Abstract
A 53-year-old man presented with cervical myelopathy. magnetic resonance imagine (MRI) revealed a predominantly extraskeletal, extradural lesion extending along the posterior aspects of the C2 to C5 vertebral bodies, with greater than 60% spinal canal compromise and severe cord compression. Bone involvement was present, but was thought to be secondary. Based on histopathology and immunohistochemical stains, the final pathologic diagnosis was chordoma. The lesion was treated with embolization, surgical resection, and proton beam radiotherapy, and there was no evidence of recurrence or metastasis after five years.
Abbreviations: MRI, magnetic resonance Imaging; STIR, short tau inversion recovery
Case Report
A 53-year-old man, in prior good health, presented with right lower extremity weakness and difficulty walking. One year earlier, the patient began noticing bilateral hand numbness and finger paresthesias. Additional complaints included inability to abduct his right arm and paresthesias along the skin overlying the spine. Physical exam was significant for profound deltoid weakness, diminished deep tendon reflexes in the right leg, and bilateral clonus with dorsiflexion of the ankles.
MRI of the cervical spine revealed a homogeneous T1 hypointense and T2 hyperintense lesion. The lesion was predominantly extraskeletal and extradural, and extended along the posterior aspect of the vertebral bodies from C2 to C5 with at least 60% spinal canal compromise and severe cord compression (Fig. 1). There was heterogeneous enhancement noted within the lesion following intravenous gadolinium. There was some involvement of the posterior aspects of the C3 and C4 vertebral bodies along the right side. The right neural foramina were involved from C3-C4 to C5-C6 and there was stenosis of the right transverse foramina from C2 to C4. There was no apparent invasion of the spinal cord.
Figure 1.
Extraskeletal chordoma of the cervical spine.
A. Sagittal T2-weighted MRI in the midline shows a lobulated mass along the posterior aspect of C1-C4 with high signal. The mass does not appear to invade the vertebral bodies. The cervical cord is severely compromised. There is also incidental degenerative disc disease with loss of height at C3-C4, and mild retrolisthesis of C3 over C4.
B. Sagittal T1-weighted post-gadolinium MRI shows mild enhancement within the mass.
C. Axial short tau inversion recovery (STIR) MRI following gadolinium injection shows the large epidural mass occupying the right side of the spinal canal, compressing the cervical cord to the left. The lesion extends into the right neural foramen.[Powerpoint Slide]
The patient underwent angiographic evaluation and neuroembolization of the right anterior cervical artery and right deep cervical artery. The patient then underwent a C2-C6 laminectomy and tumor resection. The mass was extradural with a capsular component encasing a gelatinous tumor. The tumor and its capsule were resected with sparing of the ventral portions surrounding the vertebral artery at the C4-5 level. Frozen sections at the time of surgery suggested a benign lesion of unknown etiology, possibly a nerve sheath tumor.
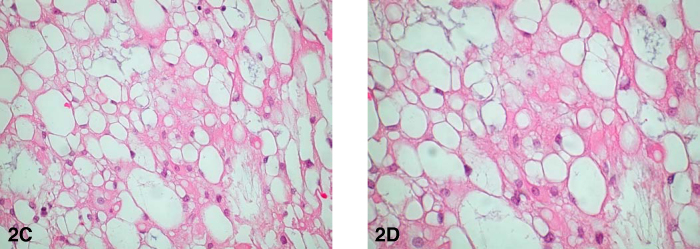
At pathology, the lesion infiltrated bone and soft tissue and was composed of islands of epithelioid cells embedded in a prominent myxoid matrix (Fig. 2A). The lesional cells contained abundant eosinophilic cytoplasm, round to oval shaped nuclei, fine chromatin, and small, inconspicuous nucleoli (Fig. 2B). Focally, the cytoplasm of the cells was extremely vacuolated and there were occasional multivacuolated physaliferous cells (large vacuolated, glycogen or mucin-containing cells) (Fig. 2C & 2D). There was occasional nuclear pleomorphism but mitotic activity was minimal. Necrosis was not present. Immunohistochemical studies revealed the lesion to be positive for cytokeratins and S-100 protein (Fig 2E). The final pathologic diagnosis was chordoma. Because of the predominance of the extraosseous extradural component and the limited, superficial involvement of bone, we believe that this case represents a chordoma of extraosseous extradural origin with secondary direct extension to bone.
