water significantly induced the expression of gene sets associated with neuronal growth, development, regulation, and neurological disorders (including idiopathic autism) [8, 9].
Recent studies have found that in utero exposure to PPs like antipsychotics, antidepressants and benzodiazepines during pregnancy may result in the development of neurologic, respiratory, gastro-intestinal and autonomic abnormalities in newly born infants [11]. These pharmaceuticals can be grouped into selective serotonin reuptake inhibitors (SSRI), such as fluoxetine; serotonin-norepinephrine reuptake inhibitors (SNRI), such as venlafaxine; mood stabilizers, such as valproate and carbamazepine; and benzodiazepines, such as diazepam, temazepam and nitrazepam [11, 15]. Studies from human subjects have reported the placental transfer of antidepressants (fluoxetine, sertraline, nortriptyline, and desmethyl clomipramine) to umbilical cord blood [16, 17] when taken from mothers at clinical dosage, providing evidence that these antidepressants can cross the intestinal and placental barriers [16, 17]. It is unknown, however, if these psychoactive pharmaceuticals can also cross intestinal and placental barriers when ingested at concentrations observed in drinking water, a question of relevance to all pregnancies where contaminated drinking water may be consumed.
In the present study, we hypothesized that PPs (fluoxetine, venlafaxine and carbamazepine) consumed by pregnant mothers at typical environmental concentrations would cross the intestinal and placental barriers, and reach the brain of the developing embryos. To determine this, we dissolved deuterium-labeled PP in the drinking water of pregnant mice and measured deuterium (2H) enrichment in the developing embryonic brains and maternal livers. We found carbamazepine, but not fluoxetine or venlafaxine in both tissues confirming its passage through biological barriers during pregnancy.
2. Materials and methods
2.1 Ethics Statement
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Idaho State University and performed in accord with the NIH (National Institutes of Health) Guide for the Care and Use of Laboratory Animals.
2.2 Drug Treatments
Fluoxetine D6- oxalate (FLX) [# F919; 98 atom (at) % 2H], Carbamazepine (CBZ) [# DLM-2806-1.2; 98 at % 2H], Venlafaxine (VNX) [# V009; 98 at % 2H] and L-glutamic acid [# DLM-556-0.05; 98 at % 2H] were obtained from Cambridge Isotope Laboratories Inc. (MA). The overall experimental design comprised five treatments: FLX (concentration = 10μg/l; n=4), VNX (50μg/l; n=4), CBZ (100μg/l; n=4), control (no treatment; n=4) and negative control (contaminated with 2H-labeled L-glutamine; n=1). To examine the possibility of embryonic tissue contamination by maternal blood during dissection, we performed control experiments by bathing dissected embryos in phosphate buffer saline (PBS) containing 0.03 mg/ml 2H-labeled L-glutamine solution followed by 7 to 8 washes with PBS. Subsequent measurements of embryonic tissue resulted in no detection of 2H leading us to adopt the same method for experimental dissections.
2.3 Animal Experiments
Female and male C57BL/6 mice (50 days old) were obtained from the Jackson Laboratory (Sacramento, CA) and placed on a standard chow diet for two weeks prior to experiments. Each experiment was ~20 days long. Starting at Day 1 and for the entire duration of the experiment, four female mice were given water containing isotope-labeled pharmaceuticals ad libitum. Water bottles were protected from light to avoid any photo-degradation of pharmaceuticals and the level of water was monitored daily confirming uniform consumption across test groups. On day 10, each female was housed with one male and examined for vaginal plug presence over the following five days. The day when a plug was observed was marked as embryonic day 0 (E0) of the pregnancy. On embryonic day 10 (E10), prior to the formation of the blood-brain barrier (BBB), pregnant mice were euthanized by CO2 asphyxiation followed by cervical dislocation. In each dissection, we collected a lobe of the mother’s liver and brains of all embryos. Each embryo was separated from extraembryonic membranes and microdissected under ice cold PBS to remove the brain. Using a disecting microscope, we removed embryonic brains using a knife. Thus, we collected one maternal liver (a lobe) and five embryonic brains from each pregnant mouse. All collected tissues were initially stored in cryogenic vials in liquid nitrogen, subsequently dried using a vacuum concentrator (Labconco) for 6 hours, and further dried by incubation at 50°C overnight. Dried samples were stored in sealed tubes within a desiccation chamber to prevent absorption of atmospheric water.
