Abstract
Abscisic acid is a phytohormone regulating plant growth, development and stress responses. PYR1/PYL/RCAR proteins are ABA receptors that function by inhibiting PP2Cs to activate SnRK2s, resulting in phosphorylation of ABFs and other effectors of ABA response pathways. Exogenous ABA induces growth quiescence of lateral roots, which is prolonged by knockout of the ABA receptor PYL8. Among the 14 members of PYR1/PYL/RCAR protein family, PYL9 is a close relative of PYL8. Here we show that knockout of both PYL9 and PYL8 resulted in a longer ABA-induced quiescence on lateral root growth and a reduced sensitivity to ABA on primary root growth and lateral root formation compared to knockout of PYL8 alone. Induced overexpression of PYL9 promoted the lateral root elongation in the presence of ABA. The prolonged quiescent phase of the pyl8-1pyl9 double mutant was reversed by exogenous IAA. PYL9 may regulate auxin-responsive genes in vivo through direct interaction with MYB77 and MYB44. Thus, PYL9 and PYL8 are both responsible for recovery of lateral root from ABA inhibition via MYB transcription factors.
As sessile organisms, plants need a sophisticated regulatory network to survive unfavorable and changing environments. When the soil environment becomes unfavorable, root system is often the first to sense it. This involves phytohormones that act quickly and accurately.
The phytohormone auxin is tightly correlated with both primary and lateral root growth and development. Auxin is perceived by a small family of F-box proteins including TRANSPORT INHIBITOR RESPONSE 1 (TIR1). Auxin gradient, which forms a sink at the root apex and just below quiescent center (QC), provides essential information for cell division, polarity and cell fate1. Lateral root initiation requires PIN-FORMED 1 (PIN1) -dependent auxin transport. During lateral root initiation, auxin determines both its position and frequency2. After initiation, auxin gradient is also required for the correct patterning of lateral root primordium3. To facilitate lateral root primordium emergence, auxin modulates cell turgor in the outer tissue layers and in the primordium4, and induces the expression of cell wall remodeling enzymes5,6,7. Besides auxin, other phytohormones, such as cytokinin, gibberellin, brassinosteroids, abscisic acid and strigolactones, also function during root growth. However, auxin acts as an integrator to them and these phytohormones either regulates polar auxin transport (PAT) or regulates auxin responsive genes.
Abscisic acid (ABA) is an isoprenoid plant hormone and a main regulator of responses to biotic and abiotic stress8. ABA biosynthesis is one of the quickest responses of plants facing stresses and ABA, in turn, will trigger downstream ABA responsive gene expression9. Besides its function on stress responses, ABA also has a role in plant development and physiological processes, including seed development and dormancy, embryo morphogenesis and stomatal movement10. The core signaling pathway of ABA includes receptors, phosphatases and kinases. The 14-member family of START domain proteins known as PYR1/PYLs/RCARs, has been identified as intracellular ABA receptors11,12. PYR1/PYLs/RCARs bind to and inhibit type 2C protein phosphatases (PP2Cs) in an ABA-dependent manner, which in turn release the inhibition of PP2Cs on SNF1-related kinase 2 (SnRK2 kinases)11,13. SnRK2 kinases are activated by activation loop autophosphorylation14 and are the key nodes in ABA signaling pathway.
High concentrations of ABA inhibit both primary and lateral root growth15,16, while low concentrations of ABA are known to promote root growth17. Studies with ABA-deficient mutants indicate that ABA is also crucial for maintenance of root growth under water-stressed and normal growth conditions18,19. This is partially due to the promotion of stem cell maintenance, which is vital for root growth, by nanomolar concentrations of ABA in the root meristem20.
Previous studies found that ABA and auxin are integrated into each other’s pathways in many developmental processes. ABA regulates auxin responses in many aspects. Loss of function of ABA-insensitive 3 leads to a reduction of auxin-induced lateral root initiation21. Overexpression of ABA-insensitive 4 (ABI4) impairs lateral root development by reducing the expression of the auxin-efflux transporter PINFORMED 1 (PIN1)22. Furthermore, Auxin Response Factor 2 (ARF2) is a transcriptional repressor involved in plant growth and directly regulates the homeodomain gene HB33. Altered auxin distribution in arf2-101 and two HB33 overexpressing lines in response to ABA treatment indicates that ABA and auxin might act synergistically in inhibiting root growth23. Furthermore, the pyl8 mutant shows a reduced sensitivity to ABA-mediated primary root growth inhibition24. Besides its role in primary root, PYL8 was found to interact with MYB DOMAIN PROTEIN 77 (MYB77) and functions in auxin-mediated lateral root growth25.
Auxin, in turn, also regulates ABA responses in many ways. During seed germination, the expression of ABI3 is controlled by auxin through the auxin response factors AUXIN RESPONSE FACTOR 10 and AUXIN RESPONSE FACTOR 1626. Some auxin responsive genes are also regulators of stress responses. Among them is MYB77 that acts as a coactivator with ARFs27. Auxin-responsive gene expression was greatly attenuated in myb77 knockout mutants and MYB77 overexpression lines mimicked the wild type treated with exogenous IAA27. In the subgroup 22 where MYB77 belongs to, there are three other members: AtMYB44/AtMYBR1, AtMYB70 and AtMYB7328. Previous research has suggested that MYB44 represses ABA signaling during drought and senescence by interacting with PYL8 and PYL929,30. Thus, these MYB proteins are associated with both auxin and stress responses31.
The members of PYR1/PYL/RCAR family have distinct properties. For example, two orthologs AtPYL13 and OsPYL12 inhibit several clade A PP2Cs in an ABA-independent manner, and most importantly, OsPYL12 is unable to bind to ABA32,33,34. PYR1 and PYL1-2 are dimers in solution, while PYL4-10 are monomers35. The diversity of PYLs may contribute to the versatility of ABA signaling.
Here, we report a function of PYL9 in regulating lateral root growth upon ABA treatment, in a manner similar to that of PYL8. Previous studies found that PYL9/RCAR1-mediated ABA signaling pathway could be modulated by ROP11 during in vitro reconstitution of ABA signaling pathway in Arabidopsis protoplasts36 and PYL9 could interact with an R2R3 MYB transcription factor, MYB4429. We found that PYL9 acts together with PYL8 in regulating the recovery of lateral root growth from inhibition after ABA treatment. Our results suggest that both PYL8 and PYL9 are nodes of crosstalk between ABA and auxin, and their signal is transduced by a group of MYB transcription factors to regulate the lateral roots.
