Sir,
We thank Vivaldi et al (2015) for their interest in reading our paper. As they noted, encouraging progression-free survival (PFS) and overall survival (OS) were found with 5.1 months and 8.8 months, respectively (Portal et al, 2015).
We argue that patients in our study were highly selected because they were able to receive a second-line therapy (CT2), even if we had more patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 2 than in the MPACT trial (Von Hoff et al, 2013). Moreover, our population was selected with other characteristics: mean age was 59 years old (63 in MPACT), 63% of patients had 1 metastatic site and only 9% had ⩾3 metastatic sites as compared with 8% and 46%, respectively, in MPACT, median Ca 19.9 was 636 U ml−1 in our patients and 2293 U ml−1 in MPACT. Finally, 14% of patients had metachronous metastases after surgery (vs 7% in MPACT) and 24.5% had previously locally advanced disease when they received FOLFIRINOX as a first-line treatment. These patients may have a better prognosis than patients with synchronous metastases.
Among patients who stopped CT2 during the follow-up period, 62.5% received a third-line chemotherapy, which reflects the selection of our population.
We agree with the remarks of Vivaldi et al (2015), concerning the necessity to identify some parameters to decide upon which subgroup may benefit of CT2. Because of the limited number of patients and already selected patients, the only significant parameter was the age of patients with a risk factor of 0.93 (95% CI 0.87–0.99).
Vivaldi et al (2015) report their prospective evaluation of mPC patients who progressed after modified FOLFIRINOX. Survival was lower than in our study despite similar characteristic, with a median PFS and OS of 2.5 months and 6.2 months, respectively. Only 18% of patients received gemcitabine+Nab-Paclitaxel, with survival in the same range. However, it suggests that all patients were not screened to receive gemcitabine+Nab-paclitaxel after progression under FOLFIRINOX, as most of patients received 5FU-based therapy, including re-challenge with FOLFIRINOX or FOLFIRI. It could lead to selection bias in the population receiving gemcitabine+Nab-paclitaxel. Moreover, the number of patients treated with gemcitabine+Nab-paclitaxel in their study remains low and may not be used to drive any conclusion.
Vivaldi et al (2015) noted that patients were not chemorefractory, with a median PFS for first-line treatment of 5.7 months. One can hypothesise that fast progression with FOLFIRINOX might signify a chemorefractory disease and that these patients might be bad candidates for CT2. However, in our study PFS under Nab-paclitaxel+gemcitabine (PFS-2) was on the contrary significantly better, whereas PFS under first-line FOLFIRINOX (PFS-1) was lower than the median.
We recently updated our data to specifically study the link between efficiency of FOLFIRINOX in first-line treatment then efficiency of Nab-paclitaxel+gemcitabine in second-line treatment.
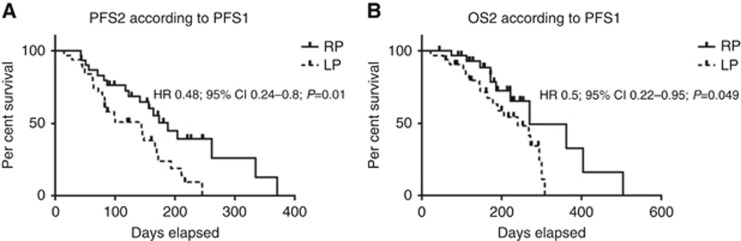
Sixty-one patients were included. PFS-1 was 248 days, with the rapidly progressive (RP) group having a PFS-1 <248 days (n=30) and the low progressive (LP) group a PFS-1 ⩾248 days (n=31). PFS-2 was significantly increased among the RP group (median of 188 days vs 144 days, HR 0.48; 95% CI 0.24–0.8; P=0.01; Figure 1A). OS from the start of CT2 (OS-2) was also significantly increased among RP group (median 270 days vs 240 days; HR 0.5; 95% CI 0.22–0.95; P=0.049; Figure 1B). Nab-paclitaxel+gemcitabine appear to be more effective in RP patients than in LP.
Figure 1.