2.4 Isotopic Analysis
Dried tissue samples (embryonic brain and maternal liver) were analyzed for 2H enrichment. Samples were weighed (typical weights~ 0.4–0.8 mg) in silver capsules and analyzed using a ThermoFinnigan High Temperature Conversion Elemental analyzer (TC/EA) interfaced to a Delta V Advantage isotope ratio mass spectrometer (IRMS) through the ConFlo IV system and using a Zero-Blank Autosampler (Costech Analytical, Valencia, CA, USA). Samples were combusted at 1400°C in a graphite crucible inside a glassy carbon reactor, and resultant gases separated in a GC column at 85°C. The resulting hydrogen isotopic ratios (2H/1H), expressed as δ2H, are reported as per mille (‰) values relative to the VSMOW scale. International/certified standards (NBS-22, IAEA-CH-7, methyl ester #Z3) were analyzed concurrent with the samples to normalize the raw data and monitor accuracy. Precision is estimated to be better than 2‰.
2.5 Statistical Analyses
Each treatment and control group contained four female mice and of each maternal mouse we analyzed five embryonic brain samples. For each female mouse, we calculated a mean δ2H VSMOW value from five embryonic brain samples, and we used that mean as a true replicate. We quantified nested effects in the experimental design (embryo in maternal mouse) using a nested mixed effect Analysis of Variance (ANOVA). Significance in omnibus pharmaceutical fixed treatment effects were followed by Dunnett post-hoc analyses comparing treatments to the control. Statistical analyses were carried out using R statistical platform [18], with heavy reliance on the packages lmer [19] and asbio [20, 21].
3. Results
3.1 Passage through the intestinal barrier: patterns of detected 2H isotope in liver samples
We hypothesized that PPs at environmental concentrations would cross the intestinal barrier and reach the maternal circulatory system. To address this question, we treated female mice with one of three pharmaceutical treatments and and compared them to control without pharmaceutical treatment. We collected one liver sample from the maternal mouse in each group.
The δ2H values in the liver samples of carbamazepine (100μg/l; n = 3; p < 0.001) were significantly higher than the control mean (Fig. 1), suggesting that carbamazepine at typical environmental concentration passed through the intestinal barrier of pregnant mice. We did not notice significant quantities of 2H in the livers of other treatments and naturally also not in the untreated controls.
Fig. 1. Liver δ2H values in samples from maternal mice.