Results
The pyl8-1pyl9 double mutant shows a reduced inhibition of primary root growth and lateral root formation to exogenous ABA compared to pyl8
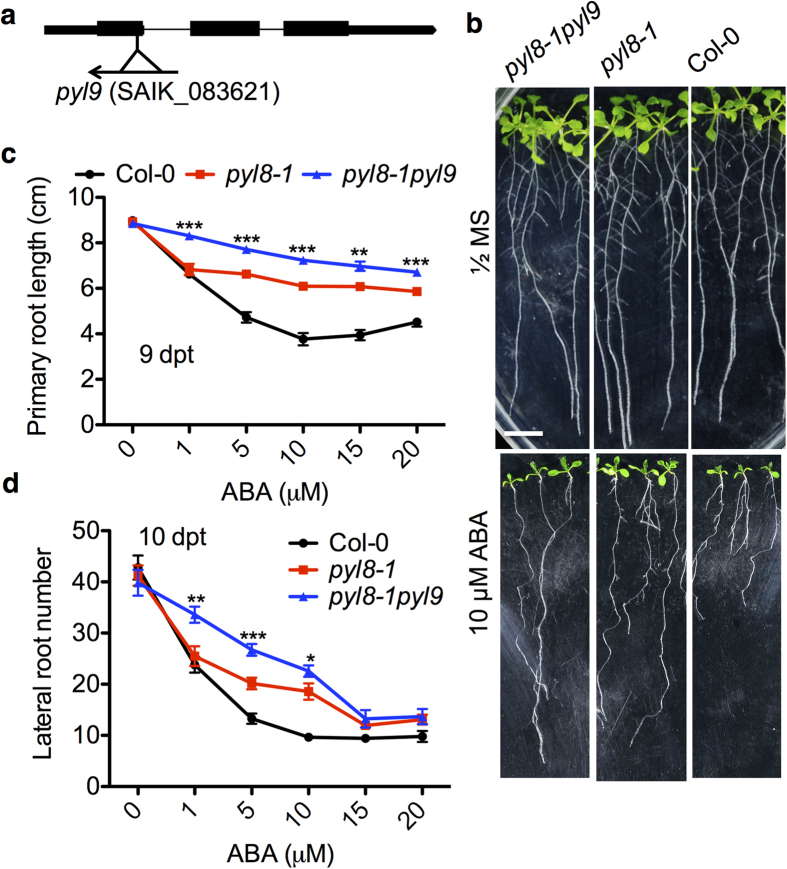
ABA is a key factor in regulating root architecture under stress16,20. To dissect its role, we analyzed Arabidopsis mutants impaired in ABA signaling pathway, especially null mutants in ABA receptors. Previous studies demonstrated that PYL8 plays an important role in ABA signaling in roots and the work was focused on primary roots24. We found that in addition to regulating the growth of primary roots, PYL8 could also promote lateral root recovery from ABA inhibition25. Since PYL9 has a very high sequence identity with PYL8 and both of them have a relative high expression level in roots37, we obtained a T-DNA knock-out mutant of pyl9 (SALK_083621) (Fig. 1a)24 and tested the root growth of pyl9 under ABA treatment. pyl9 mutant did not have an obvious lateral root growth phenotype on ABA medium (see Supplementary Fig. S1). We also quantified the primary root length and the lateral root number as well as average lateral root length both on the control medium and on ABA medium, still found no significant difference between pyl9 and wild type (WT) (see Supplementary Fig. S1). PYL8 and PYL9 are in the same clade of the PYL phylogenetic tree11. We therefore analyzed the root growth of the pyl8-1pyl9 double mutant38 (see Supplementary Fig. S2). Consistent with its visible phenotypes (Fig. 1b), the pyl8-1pyl9 double mutant showed a faster primary root growth than the py8-1 single mutant after treatment with ABA. On the 9th day post transfer (dpt) to plates with ABA, the pyl8-1pyl9 double mutant showed a longer primary root than pyl8-1 single mutant at all concentrations of ABA tested (Fig. 1c). We also quantified the lateral root number of both mutants (Fig. 1d). On the 7 dpt to different concentrations of ABA, pyl8-1pyl9 had more lateral roots than pyl8-1 on 1, 5 and 10 μM ABA medium, and both of them differ significantly from WT at all concentrations except 1 μM ABA medium. Thus pyl8-1pyl9 double mutant was less sensitive to ABA than pyl8-1 single mutant in primary root growth and lateral root formation.
Figure 1. The primary root and lateral root formation of pyl8-1pyl9 double mutant are more insensitive upon ABA treatment.
(a) A schematic diagram of T-DNA insertions in the PYL9 gene. The T-DNA insertion in the pyl9 mutant is inserted in exon, which is presented as closed box. (b) Root architecture of Col-0, pyl8-1 and pyl8-1pyl9 mutants under ABA treatment. Root architecture of seedlings was documented at 9 dpt (days post transfer). Seedlings were transferred at 4 dpg (days post germination) to the control medium (1/2 MS, 1% sucrose) or medium with 10 μM ABA. Bar, 1 cm. (c,d) The primary root length and lateral root number of Col-0, pyl8-1 and pyl8-1pyl9 mutants were documented with different concentrations of ABA. The concentrations of ABA in the medium are as indicated. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). Asterisks indicate comparison between pyl8-1 and pyl8-1pyl9. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test. P-values were adjusted for multiple comparisons by “Benjamini & Hochberg” method. Primary root length and lateral root number of pyl8-1 (at 5 μM, 10 μM, 15 μM, 20 μM) and pyl8-1pyl9 (at all ABA concentrations) were significantly larger than Col-0, P < 0.05.
