Abstract
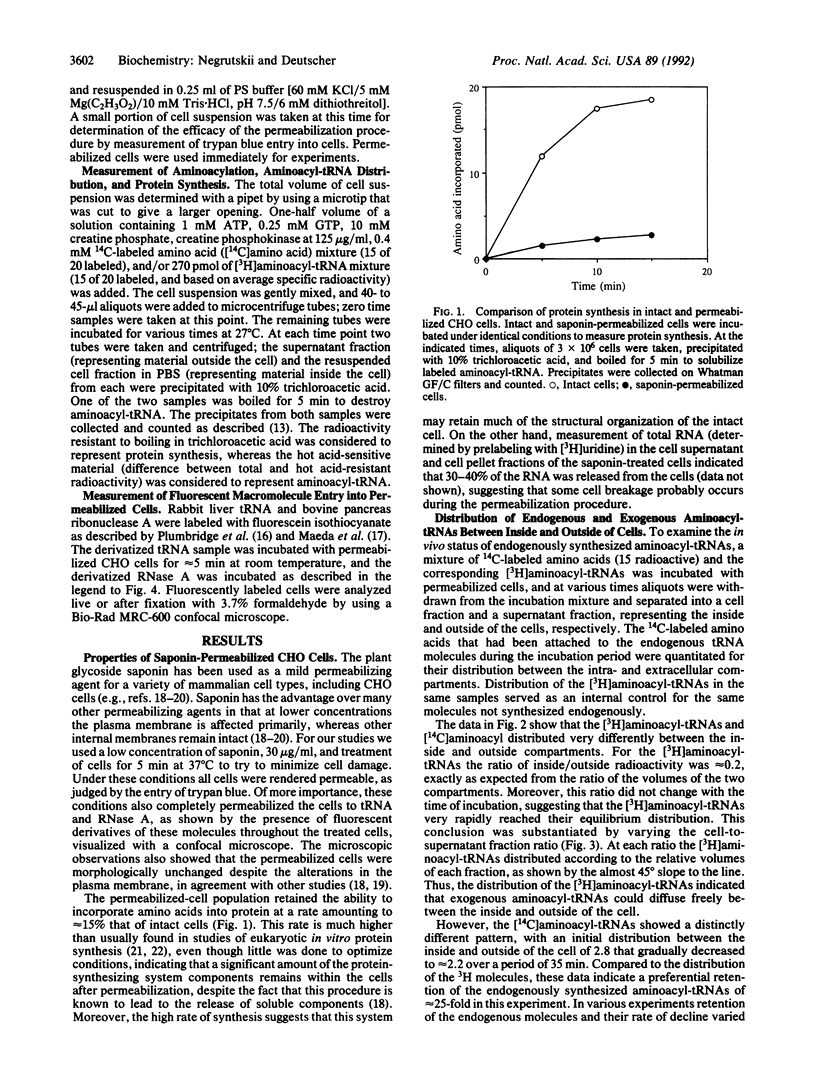
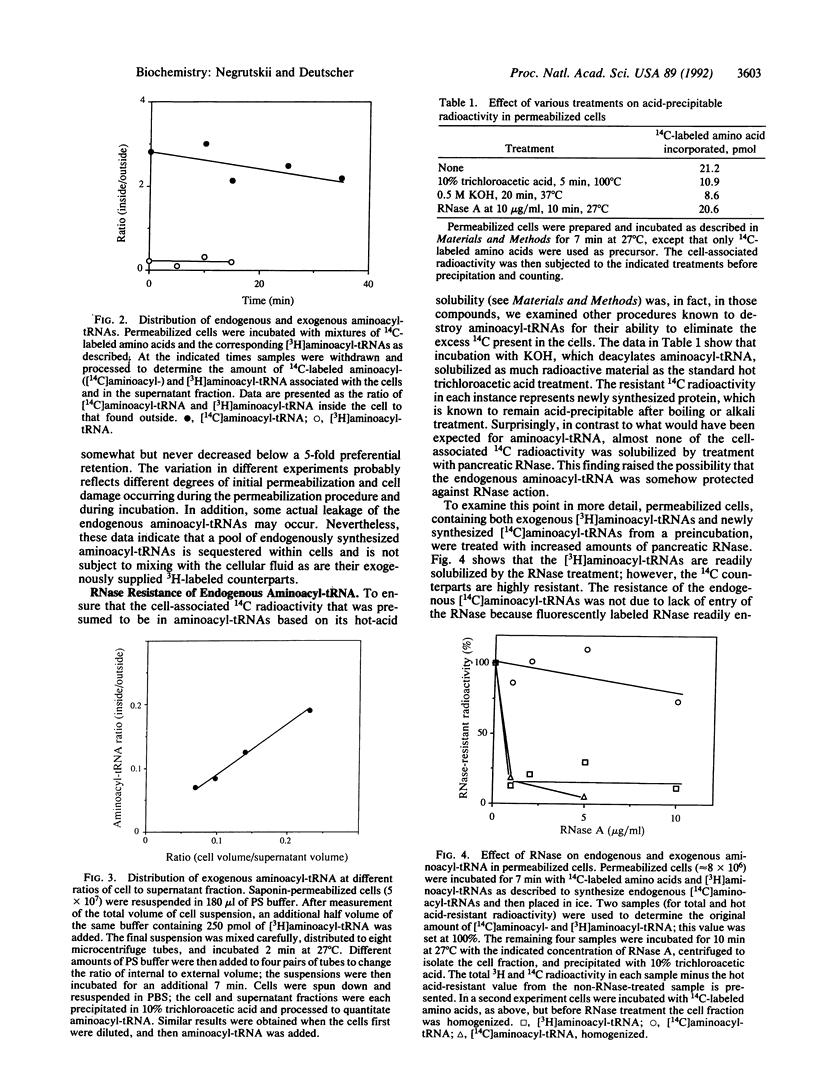
We have recently proposed that aminoacyl-tRNA is channeled during protein synthesis in vivo--i.e., it is directly transferred among the components of the protein-synthesizing machinery and does not mix with aminoacyl-tRNA molecules introduced from outside the cell. To understand the structural basis for these functional properties, we have examined the disposition of aminoacyl-tRNA within the cell. To do this we have developed a Chinese hamster ovary (CHO) permeabilized-cell system using the plant glycoside saponin. We show, using a mixture of free 14C-labeled amino acids and 3H-labeled aminoacyl-tRNAs, that 14C-labeled aminoacyl-tRNAs synthesized endogenously from the free amino acids are preferentially sequestered within the cell, whereas their exogenous 3H counterparts distribute between the inside and outside of the cell based solely on the relative volumes of the two compartments. Furthermore, the endogenous 14C-labeled aminoacyl-tRNA population is resistant to pancreatic ribonuclease action, whereas the 3H molecules are rapidly degraded. We conclude, based on these observations, that aminoacyl-tRNAs synthesized in vivo are continually associated with components of the protein synthesis machinery and are thereby retained within the permeabilized cell and are also protected from RNase action. These data provide independent evidence for the channeling model of protein biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bec G., Kerjan P., Zha X. D., Waller J. P. Valyl-tRNA synthetase from rabbit liver. I. Purification as a heterotypic complex in association with elongation factor 1. J Biol Chem. 1989 Dec 15;264(35):21131–21137. [PubMed] [Google Scholar]
- Bonneau A. M., Darveau A., Sonenberg N. Effect of viral infection on host protein synthesis and mRNA association with the cytoplasmic cytoskeletal structure. J Cell Biol. 1985 Apr;100(4):1209–1218. doi: 10.1083/jcb.100.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. The eucaryotic aminoacyl-tRNA synthetase complex: suggestions for its structure and function. J Cell Biol. 1984 Aug;99(2):373–377. doi: 10.1083/jcb.99.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf H. Intraction of aminoacyl-tRNA synthetases with ribosomes and ribosomal subunits. Biochim Biophys Acta. 1976 Mar 4;425(2):175–184. doi: 10.1016/0005-2787(76)90023-x. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Irvin J. D., Hardesty B. Binding of aminoacyl transfer ribonucleic acid synthetases to ribosomes from rabbit reticulocytes. Biochemistry. 1972 May 9;11(10):1915–1920. doi: 10.1021/bi00760a028. [DOI] [PubMed] [Google Scholar]