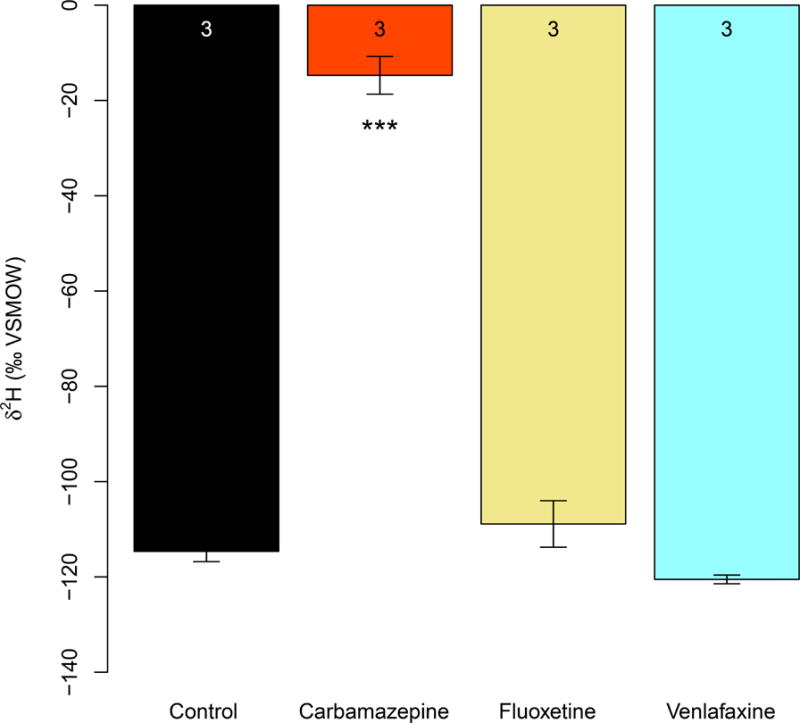
Pregnant mice were treated with CBZ, FLX, VNX and compared to untreated controls. Liver samples from CBZ (100μg/l; n = 3; p <0.001) were significantly 2H enriched relative to controls. Data were analyzed using mixed effect ANOVAs followed by Dunnett’s post-hoc tests. Error bars represent SEMs. Asterisks indicate significant differences compared to controls after Dunnett’s adjustment for familywise type I error at significance level: “***” = 0.001. Numbers at the top of each bar represent the sample size.
3.2 Passage through the placental barrier: patterns of 2H enrichment in grouped brain samples of developing embryos
Next, we determined the extent to which PPs at environmental concentrations could cross the placental barrier and reach the embryonic brain. To accomplish this, we removed and measured the δ2H values for five embryonic brains at E10 from each pregnant mouse (true replicates n = 3). We chose as collection day E10 to preempt the formation of the blood-brain barrier (BBB), which in mice starts at ~E12 [22]. Consequently, pregnant females were sacrificed prior to E12 to avoid any stoppage of pharmaceuticals by the BBB and mimic the physiology of the early human fetus where the BBB does not develop until gestational week 28 [23].
In factor level comparisons at the level of true replicate, δ2H values in the brain samples of the carbamazepine (100μg/l; n = 3) treatment were significantly higher than the control (no treatment; n = 3; p < 0.001; Fig. 2). Detection of labeled carbamazepine at typical environmental concentration confirms that the pharmaceutical crossed the placental barrier and reached the embryonic brains.
Fig. 2. Levels of δ 2H in embryonic brain samples.
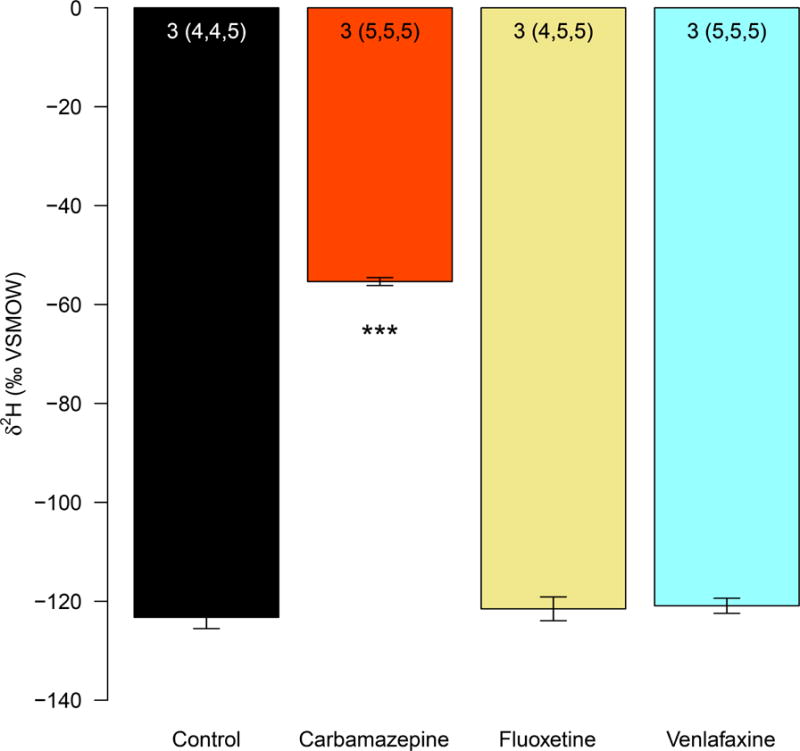
In different treatments, female pregnant mice were treated with CBZ, FLX, VNX and untreated controls. Embryonic brain samples from CBZ treatment (100μg/l; n = 3) were significantly 2H-enriched (significant level “***” = 0.001) relative to controls. Data were anlyzed using a mixed effect ANOVA followed by Dunnett’s post-hoc comparisons of treatments to controls. Error bars represent SEM. Numbers at the top of each bar indicate the number of maternal mice and the number of analyzed embryos (in parentheses).