Lateral root growth stays in a longer quiescence in pyl8-1pyl9 double mutant
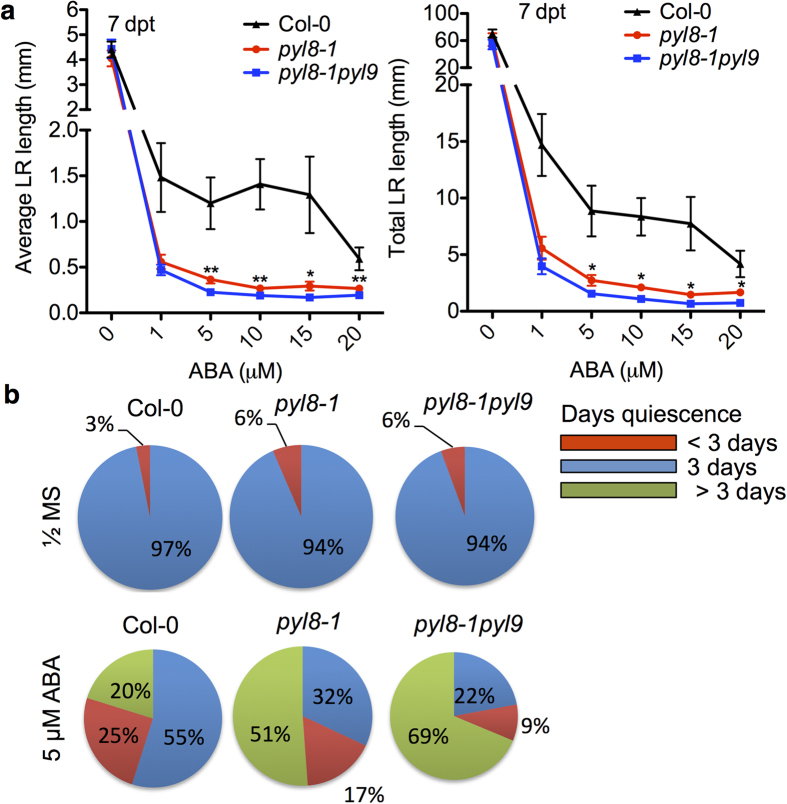
Besides primary root growth phenotypes, we have also observed that the pyl8-1pyl9 double mutant had shorter lateral roots (Fig. 1b). Consistent with its visible phenotypes, the pyl8-1pyl9 double mutant showed a slower lateral root growth than the pyl8-1 single mutant after treatment with ABA. Lateral root growth was severely suppressed in both mutants exposed to ABA especially in the double mutant. Average lateral root length and total lateral root length were both decreased (Fig. 2a). The pyl8 single mutant was reported to have a longer quiescent phase on ABA plates before recovery25. Our results here suggest that PYL9 may promote growth recovery of lateral root under ABA treatment together with PYL8. Lateral root with a length shorter than 0.5 mm is considered to be quiescent39. We found that the two mutants and the wild type had similar quiescent days on control medium. However both mutants have a prolonged quiescent phase than Col-0 under ABA treatment. Moreover, the pyl8-1pyl9 double mutant had an even longer quiescent phase than the py8-1 single mutant (Fig. 2b). This suggests that PYL9 functions together with PYL8 in promoting growth recovery of lateral roots under ABA treatment.
Figure 2. pyl8-1pyl9 double mutant shows a prolonged quiescence phase compared to pyl8-1 on ABA-containing medium.
(a) Average and total lateral root lengths of Col-0, pyl8-1 and pyl8-1pyl9 mutants under different concentrations of ABA. Lateral root length of 7 dpt seedlings grown on ABA media were measured. The concentrations of ABA in the medium are as indicated. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). *P < 0.05, **P < 0.01, Student’s t test. Asterisks indicate comparison between pyl8-1 and pyl8-1pyl9. P-values were adjusted for multiple comparisons by “Benjamini & Hochberg” method. (b) Pie charts of the percentage of quiescent lateral roots for the indicated number of days on seedlings on control medium and medium with ABA (n = 25 seedlings per condition, 5 independent experiments). The proportion of quiescence greater than 3 days of pyl8-1 is significantly less than that of pyl8-1pyl9 (p-value < 0.05, one-tailed binomial test).
IAA suppresses ABA-induced growth inhibition of lateral roots in pyl8-1pyl9
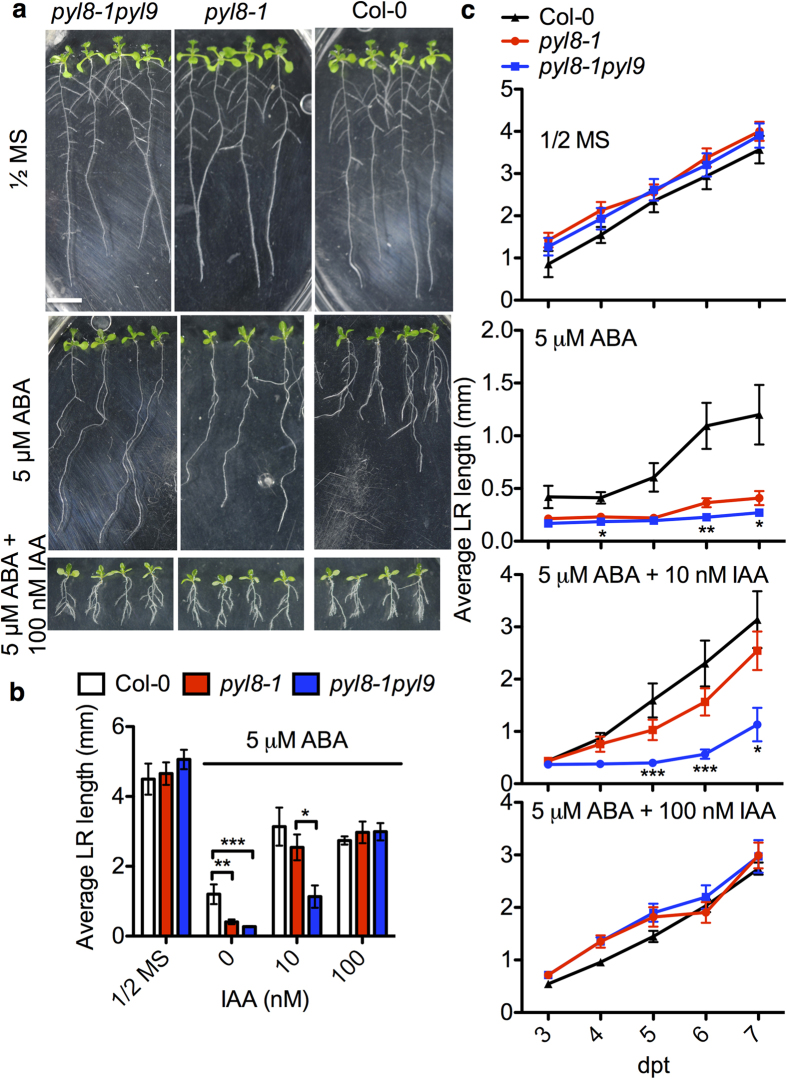
Exogenous IAA can release growth inhibition of lateral roots under ABA treatment25. To determine whether this happens in the pyl8-1pyl9 double mutant, we analyzed the lateral root growth of pyl8-1pyl9 seedlings grown on ABA-containing medium supplemented with IAA (Fig. 3a). We found that the ABA-dependent lateral root growth suppression of pyl8-1pyl9 was partially overcome by 10 nM IAA, and was fully rescued by 100 nM IAA (Fig. 3b). We also determined lateral root growth over the course of time. We found that both pyl8-1 and pyl8-1pyl9 mutants had a slower lateral root elongation than wild type under ABA treatment but not under control conditions. Exogenous application of 10 nM IAA rescued the ABA-dependent growth defects of lateral root in pyl8-1 but not in pyl8-1pyl9. In contrast, in the presence of 100 nM IAA, the lateral root growth defects of both mutants were fully rescued (Fig. 3c). This means that IAA could also rescue the ABA-dependent inhibition of lateral root elongation caused by loss of PYL8 and PYL9. However, the pyl8-1pyl9 double mutant requires a higher concentration of IAA than pyl8-1 single mutant. These results suggest that the lateral root growth defect of pyl8-1pyl9 mutant may be caused by auxin deficiency.
Figure 3. High exogenous IAA complements the lateral root growth defect of pyl8-1pyl9 on ABA-containing medium.