Figure 2A.

Extraskeletal chordoma of the cervical spine. Photomicrograph (200x, hematoxylin and eosin) – Some areas of the neoplasm revealed sheets of epithelioid cells admixed with pools of mucin. Some of the cells contain vacuolated cytoplasm. [Powerpoint Slide]
Figure 2B.

Extraskeletal chordoma of the cervical spine. Photomicrograph (400x, hematoxylin and eosin) – Higher power of the area from part A showing sheets of cells with brightly eosinophilic cytoplasm and some vacuolization. Note the focal cytologic pleomorphism. [Powerpoint Slide]
Figure 2.
Extraskeletal chordoma of the cervical spine. C. Photomicrograph (200x, hematoxylin and eosin) – Other areas of the neoplasm contained cells with extensive cytoplasmic vacuolization. D. Photomicrograph (400x, hematoxylin and eosin) – Higher power view of the area from part C revealing multivacuolated physaliferous cells in areas with extensive cytoplasmic vacuolization. [Powerpoint Slide]
Figure 2E.
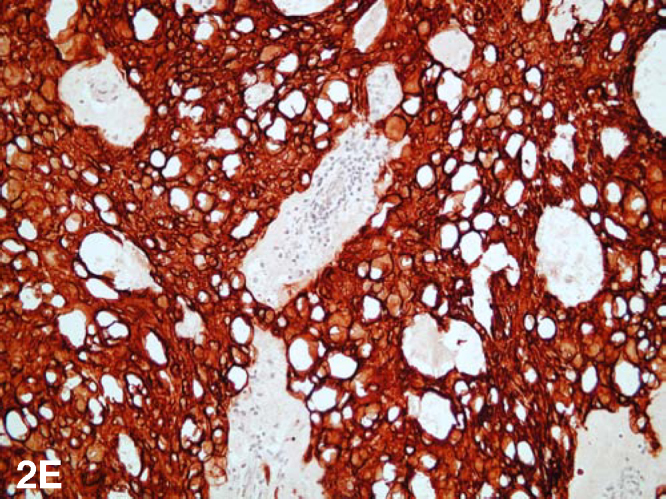
Extraskeletal chordoma of the cervical spine. Photomicrograph (200x) – Immunohistochemical study for cytokeratin revealed the cells to be extensively positive for cytokeratin. The lesional cells were also positive for S-100 protein (not shown). [Powerpoint Slide]
Discussion
Chordomas are rare tumors that arise from the embryologic remnants of the notochord. Most chordomas occur in the sacrococcygeal (50%) and sphenooccipital regions (35%), with a much smaller percentage occurring in the cervical, thoracic, and lumbar spine (15%) (1). Of those that arise in the mobile segments of the spine, approximately 50% will be in the cervical region (2). Chordomas comprise 1 to 5% of all primary malignant bone tumors (3). Most patients with chordomas present in middle adulthood, but chordomas have been described in a wide age range from childhood to senescence (3)
The notochord represents the embryological origin of the axial skeleton. It is surrounded by cartilage which eventually ossifies into the clivus, vertebral bodies, and sacrum (4). With ossification of the surrounding cartilage, extrusion of the notochord takes place, eventually forming the nucleus pulposus. Occasionally, extrusion can occur in aberrant locations, and smaller remnants of the notocord (notocord rests) may persist in other locations along the cranio-spinal axis. It is believed that most notochordal rests occur within osseous structures, but complete extrusion into the extradural or intradural spaces would allow for growth without bony involvement.