For the FLX and VNX treatments, we observed no significant change in δ2H values relative to the control.
4. Discussion
The risks of exposure to commonly available PPs, not through direct administration, but rather through secondary sources such as drinking water at much lower concentrations remain largely unexplored. Nonetheless, risks need to be assessed, as concentration of these compounds in the environment experience a continuous rise [1]. Our study explored the hypothesis that PPs can cross biological barriers in pregnant mice and reach the developing brain, potentially posing a liability in the incidence of neurodevelopmental disorders. Consistent with this hypothesis, we detected isotope-labeled carbamazepine in the liver of dams and brains of developing embryos if given carbamazepine in the drinking water at typical environmental concentration (100 μg/l) (Fig. 3).
Fig. 3. Pictorial representation of the central hypothesis.
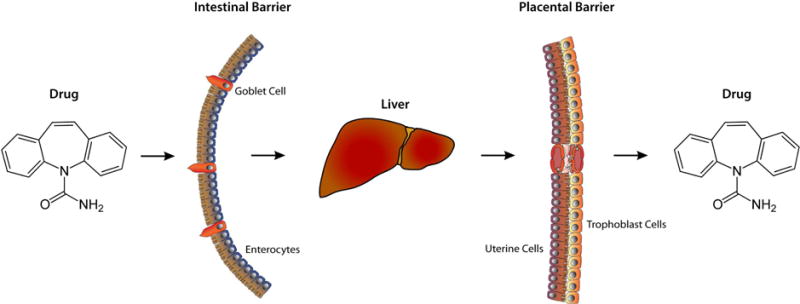
This conceptual diagram represents the passage of psychoactive pharmaceuticals at environmental concentrations through biological barriers.
Carbamazepine is a sodium channel blocker used as an anticonvulsant to treat bipolar disorder and to inhibit epileptic seizures [24]. It is also used as a mood stabilizer during pregnancy and many studies have documented poor neonatal adaptation in newly born infants following its consumption by their mothers [11]. Previous studies had looked at the transfer of carbamazepine at clinical doses, but not at very low concentrations. In a landmark 1995 pharmacokinetics study, carbamazepine (CBZ) was shown not only to cross the intestinal barrier but also the blood brain barrier in rats [25]. More worrisome, studies have also identified teratogenic effects of carbamazepine including abnormal embryonic eye development upon administartion at clinical doses to pregnant mice [26]. These studies suggest that carbamazepine could be a potent teratogen during pregnancy. Furthermore, carbamazepine has also been identified as an environmental contaminant, on occasion detected in the drinking water in concentrations even higher than 100 μg/l, which may contribute to neurological disorders [8].