(a) Root architecture of Col-0, pyl8-1 and pyl8-1pyl9 mutants under ABA and ABA with IAA treatment. Root architecture of seedlings was documented at 7 dpt. Seedlings were transferred at 4 dpg to the control medium (1/2 MS, 1% sucrose) (upper panel) or medium supplemented with 5 μM ABA (middle panel) or 5 μM ABA and 100 nM IAA (bottom panel). Bar, 1 cm. (b) Average lateral root length of Col-0, pyl8-1 and pyl8-1pyl9 mutants grown with or without ABA treatment or ABA plus IAA treatment. Lateral root length of seedlings grown on ABA-containing medium was measured at 7 dpt. The concentrations of ABA and IAA in the medium are as indicated. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test. (c) The lateral root length of seedlings was measured at the indicated days after transfer to media with or without ABA or ABA plus IAA. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test. Asterisks indicate comparison between pyl8-1 and pyl8-1pyl9.
ABA-induced overexpression of PYL9 alters both primary and lateral root growth
Since the function of PYL9 on root growth was relatively weaker than that of PYL8, we analyzed the root architecture of PYL9 overexpression lines. The PYL9 gene, under the control of RD29A promoter, was transformed into Arabidopsis. This RD29A gene was first discovered as a cold-induced gene. Later it was found to be responsive to not only cold but also dehydration and ABA40. Two homozygous pRD29A:PYL9 transgenic lines with high PYL9 expression were used for further analysis38. Both transgenic lines had a dramatic increase in PYL9 expression compared to Col-0 wild type under ABA treatment (see Supplementary Fig. S3).
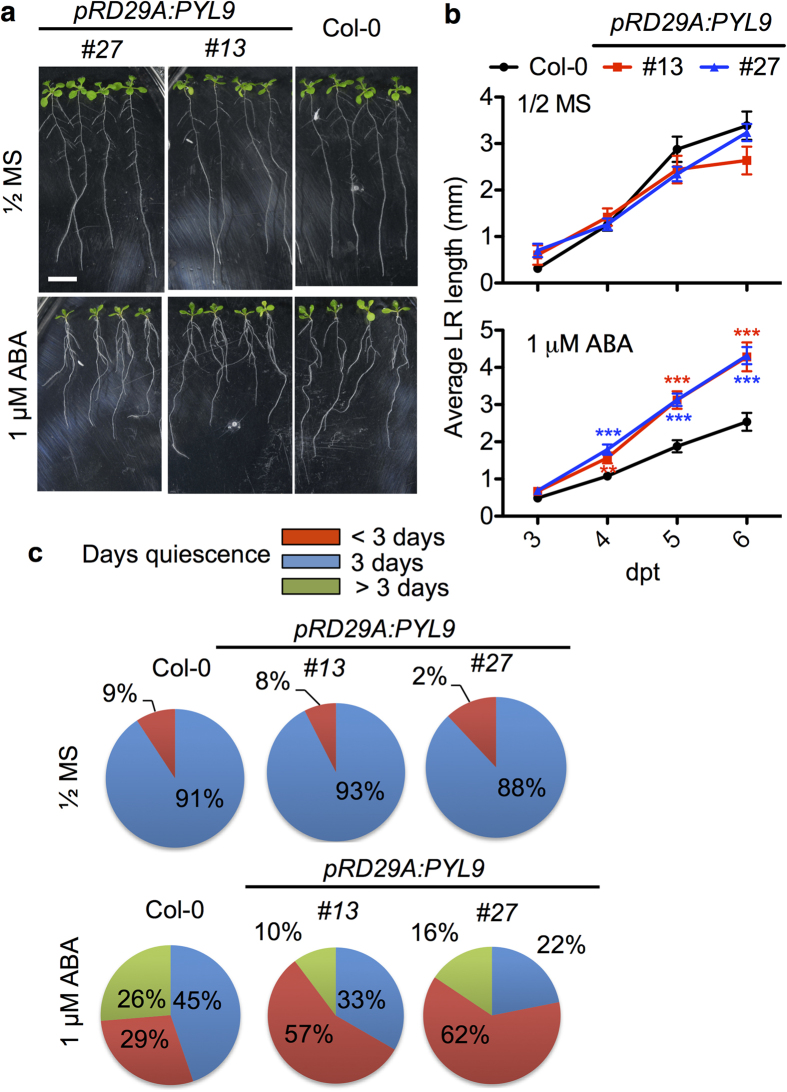
We transferred 4-day-old seedlings to both control medium and medium with 1 μM ABA (Fig. 4a). We monitored the lateral root length over time (Fig. 4b). The two transgenic lines did not have any obvious difference from wild type on control medium since the PYL9 gene was not induced. When 1 μM ABA was applied, the transgenic lines produced longer lateral root than the wild type after 3 days and the difference was more significant over time (Fig. 4b). As loss of PYL9 caused a prolonged quiescent phase, we wondered whether this increase in average lateral root length was due to a shortened quiescent phase. Under 1 μM ABA treatment, more than half of the lateral roots had a quiescent phase shorter than 3 days in both transgenic lines (Fig. 4c). Thus, overexpression of PYL9 promotes lateral roots to escape from ABA-dependent inhibition.
Figure 4. Induced expression of PYL9 increases the average lateral root length and shortens the quiescent phase.
(a) Root architecture of pRD29A:PYL9#13, pRD29A:PYL9#27 and Col-0 under ABA treatment. Seedlings were transferred at 4 dpg to the control medium (1/2 MS, 1% sucrose) (upper panel) or medium supplemented with 1 μM ABA (bottom panel). Root architecture of seedlings was documented at 7 dpt. Bar, 1 cm. (b) The lateral root length of Col-0, pRD29A:PYL9#13 and pRD29A:PYL9#27 was measured at the indicated days after transfer to media supplemented with ABA. Seedlings were transferred at 4 dpg. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). **P < 0.01, ***P < 0.001, Student’s t test. Asterisks indicate comparison between Col-0 and pRD29A:PYL9#13, Col-0 and pRD29A:PYL9#27. (c) Pie charts of the percentage of quiescent lateral roots for the indicated number of days on seedlings on control medium and medium with ABA (n = 25 seedlings, 5 independent experiments). The proportion of quiescence less than 3 days of Col-0 is significantly less than that of pRD29A:PYL9#13 and pRD29A:PYL9#27, respectively (p-value < 0.001, one-tailed binomial test).
To test whether overexpression of ABA receptor PYL9 conferred hypersensitivity to ABA in primary root growth, we analyzed root growth of pRD29A:PYL9 transgenic lines with treatment of different concentrations of ABA. After transferring to medium supplemented with ABA and growing for a week, the primary root of transgenic lines showed an obvious reduction (Fig. 5a). After ABA treatment, both lines showed a significant reduction of lateral root number compared to the wild type (Fig. 5b). This suggests that PYL9 functions in the promotion of lateral root escaping from quiescence as well as the inhibition of primary root growth and lateral root formation under ABA treatment.