Histologically, chordomas are composed of mucinous fatty material, which is surrounded by a fibrous pseudocapsule that results in a lobulated radiographic appearance. They are well demarcated from adjacent soft tissue structures, but may be associated with local bone destruction (3, 4).
MRI features of chordomas include hypointense signal in T1 weighted images, with moderate-to-high signal intensity on T2 weighted images, owing to high fluid content. Heterogeneous appearance is frequent on T1 and T2 weighted images, explained by high protein content or high ferritin from old blood products, respectively. Enhancement patterns are variable (4, 5, 6). Vascular encasement is a common finding, both intra and extra-cranially. However, chordomas rarely cause arterial stenosis in the spine (7).
Chordomas arising in the cervical spine have been reported in very small series (8) or as individual cases. Many of them arise within bone or involve bone (9, 10, 11, 12, 13, 14, 15, 16, 17). Multiple-level involvement is common, as is an extraosseous component (9, 10, 11, 12, 13, 14, 15, 16, 17). Unusual presentations include morphologic resemblance to nerve sheath tumors (18, 19, 20, 21), Horner's syndrome (22), and oral cavity mass (23). Involvement of the upper levels may be more common than the lower levels (8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23). The challenge of surgical resection with adequate margins has been emphasized by many authors (24, 25).
There have been a few case reports of extraosseous, intradural cervical chordomas (26, 27, 28, 29, 30). Intradural notochordal remnants may occur anywhere in the spinal axis, but are more common in the retroclival space, and have been reported to occur in up to 2% of autopsy specimens (31). Usually, these remnants are associated with a thin stalk that penetrates the dura. These were initially described by Virschow as “eccordosis physaliphora.” Eccordosis physaliphora are currently thought to be distinct from chordomas, in that they are clinically silent, and represent developmental vestiges rather than tumors (32). Histologically, however, chordomas and eccordosis physaliphora are almost identical. Whether chordomas arise from eccordosis physaliphora is still under debate.
There have been a few case reports of extraosseous, extradural cervical chordomas (33, 34, 35). In these cases, the lesions were located in the loose areolar tissue of the spinal epidural space. Jallo et al. have developed a classification of spinal chordomas, based on location and osseous involvement (35). Type I are osseous and extradural and comprise the majority of chordomas. Type II are extraosseous and extradural, as may have been this case. Type III chordomas are osseous and intradural, and Type IV are extraosseous and intradural (Table 1). Type III lesions would presumably arise in bone and involve the dura secondarily by direct extension. Jallo hypothesizes that the classification serves as a prognostic indicator, because extraosseous location (Type II and Type IV) is associated with favorable resectability and therefore lower rates of recurrence. The extreme rarity of these cases makes this hypothesis difficult to test.
TABLE 1.
Classification of Spinal Chordomas Based on Anatomic Location
| Extradural | Intradural | |
|---|---|---|
| Osseous | Type I | Type III |
| Extraosseous | Type II | Type IV |
Footnotes
Published: December 27, 2006
References