Survival under second-line chemotherapy with gemcitabine + Nab-paclitaxel, according to first-line PFS. (A) PFS under second-line chemotherapy with gemcitabine+Nab-paclitaxel (PFS-2): RP patients vs low progressive patients. (B) OS under second-line chemotherapy with gemcitabine+Nab-paclitaxel (OS-2): RP patients vs low progressive patients.
We have some hypotheses to explain this:
Long exposure to FOLFIRINOX could lead to selection of more aggressive clones.
Long exposure to oxaliplatin can cause increased neuropathy, which may result in lower dose-intensity of Nab-paclitaxel.
The RP patients under FOLFIRINOX could be refractory to fluorouracil-based chemotherapy but not gemcitabine+Nab-paclitaxel.
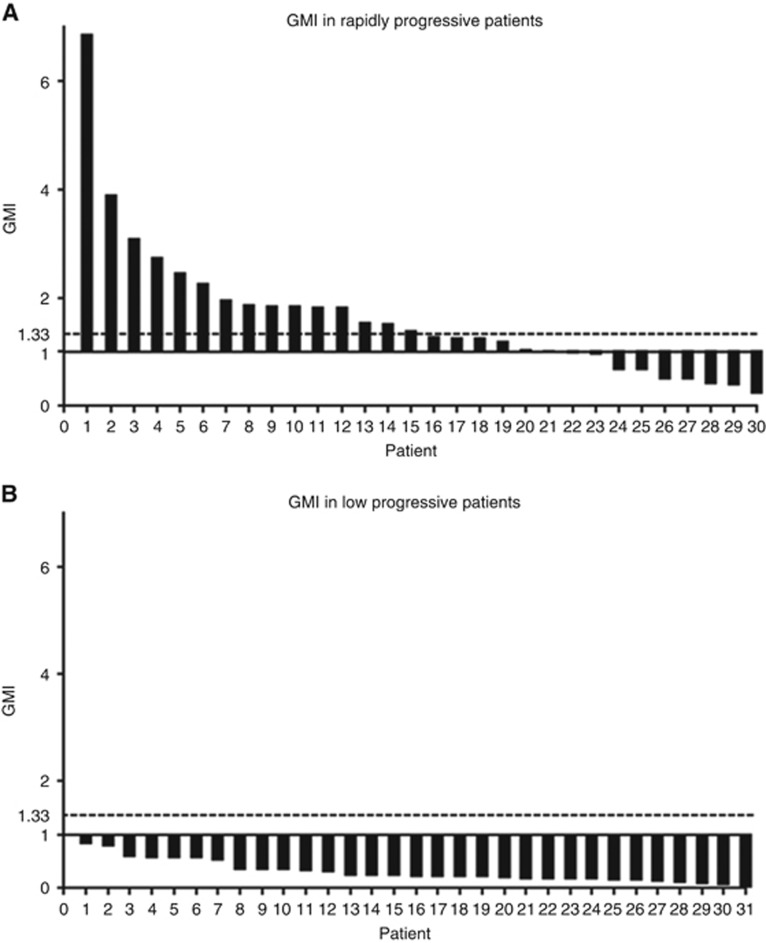
Among RP patients, PFS-2 was significantly longer than PFS-1 (median 188 days vs 98 days, P=0.0005), supporting this last hypothesis. We can evaluate the benefit of CT2 using the growth modulation index (GMI). Using each patient as his own control, the GMI was defined as the ratio of PFS-2 to PFS-1 (Von Hoff, 1998). A GMI >1 indicates that PFS was longer with CT2, whereas a GMI >1.33 suggests that CT2 may be considered to have significant benefit. The GMI has been used in previous studies in mPC or colorectal cancer (Bachet et al, 2009). In our study, 24.5% and 34.4% of patients had a GMI of >1.33 and >1, respectively, reaching 50% and 70% in the RP patients, whereas none of the LP patients had a GMI of >1 (Figure 2).
Figure 2.
Growth modulation index. (A) Rapidly progressive (RP) patients and (B) low progressive (LP) patients.