In previous work from our laboratory, treating fathead minnow fish with PP at environmental concentrations [8], we determined that carbamazepine had the capacity to disrupt a greater set of protein interaction networks than other pharmaceuticals [27]. The study employed RNA-Seq based expression profiling of treated versus control brains and was biased for gene sets enriched for ASD associated genes. Other transcriptome studies from our laboratory in human neuronal cells examined whether valproate at clinical dosage can induce changes in the expression of genes associated with neurological disorders including autism [27]. Valproate, an anticonvulsant with similar properties to carbamazepine [28], has been previously found linked to ASD by inducing autism-like phenotypes in mice [29]. Furthermore, studies in humans have found that in utero exposure to valproate is associated with higher risk of ASD [29]. Taken together, these studies suggest that exposure to carbamazepine during pregnancy, even at very low concentrations, may pose a liability to the embryo/fetus towards the development of ASD.
In maternal liver samples (Fig. 1), we did not see enriched δ2H values in treatments other than for carbamazepine. This implies that fluoxetine and venlafaxine were not metabolized but this may be limited by the short experimental period. Mice may metabolize these drugs differently than carbamazepine as observed with other pharmaceuticals [30]. It is also known that deuterated pharmaceuticals are more metabolically stable due to the increased strength of carbon-deuterium bonds, and thus may be metabolized slower but persist longer [31]. In the present study, in vivo detection of carbamazepine traces in the maternal liver and brains occurred in the offspring of mice given water at typical environmental concentrations found in the drinking water. This suggests that carbamazepine could act as an environmental contaminant and potentially trigger ASD-like symptoms in the developing fetus during pregnancy.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contribution of the late Dr. Chris Cretekos and thank him for supporting and providing methodological and intellectual expertise to the present study, and we dedicate this publication to his memory. We also thank Million Hailemichael for the stable isotope analysis; Harmandeep Sharma for helping in the preparation of samples. The project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare no conflict of interest.
Contributor Information
Gaurav Kaushik, Email: kausgaur@isu.edu.
David P. Huber, Email: hubedavi@isu.edu.
Ken Aho, Email: ahoken@isu.edu.
Bruce Finney, Email: finney@isu.edu.
Shawn Bearden, Email: bearshaw@isu.edu.
Konstantinos S. Zarbalis, Email: kzarbalis@ucdavis.edu.
Michael A. Thomas, Email: mthomas@isu.edu.
References
- 1.Landrigan PJ. What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics. 2010;22(2):219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 2.Pericak-Vance MA, Hussman JP, Chung R-H, Griswold AJ, Jaworski JM, Salyakina D, Ma D, Konidari I, Whitehead PL, Vance JM, et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. 2:1. Molecular Autism; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, Coleman M, Leboyer M, Gillberg C, Bourgeron T. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends in Genetics. 2010;26(8):363–372. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind DH. Autism: Many Genes, Common Pathways? Cell. 2008;135(3):391–395. doi: 10.1016/j.cell.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geschwind DH. Genetics of autism spectrum disorders. Trends in cognitive sciences. 2011;15(9):409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nature Neuroscience. 2011;14(12) doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: Evidence of genes involved in autism. Neuroscience and Biobehavioral Reviews. 2011;35(5):1254–1265. doi: 10.1016/j.neubiorev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MA, Joshi PP, Klaper RD. Gene-class analysis of expression patterns induced by psychoactive pharmaceutical exposure in fathead minnow (Pimephales promelas) indicates induction of neuronal systems. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2012;155(1) doi: 10.1016/j.cbpc.