- Kawauchi H., Tuzimura K., Maeda H., Ishida N. Reaction of fluorescein-isothiocyanate with proteins and amino acids. II. Preparation of fluorescein-thiohydantoin amino acids and their thin-layer chromatography. J Biochem. 1969 Dec;66(6):783–789. doi: 10.1093/oxfordjournals.jbchem.a129208. [DOI] [PubMed] [Google Scholar]
- Lin A., Krockmalnic G., Penman S. Imaging cytoskeleton--mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8565–8569. doi: 10.1073/pnas.87.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin YuA, Wolfson A. D., Orlovsky A. F., Gladilin K. L. Mammalian valyl-tRNA synthetase forms a complex with the first elongation factor. FEBS Lett. 1988 Oct 10;238(2):262–264. doi: 10.1016/0014-5793(88)80492-7. [DOI] [PubMed] [Google Scholar]
- Negrutskii B. S., Deutscher M. P. Channeling of aminoacyl-tRNA for protein synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4991–4995. doi: 10.1073/pnas.88.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Bäumert H. G., Ehrenberg M., Rigler R. Characterisation of a new, fully active fluorescent derivative of E. coli tRNA Phe. Nucleic Acids Res. 1980 Feb 25;8(4):827–843. [PMC free article] [PubMed] [Google Scholar]
- Ryazanov A. G., Ovchinnikov L. P., Spirin A. S. Development of structural organization of protein-synthesizing machinery from prokaryotes to eukaryotes. Biosystems. 1987;20(3):275–288. doi: 10.1016/0303-2647(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Sivaram P., Deutscher M. P. Existence of two forms of rat liver arginyl-tRNA synthetase suggests channeling of aminoacyl-tRNA for protein synthesis. Proc Natl Acad Sci U S A. 1990 May;87(10):3665–3669. doi: 10.1073/pnas.87.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991 Sep 13;253(5025):1275–1277. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- Voelker D. R. Characterization of phosphatidylserine synthesis and translocation in permeabilized animal cells. J Biol Chem. 1990 Aug 25;265(24):14340–14346. [PubMed] [Google Scholar]
- Wassler M., Jonasson I., Persson R., Fries E. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem J. 1987 Oct 15;247(2):407–415. doi: 10.1042/bj2470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Demma M., Warren V., Dharmawardhane S., Condeelis J. Identification of an actin-binding protein from Dictyostelium as elongation factor 1a. Nature. 1990 Oct 4;347(6292):494–496. doi: 10.1038/347494a0. [DOI] [PubMed] [Google Scholar]