Despite this increase in PFS-2, RP patients had still a worse OS from the start of FOLFIRINOX (OS 1+2) with a median of 502 days vs 565 days, (P=0.0042).
It would be therefore of great interest to identify patients which could have a better sensitivity to Nab-paclitaxel+gemcitabine than to FOLFIRINOX. Molecular marker should be validated as expression of HENT-1 as a marker of efficacy of gemcitabine-based chemotherapy (Maréchal et al, 2012).
However, to date, no biomarker has been validated, and a sequential regimen alternating 5FU-based and gemcitabine-based therapies might allow to use precociously two potentially efficient regimens. The sequential strategy was first tested in mPC with alternating 2 months of gemcitabine and 2 months of FOLFIRI-3 (Trouilloud et al, 2014), which resulted in increased PFS and OS compared with gemcitabine. This regimen is currently being tested vs FOLFIRINOX (PRODIGE 35, NCT02352337), and has been extrapolated with gemcitabine+Nab-paclitaxel in the FIRGEMAX and the GABRINOX trial (NCT01964287). In these ongoing trials, patients receive gemcitabine+Nab-paclitaxel alternating with FOLFIRI-3 or FOLFIRINOX, respectively.
In conclusion, we think that clinical parameters to choose the right patient for gemcitabine+Nab-paclitaxel after failure of FOLFIRINOX could include a limited number of metastatic site, metachronous metastases, and low Ca 19.9 level. Fast progression under FOLFIRINOX in first-line treatment is predictive of efficacy of gemcitabine+Nab-paclitaxel. However, these observations should be confirmed with prospective data and research for validated biomarkers remains an emergency to optimise mPC treatment.
The authors declare no conflict of interest.
References
- Bachet J-B, Mitry E, Lièvre A, Lepère C, Vaillant J-N, Declety G, Parlier H, Emile J-F, Julié C, Rougier P (2009) Second- and third-line chemotherapy in patients with metastatic pancreatic adenocarcinoma: feasibility and potential benefits in a retrospective series of 117 patients. Gastroenterol Clin Biol 33: 1036–1044. [DOI] [PubMed] [Google Scholar]
- Maréchal R, Bachet J-B, Mackey JR, Dalban C, Demetter P, Graham K, Couvelard A, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet A, Louvet C, Paye F, Rougier P, Penna C, André T, Dumontet C, Cass CE, Jordheim LP, Matera E-L, Closset J, Salmon I, Devière J, Emile J-F, Van Laethem J-L (2012) Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology 143: 664–674. [DOI] [PubMed] [Google Scholar]
- Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, Bachet JB, Dubreuil O, Marthey L, Dahan L, Tchoundjeu B, Locher C, Lepère C, Bonnetain F, Taieb J (2015) Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 113(7): 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouilloud I, Dupont-Gossard A-C, Malka D, Artru P, Gauthier M, Lecomte T, Aparicio T, Thirot-Bidault A, Lobry C, Asnacios A, Manet-Lacombe S, Fein F, Dubreuil O, Landi B, Zaanan A, Bonnetain F, Taïeb J (2014) Fixed-dose rate gemcitabine alone or alternating with FOLFIRI.3 (irinotecan, leucovorin and fluorouracil) in the first-line treatment of patients with metastatic pancreatic adenocarcinoma: an AGEO randomised phase II study (FIRGEM). Eur J Cancer 50: 3116–3124. [DOI] [PubMed] [Google Scholar]
- Vivaldi C, Caparello C, Pasquini G, Musettini G, Catanese S, Lencioni M, Falcone A, Vasile E (2015) Second-line treatment after disease progression following first-line chemotherapy with modified FOLFIRINOX in advanced pancreatic cancer patients: a single institution retrospective cohort study. Ann Oncol Off J Eur Soc Med Oncol 26(Supp 4): iv52. [Google Scholar]
- Von Hoff DD (1998) There are no bad anticancer agents, only bad clinical trial designs—twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res Off J Am Assoc Cancer Res 4: 1079–1086. [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]