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MA, Klaper RD. Psychoactive Pharmaceuticals Induce Fish Gene Expression Profiles Associated with Human Idiopathic Autism. Plos One. 2012;7(6) doi: 10.1371/journal.pone.0032917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calisto V, Esteves VI. Psychiatric pharmaceuticals in the environment. Chemosphere. 2009;77(10):1257–1274. doi: 10.1016/j.chemosphere.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Kieviet N, Dolman KM, Honig A. The use of psychotropic medication during pregnancy: how about the newborn? Neuropsychiatric Disease and Treatment. 2013;9:1257–1266. doi: 10.2147/NDT.S36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant Use During Pregnancy and Childhood Autism Spectrum Disorders. Archives of General Psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 13.Rasalam AD, Hailey H, Williams JHG, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JCS. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental Medicine and Child Neurology. 2005;47(8):551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 14.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, et al. Cognitive Function at 3 Years of Age after Fetal Exposure to Antiepileptic Drugs. New England Journal of Medicine. 2009;360(16):1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray S, Stowe ZN. The use of antidepressant medication in pregnancy. Best Practice & Research Clinical Obstetrics & Gynaecology. 2014;28(1):71–83. doi: 10.1016/j.bpobgyn.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. American Journal of Psychiatry. 2003;160(5):993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 17.Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, DeVane CL, Owens MJ. Placental passage of tricyclic antidepressants. Biological Psychiatry. 2006;59(3):287–290. doi: 10.1016/j.biopsych.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2012. [Google Scholar]
- 19.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. CRAN; 2014. [Google Scholar]
- 20.Aho K. asbio: A collection of statistical tools for biologists. R package version 1.1-3. CRAN; 2014. [Google Scholar]
- 21.Aho K. Foundational and Applied Statistics for Biologists Using R. Chapman and hall/CRC Press; 2014. [Google Scholar]
- 22.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview-Structure, regulation, and clinical implications. Neurobiology of Disease. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Hough CJ, Irwin RP, Gao XM, Rogawski MA, Chuang DM. Carbamazepine inhibition of N-methyl-D-aspartate-evoked calcium influx in rat cerebellar granule cells. Journal of Pharmacology and Experimental Therapeutics. 1996;276(1):143–149. [PubMed] [Google Scholar]
- 25.Vanbelle K, Sarre S, Ebinger G, Michotte Y. BRAIN, LIVER AND BLOOD DISTRIBUTION KINETICS OF CARBAMAZEPINE AND ITS METABOLIC INTERACTION WITH CLOMIPRAMINE IN RATS – A QUANTITATIVE MICRODIALYSIS STUDY. Journal of Pharmacology and Experimental Therapeutics. 1995;272(3):1217–1222. [PubMed] [Google Scholar]
- 26.Afshar M, Moallem SA, Mohammadpour AH, Shiravi A, Jalalian SM, Golalipour MJ. Teratogenic effects of carbamazepine on embryonic eye development in pregnant mice. Cutaneous and Ocular Toxicology. 2010;29(1):10–15. doi: 10.3109/15569520903380353. [DOI] [PubMed] [Google Scholar]
- 27.Kaushik G, Thomas MA, Aho KA. Psychoactive pharmaceuticals as environmental contaminants may disrupt highly inter-connected nodes in an Autism-associated protein-protein interaction network. Bmc Bioinformatics. 2015;16 doi: 10.1186/1471-2105-16-S7-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattson RH, Cramer JA, Collins JF. A Comparison of Valproate with Carbamazepine for the Treatment of Complex Partial Seizures and Secondarily Generalized Tonic-Clonic Seizures in Adults. N Engl J Med. 1992:765–771. doi: 10.1056/NEJM199209103271104. [DOI] [PubMed] [Google Scholar]
- 29.Harden CL. Utero Valproate Exposure and Autism: Long Suspected, Finally Proven. Epilepsy Currents. 2013;13(6):282–+. doi: 10.5698/1535-7597-13.6.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciraulo DA, Tsirulnik-Barts L, Shader RI, Greenblatt DJ. Clinical pharmacology and therapeutics of antidepressants. Pharmacotherapy of Depression. 2004:33–117. [Google Scholar]
- 31.Timmins GS. Deuterated drugs: where are we now? Expert Opinion on Therapeutic Patents. 2014;24(10) doi: 10.1517/13543776.2014.943184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