Figure 5. Induced expression of PYL9 confers hypersensitivity of primary root and lateral root initiation.
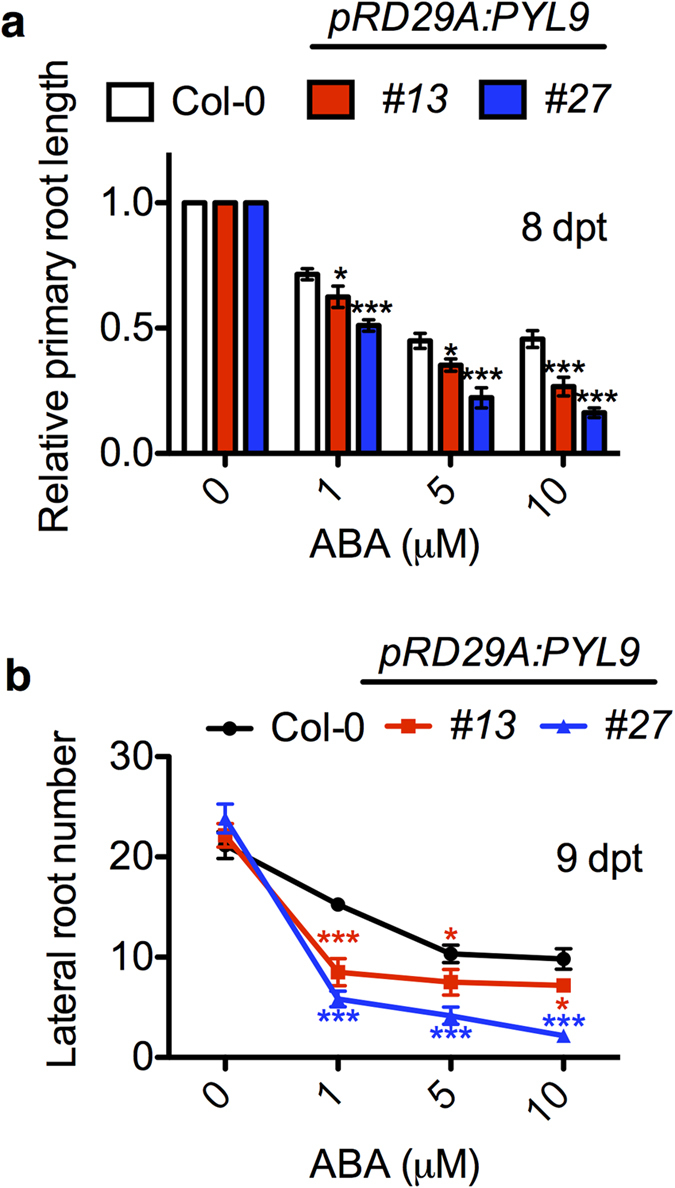
(a,b) Relative primary root length and lateral root number of Col-0, pRD29A:PYL9#13 and pRD29A:PYL9#27 under ABA treatment. The concentrations of ABA in the medium are as indicated. Measurements in a are expressed as a percentage of the length under 1/2 MS conditions. Error bars indicate s.e.m. (n = 25 seedlings, 5 independent experiments). *P < 0.05, ***P < 0.001, Student’s t test. Asterisks indicate comparison between Col-0 and #13, Col-0 and #27.
PYL9 interacts with MYB transcription factors
Recently, interactions between PYLs and MYBs have been reported25,29,30. In our study, several different clones in each combination were tested on yeast growth medium deprived of Trp, His and Leu (Fig. 6a). All of these clones with MYB44/PYL8 had visible colonies, suggesting that PYL8 strongly interacts with MYB44. However, only one of these clones with MYB44/PYL9 had visible colonies (Fig. 6a), suggesting that PYL9 may weakly interact with MYB44. Other MYB proteins were also tested using multiple independent colonies. PYL6 was used as a negative control and had no interactions with any of the three tested MYBs (Fig. 6b, bottom panel). PYL8, as a positive control, had interactions with MYB77 and MYB73 (Fig. 6b, upper panel). Similar to clones with MYB44/PYL9, the minority of these independent clones with MYB77/PYL9 had visible colonies. However, we did not detect any interaction between MYB73 and PYL9 in the yeast two-hybrid assay (Fig. 6b, middle panel). These differences between our results and previous published results might be caused by the expression level of different plasmids. Li et al. used pGADT7-MYBs and the higher expressing pGBKT7-PYLs29, while Jaradat et al. used pGADT7-PYLs and the lower expressing pGBT9-MYBs30. We used pGADT7-MYBs and pBD-GAL4-PYLs. Our results and the previous findings suggest that PYL8 strongly interacts with MYBs, while PYL9 has a relatively weak interaction with MYBs.
Figure 6. PYL9 interacts with MYB transcription factors.