- 1.Rich TA, Schiller A, Suit HD, Mankin HJ. Clinical and pathologic review of 48 cases of chordoma. Cancer. 1985;56:182–187. doi: 10.1002/1097-0142(19850701)56:1<182::aid-cncr2820560131>3.0.co;2-j. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson J, Wold LE, Ebersold MJ, Laws ER. Chordoma of the mobile spine: a clinicopathologic analysis of 40 patients. Cancer. 1993 Feb 1;71(3):735–740. doi: 10.1002/1097-0142(19930201)71:3<735::aid-cncr2820710314>3.0.co;2-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Unni KK. Dahlin's bone tumors : general aspects and data on 11,087 cases. Fifth edition. Lippincott-Raven; New York: 1996. [Google Scholar]
- 4.Weber AL, Liebsch NJ, Sanchez R, Sweriduk ST., Jr Chordomas of the skull base. Radiologic and clinical evaluation. Neuroimaging Clin N Am. 1994;4(3):515–527. [PubMed] [PubMed] [Google Scholar]
- 5.Sze G, Vichanco LS, III, Brant-Zawadski MN. Chordomas: MR imaging. Radiology. 1988;166:187–191. doi: 10.1148/radiology.166.1.3336677. [Pubmed] [DOI] [PubMed] [Google Scholar]
- 6.Ducou le Pointe H, Brugieres P, Chevalier X, Meder JF, Voisin MC, Gaston A. Imaging of chordomas of the mobile spine. J Neuroradiol. 1991;18(3):267–276. [PubMed] [PubMed] [Google Scholar]
- 7.Bjornsson J, Wold LE, Ebersold MJ, Laws ER. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993 Feb 1;71(3):735–740. doi: 10.1002/1097-0142(19930201)71:3<735::aid-cncr2820710314>3.0.co;2-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Wippold FJ, 2nd, Koeller KK, Smirniotopoulos JG. Clinical and imaging features of cervical chordoma. AJR Am J Roentgenol. 1999 May;172(5):1423–1426. doi: 10.2214/ajr.172.5.10227531. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Anegawa T, Rai M, Hara K, Yamamoto K, Narumi O, Hashimoto K, Kusaka H. An unusual cervical chordoma: CT and MRI. Neuroradiology. 1996 Jul;38(5):466–467. doi: 10.1007/BF00607279. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Winants D, Bertal A, Hennequin L, Fays J, Bernadac P. Imaging of cervical and thoracic chordoma. Apropos of 2 cases. J Radiol. 1992 Mar;73(3):169–174. [PubMed] [PubMed] [Google Scholar]
- 11.Zacay G, Eyal A, Shacked I, Hadani M, Faibel M, Kronenberg J, Talmi YP. Chordoma of the cervical spine. Ann Otol Rhinol Laryngol. 2000 Apr;109(4):438–440. doi: 10.1177/000348940010900417. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Coraddu M, Floris F, Nurchi GC, Rachele MG, Marrosu F, Todde PF. Chordoma of the cervical spine. Case report. J Neurosurg Sci. 1994 Mar;38(1):51–53. [PubMed] [PubMed] [Google Scholar]
- 13.Booi GA, van Horn JR. Cervical chordoma. A case report. Acta Orthop Belg. 1987;53(4):520–522. [PubMed] [PubMed] [Google Scholar]
- 14.Kawai K, Sasaki T, Yanai A, Teraoka A. High cervical chordoma–case report. Neurol Med Chir (Tokyo) 1995 Mar;35(3):165–167. doi: 10.2176/nmc.35.165. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Strayer A. Upper cervical spine chordoma: a case study. J Neurosci Nurs. 2003 Oct;35(5):276–280. doi: 10.1097/01376517-200310000-00006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.D'Haen B, De Jaegere T, Goffin J, Dom R, Demaerel P, Plets C. Chordoma of the lower cervical spine. Clin Neurol Neurosurg. 1995 Aug;97(3):245–248. doi: 10.1016/0303-8467(95)00043-j. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Nina P, Franco A, Barbato R, De Gregorio A, Schisano G. Extradural low cervical chordoma. Case report. J Neurosurg Sci. 1999 Dec;43(4):305–309. [PubMed] [PubMed] [Google Scholar]