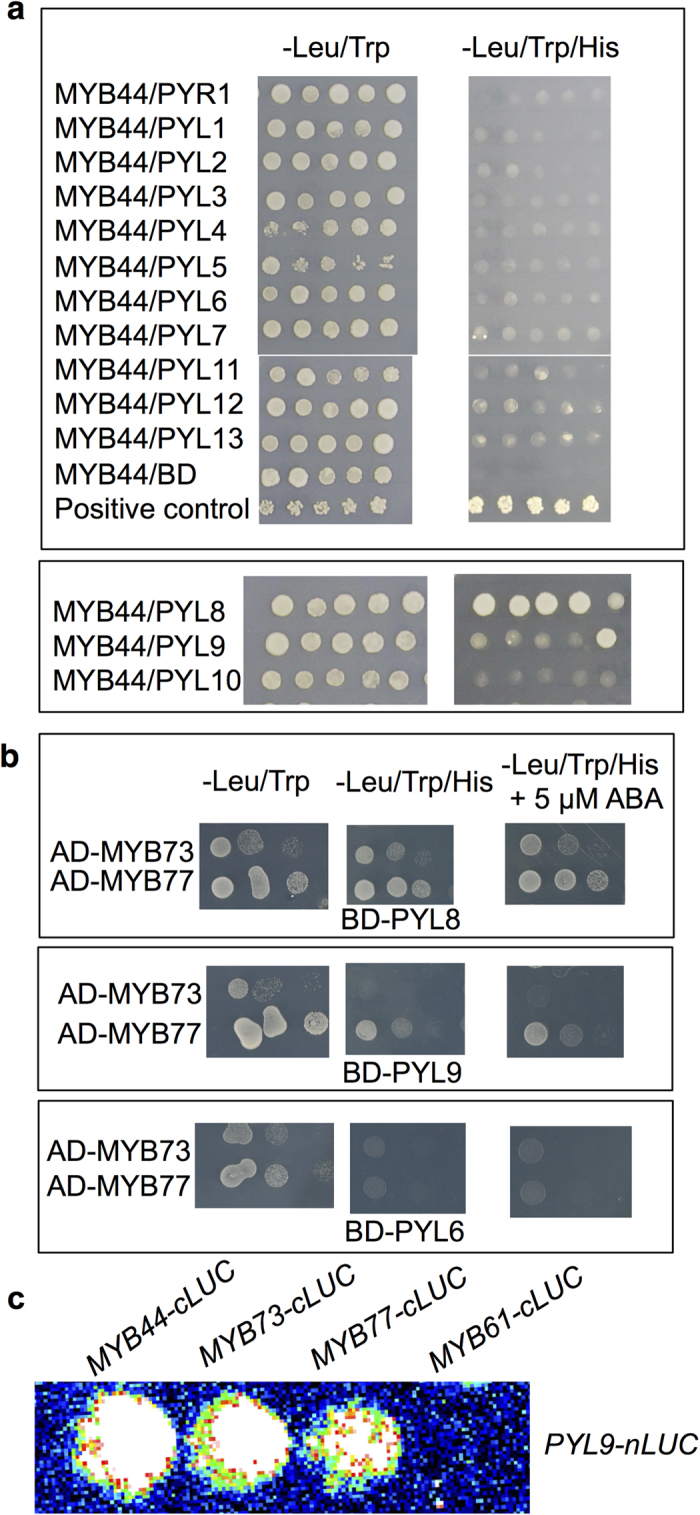
(a) PYLs interact with MYB44 in the yeast two-hybrid assay. PYLs fused to the GAL4-DNA-binding domain (BD) were used as bait. MYB44 fused to the GAL4-activating domain (AD) were used as preys. Interaction was determined by yeast growth on media lacking His, Leu and Trp. BD-PYL10 and AD-ABI1 was used as a positive control. (b) PYL9 interacts with MYBs in the yeast two-hybrid assay. PYL8 and MYB combinations were used as positive controls and PYL6 and MYBs were used as negative controls. Interaction was determined by growth on medium lacking His, Leu and Trp with or without 5 μM ABA. Dilutions (10−1, 10−2, and 10−3) of saturated cultures were spotted onto the plates, which were photographed after 5 days. (c) Co-transform of PYL9-nLUC with MYB44-cLUC, MYB73-cLUC, MYB77-cLUC and MYB61-cLUC in Col-0 wild-type protoplasts in LUC complementation assay.
To further analyze the interactions between PYL9 and MYBs in plant cells, we used the firefly luciferase (LUC) complementation assay in Arabidopsis protoplasts. PYL9 was fused to the N-terminal domain of firefly luciferase (LUC) and MYB proteins were fused to the C-terminal domain of LUC. We co-transformed PYL9-nLUC with the MYB-cLUC into Arabidopsis protoplasts (Fig. 6c). Coexpression of PYL9-nLUC with MYB44/73/77-cLUC, but not MYB61-cLUC produced measurable luciferase activity. These results suggest that PYL9 also interacts with some MYBs in vivo.
PYL9 enhances the activity of MYBs in the presence of ABA and IAA
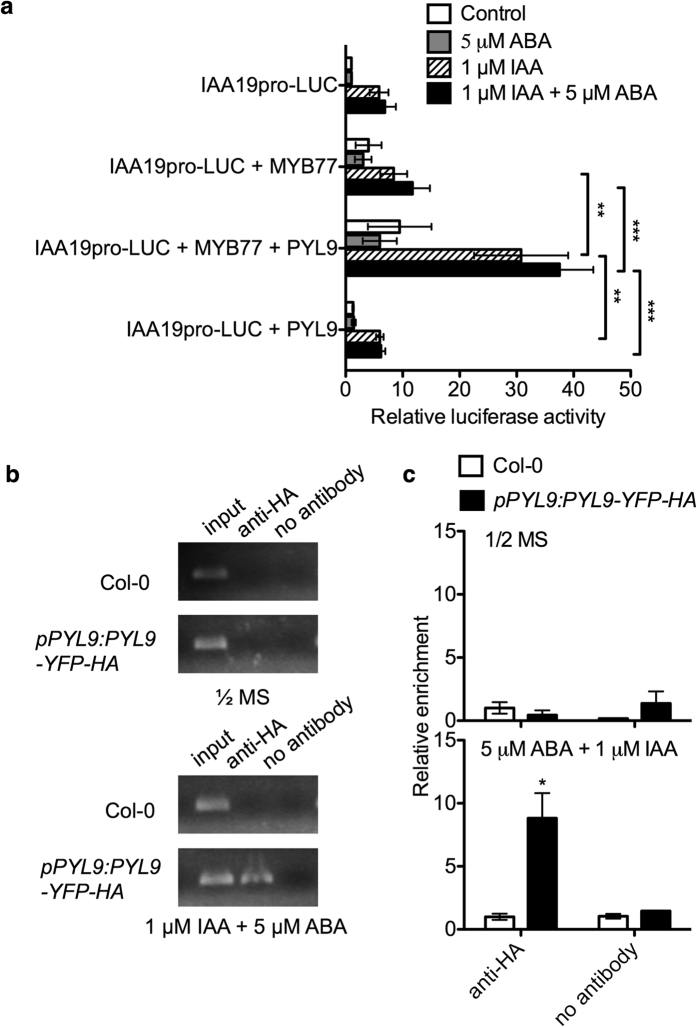
We asked whether the interaction with PYL9 might affect the regulation of downstream genes by MYBs. Previous studies showed that MYB77 could recognize cis-elements MBSI (CNGTTR) and MBSII (GTTAGTTA) and preferentially binds to the MBSI motif 25. IAA19 is one of these downstream genes regulated by MYB7727. pIAA19:LUC was used as a reporter in the Arabidopsis protoplasts transient expression assay25. We co-expressed PYL9, MYB77 with pIAA19:LUC in the protoplasts (Fig. 7a). As expected, the luciferase signal was induced by IAA and was enhanced by MYB77. Similar to PYL8, PYL9 enhanced the activity of MYB77 to increase the expression of pIAA19:LUC in the presence of IAA (Fig. 7a).
Figure 7. PYL9 directly regulates MYB77 transcriptional activity in vivo.
(a) PYL9 enhances the ability of MYB77 to activate IAA19 expression in Col-0 protoplasts. PYL9, MYB77, IAA19-LUC and ZmUBQ-GUS were co-expressed in protoplasts. IAA19-LUC was used as the auxin-responsive reporter. ZmUBQ-GUS was used as the internal control. After transfection, protoplasts were incubated for 12 h under light in the absence of hormone (open bars) or in the different combinations of 5 μM ABA and 1 μM IAA. Error bars indicate s.e.m. (n ≥ 3 experiments). **P < 0.01, ***P < 0.001, Student’s t test. (b,c) ChIP-PCR and ChIP-quantitative-PCR for IAA7 promoter. The 500-bp region of the translational start of IAA7 contains MBSI elements recognized by MYB77 and MYB44. pPYL9:PYL9-YFP-HA was treated with ABA and IAA before conducting ChIP assay. Col-0 was used as control. Numbers are folds compared to Col-0 adding anti-HA. Error bars indicate s.e.m. (n = 3 experiments). *P < 0.05, Student’s t test.
Previous studies demonstrated that PYL8 enhances the interaction between MYB77 and MBSI motifs in vitro25. To further understand whether PYL9 and MYB77 regulate their target genes as a protein complex, we used a transgenic line with tagged PYL9 driven by PYL9 native promoter38. pPYL9:PYL9-YFP-HA was introduced into the quadruple mutant pyr1pyl1pyl2pyl4 and this tagged line was used in chromatin immunoprecipitation assay (see Supplementary Fig. S4). We pre-incubated the seedlings with 5 μM ABA and 1 μM IAA for 5 hours, and the assay was performed as described41. We added extra EGS or ethylene glycol-bis (succinic acid N-hydroxysuccinimide ester) to strengthen the PYL9-MYBs interaction during cross-linking. Because MYB77 preferentially binds to MBSI, we filtered candidate genes using this criterion. We checked several DNA fragments that harbored this cis-element, including the promoter region of IAA1, IAA7, IAA17, IAA19 and HAT2. Different primer pairs were designed flanking this motif. The ChIP-PCR result showed that the promoter region of IAA7 was enriched with PYL9-YFP-HA after treatment of IAA and ABA (Fig. 7b). The ChIP-quantitative-PCR result showed nearly 10-fold enrichment in pPYL9:PYL9-YFP-HA transgenic lines than in the wild type negative control after the treatment of ABA and IAA. While in the control, there was no such enrichment (Fig. 7b,c). The motif in this promoter fragment was CTGTTG, belonging to MBSI. We also found another CTGTTG in the promoter of HAT2 but this one did not show enrichment in ChIP-quantitative-PCR. This might be due to the position of the motif. The one in IAA7 is located around 500 bp upstream from the start codon, however, the one in HAT2 is located within 100 bp from its start codon. This suggests that the transcription complex of PYL9 and MYB proteins may bind to promoters around 500 bp from where transcription starts. This in vivo evidence strongly supports the hypothesis that PYL9 regulates the transcriptional activity of MYB77 or other MYB proteins directly in vivo and participates in the auxin signal pathway.
Discussion
ABA and auxin are two important phytohormones; one is well known for its function under stress, and the other one is well known for its growth-inducing activity. Our study here reveals a crosstalk node between them in regulating root growth.
Roots provide a tight connection between plants and the soil environment, and are often the first to sense the soil environment. Root systems of ABA-deficient mutants like aba2-1 and aba3-1 are less affected than wild type seedlings upon osmotic stress42. ABA at low concentrations has been reported to promote root growth17. The endogenous ABA controls root architecture both in the presence and absence of osmotic stress through maintaining meristem in dormancy and inhibiting QC division and suppressing the differentiation of stem cells in the primary root20. High concentrations of ABA are well known to inhibit the growth of both primary and lateral root (Fig. 1c,d). ABA has a much stronger effect on lateral root than on the primary root, suggesting that different signaling mechanisms in the two types of roots39. In our study, the lateral root number in all the tested ABA concentrations showed a decrease compared to control medium (Fig. 1d,b). What’s more, the lateral root elongation is more sensitive to ABA than the inhibition of seed germination and this reversible growth arrest occurs at a specific developmental stage, that is right after the lateral root emergence with a length less than 0.5 mm16. As shown in Fig. 2b, longer quiescence was observed on ABA-containing medium and this might be due to a dormant state of lateral roots. Although ABA is well known for its function in seed dormancy and growth inhibition, different ABA sensitivities suggest different pathways in lateral roots compared with other tissues.
Salt stress induces a quiescence phase in post emergence lateral root growth and then recovery takes place several days later, which involves genes of ABA biosynthesis, signaling and transcription regulation39. PYL8 is responsible for this quiescence upon ABA treatment25. PYL9 has a 77% amino acid sequence identity with PYL8 and particularly high GUS activity can be detected in stele cells of pPYL9:GUS transgenic plants24. Although pyl9 single mutant did not show obvious difference from wild type on ABA medium (see Supplementary Fig. S1), pyl8-1pyl9 double mutant had an even longer primary root and more lateral roots than pyl8-1 on ABA medium (Fig. 1c,d). The induced overexpression of PYL9 upon ABA treatment led to a shorter primary root and fewer lateral roots (Fig. 5a,b). These results suggest that PYL9 functions in the repression of lateral root formation and primary root elongation by ABA. PYL9 is a functional ABA receptor11 and the ABA signal perceived by it and other PYLs is passed down eventually to ABA-RESPONSIVE ELEMENT (ABRE)-BINDING FACTOR (ABF) proteins. And overexpression of ABF2 confers hypersensitivity to ABA in primary root43. Other mutants impaired in ABA signaling pathway have also been tested including abi1-1, pyr1/pyl1/2/4 and snrk2.2/3/625. All these ABA-resistant mutants showed a reduced repression of primary root growth and lateral root formation upon ABA treatment25 and this correlates with our results in pyl8-1pyl9 double mutant. However, these ABA-resistant mutants have shorter quiescence compared to WT on ABA medium, which is opposite to our pyl8-1pyl9 double mutant. This suggests a different pathway involving PYL9. Recently, interactions between PYLs and MYBs have been reported25,29,30. PYL8 strongly interacts with the MYBs25,30. Further studies found that PYL9 and probably PYL7 also interact with these MYBs29, which is different from the previous published result30. Our results also showed that PYL9 interacted with MYB77 and MYB44 (Fig. 6), however in our Y2H assay there was no obvious interaction between PYL9 and MYB73 (Fig. 6b), which is different from previous reports29. This is probably due to different yeast systems and plasmids. MYB77 is a positive regulator of lateral root growth and its interaction with ARF7 suggests that MYB77 may function through auxin signal transduction pathway27. The lateral root growth of myb77 mutants is more sensitive upon ABA treatment and exogenous IAA could reverse this like it could reverse in pyl8-1 and pyl8-1pyl925. So the interaction between PYL9 and MYB77 suggests they may function together. MYB77 preferentially recognizes cis-elements MBSI (CNGTTR) and previous EMSA data showed that PYL8 protein enhances MYB77 binding to MBSI in an ABA-dependent manner25,27. MYB44 has a clear preference for MBSII (GTTAGTTA) type but still binds to MBSI44. Our chromatin immunoprecipitation assay indicated that anti-HA antibody pulls down the protein complex of PYL9-MYB proteins (Fig. 7b,c). The exogenous IAA and ABA enhanced their interaction and the effect on the downstream gene promoters25 (Fig. 7). Quantitative PCR showed that one fragment of IAA7 promoter, containing MBSI motif, has a huge enrichment (Fig. 7b,c). IAA7 is a member of the Aux/IAA protein family, which is induced by auxin45. Besides PYL8 and PYL9, PYL7 is also an interacting protein of MYB44 in Y2H assays29. This implies that more PYLs might be involved in the PYL-MYB pathway.