- 18.Wang AM, Joachim CL, Shillito J, Jr, Morris JH, Zamani AA, Rumbaugh CL. Cervical chordoma presenting with intervertebral foramen enlargement mimicking neurofibroma: CT findings. J Comput Assist Tomogr. 1984 Jun;8(3):529–532. doi: 10.1097/00004728-198406000-00030. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Mortele B, Lemmerling M, Mortele K, Verstraete K, Defreyne L, Kunnen M, Vandekerckhove T. Cervical chordoma with vertebral artery encasement mimicking neurofibroma: MRI findings. Eur Radiol. 2000;10(6):967–969. doi: 10.1007/s003300051046. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Karakida O, Aoki J, Seo GS, Ishii K, Sone S, Nakakouji T, Otsuka K. Epidural dumbbell-shaped chordoma mimicking a neurinoma. Pediatr Radiol. 1996;26(1):62–64. doi: 10.1007/BF01403709. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Gunnarsson T, Leszniewski W, Bak J, Davidsson L. An intradural cervical chordoma mimicking a neurinoma. Case illustration. J Neurosurg. 2001 Jul;95(1):144. doi: 10.3171/jns.2001.95.1.0144. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Leone A, Cerase A, Tarquini E, Mule A. Chordoma of the low cervical spine presenting with Horner's syndrome. Eur Radiol. 2002 Dec;12(Suppl 3):S43–S47. doi: 10.1007/s00330-002-1590-0. [PubMed] Epub 2002 Sep 11. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita A, Hatakeyama K, Marutsuka K, Fujita S, Ono S, Torihara K, Tamura S, Komune S, Asada Y. Chordoma of cervical vertebra protruding into the oral cavity. Pathol Int. 2002 Jan;52(1):59–62. doi: 10.1046/j.1440-1827.2002.01315.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Bailey CS, Fisher CG, Boyd MC, Dvorak MF. En bloc marginal excision of a multilevel cervical chordoma. Case report. J Neurosurg Spine. 2006 May;4(5):409–414. doi: 10.3171/spi.2006.4.5.409. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Rhines LD, Fourney DR, Siadati A, Suk I, Gokaslan ZL. En bloc resection of multilevel cervical chordoma with C-2 involvement. Case report and description of operative technique. J Neurosurg Spine. 2005 Feb;2(2):199–205. doi: 10.3171/spi.2005.2.2.0199. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Vaz RM, Pereira JC, Ramos U, Cruz CR. Intradural cervical chordoma without bone involvement. J Neurosurg. 1995;82:650–653. doi: 10.3171/jns.1995.82.4.0650. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Nishigaya K, Kaneko M, Ohashi Y, Nukui H. Intradural retroclival chordoma without bone involvement: no tumor regrowth 5 years after operation. J Neurosurg. 1998;88:764–768. doi: 10.3171/jns.1998.88.4.0764. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Badwal S, Pal L, Basu A, Saxena S. Multiple synchronous spinal extra-osseous intradural chordomas: is it a distinct entity? Brit J Neurosurg. 2006 Apr;20(2):99–103. doi: 10.1080/02688690600682614. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Steenberghs J, Kiekens C, Menten J, Monstrey J. Intradural chordoma without bone involvement. J Neurosurg (Spine1) 2002;97:94–97. [PubMed] [PubMed] [Google Scholar]
- 30.Gelabert-Gonzalez M, Pintos-Martinez E, Caparrini-Escondrillas A, Martinez-Rumbo R. Intradural cervical chordoma. Case report. J Neurosurg Sci. 1999 Jun;43(2):159–162. [PubMed] [PubMed] [Google Scholar]
- 31.Congdon CC. Benign and malignant chordomas: a clinico-anatomical study of 22 cases. Am J Pathology. 1952;28:793–821. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehnert F, Beschorner R, Kueker W, Hahn U, Naegele T. Retroclival ecchordosis physaliphora: MR imaging and review of the literature. AJNR Am J Neuroradiol. 2004;25(10):1851–1855. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomlinson FH, Scheithauer BW, Miller GM, Onofrio BM. Extraosseous spinal chordoma. J Neurosurg. 1991;75:980–984. doi: 10.3171/jns.1991.75.6.0980. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Sebag G, Dubois J, Beniaminovitz A, Lelouch-Tubiana A, Brunelle F. Extraosseous spinal chordoma: radiographic appearance. AJNR Am J Neuroradiol. 1993 Jan-Feb;14(1):205–207. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 35.Jallo J, Nathan D, Bierbraur K, Farber E. Chordoma: a case report. Surg Neurol. 1997;48:46–48. doi: 10.1016/s0090-3019(96)00430-2. [PubMed] [DOI] [PubMed] [Google Scholar]