Auxin and ABA are widely accepted as regulators of root growth. These two phytohormones have a lot of interactions and previous studies have revealed that auxin could potentiate ABA response in roots46. Unlike in p35S:VP1 where ABA fully inhibits auxin induced lateral root initiation47, the prolonged quiescence phase of pyl8-1 on ABA medium was shortened by application of IAA25. Osmotic stress represses lateral root development which could be overcome by auxin42, however exogenous ABA represses lateral root formation, that is the initiation of lateral root primordia, which could not be rescued by auxin16. In our study, the quiescence in pyl8-1 and pyl8-1pyl9 was overcome by application of IAA (Fig. 3). What’s more, the severe defects in the double mutant require more IAA to be rescued (Fig. 3b,c). Whether auxin rescues or not might be dependent on the state of lateral roots and these activities might be mediated by different mechanisms. Lateral root primordia initiation is highly dependent on auxin48, however in our study, we only considered the lateral roots that are already formed when we transferred seedlings to ABA medium.
The function of PYL9 and MYB77 complex is dependent on IAA treatment. In Arabidopsis protoplasts, the reporter (pIAA19:LUC) showed a strong signal when PYL9 was co-transformed with MYB77 in the presence of IAA (Fig. 7a). In transgenic seedlings, PYL9 can pull down auxin responsive promoter fragments in the presence of IAA and ABA (Fig. 7b,c). Besides, PYL9 itself is a functional ABA receptor. Taken together, this suggests that PYL9 connects ABA and auxin signaling pathway. MYB77 modulates auxin response by forming a heterodimer with ARF7 and their double mutant myb77-2arf7 (myb77-2nph4-1) has even smaller lateral root density than nph4-1, which is already smaller than wild type and myb77-227. In myb77 mutants, auxin-responsive genes are attenuated while they are increased in over-expression lines27. Similar results were obtained in p35S:MYB44 transgenic lines where auxin-responsive genes are increased, suggesting that salt response and auxin signaling cross-talk at the transcriptional level31. This suggests that MYB77 and MYB44 might have some overlapping functions in regulating auxin-responsive genes. IAA7, which might be the target of PYL9-MYB77 complex, is also a key component regulating ABA and auxin-dependent post-embryonic growth49. It is suppressed when ABA represses embryonic axis elongation by potentiating auxin signaling in the elongation zone49.
PYL9 binds type 2C protein phosphatases and was found to interact with R2R3 MYB proteins recently. Our results show that PYL9 not just binds to MYB transcription factors, but also regulates their transcriptional activity, and that this is a point of convergence between ABA and auxin pathways.
Methods
Plant materials and growth condition
The pyl8-1 (SAIL_1269_A02)24, pyl9 (SALK_083621)24, pyr1pyl1/2/411 quadruple mutant were in Col-0 background. The pyl8-1pyl9 double mutant was obtained by crossing pyl8-1 with pyl9. The pRD29A::PYL9 construct was transformed into Col-0, and pPYL9::PYL9-YFP-HA construct was transformed into pyr1pyl1/2/4 by Agrobacterium tumefaciens-mediated floral-dip transformation.
Seeds were surface-sterilized for 8 min in 10% bleach and then rinsed in sterile deionized water for four times. Sterilized seeds were grown on 0.6% Phytagel (Sigma) or 0.8% agar media containing 1/2 MS nutrients (catalog no. M524, PhytoTechnology Laboratories), 1% sucrose adjusted to pH 5.7 (control media), and kept at 4 °C for 2 days. Seedlings were grown vertically before transfer to control media or media supplemented with the indicated concentrations of ABA (Sigma, A1049) or IAA (Sigma, I2886). Seedlings were grown at 22 °C under 16-h light/8-h dark cycles.
For protoplast analysis, seedlings were grown on Jiffy 7 peat soil (42 mm Pellets) in a Percival chamber with a relatively short photoperiod (12 hours of light at 23 °C, 12 hours of dark at 20 °C) under low light (about 100 μE m−2 s−1) and 50 to 70% relative humidity under well-watered conditions.
Phenotype analysis
The primary root length was measured from the first day after transfer. The results of lateral root were observed using HIROX KH-7700 digital microscope with MX-5040RZ lens. The total and average lateral root lengths of individual plants were quantified by summing or averaging the lengths of all the lateral roots on each plant. Lateral roots shorter than 0.5 mm were categorized as quiescent39.
Plasmid construction
The pIAA19-LUC and pGADT7-MYBs constructs were generated as described25. The pBD-GAL4-PYLs constructs were generated as described11.
Yeast two-hybrid assay
Yeast two-hybrid assays were performed as described25. PYLs fused to the GAL4 DNA binding domain were used as baits. MYB44, MYB73 and MYB77 fused to the GAL4-activating domain were used as preys. Interaction was determined by growth assay on media lacking His or His and Ade with or without 5 μM ABA. Dilutions (10−1, 10−2 and 10−3) of saturated cultures were spotted onto the plates and photographed after 5 days.
Transient expression assay in Arabidopsis
Assays for transient expression in protoplasts were performed as described25. All steps were at room temperature. pIAA19::LUC was used as the auxin-responsive reporter. ZmUBQ-GUS was used as the internal control. After transforming, protoplasts were incubated in washing and incubation solution (0.5 M mannitol, 20 mM KCl, 4 mM MES, pH 5.7) with or without ABA and IAA at the indicated concentrations for 12 hours of light and LUC luminescence was measured with a plate reader (Wallac VICTOR2 plate reader). LUC complementation assays were performed as described25.
Chromatin immuoprecipitation assay
Ten-day-old transgenic seedlings and anti-HA antibodies (1:100 for ChIP assay, HA-Tag, 26D11, Mouse mAb, M20003, Abmart, Shanghai, China) were used for ChIP experiments50. Briefly, the transgenic seedlings were ground into a fine powder with liquid nitrogen and resuspended in nuclei isolation buffer. 1.5 mM EGS was added into the nuclei isolation buffer for protein-protein cross-linking. The nucleus were then collected by centrifugation and resuspended with nuclei lysis buffer. The resuspended chromatin was sonicated to fragments with various sizes (250 bp–1 kb) subsequently. PYL9-HA-YFP-MYBs protein complex was precipitated from input DNA with or without anti-HA antibodies. Protein A agarose beads (Millipore, USA) were added into the incubation mixture for additional 2 h at 4 °C. The immune complexes were eluted from the washed protein A beads. The DNA was purified with phenol/chloroform (1:1, v/v) and precipitated. The purified DNA and input DNA were used as templates. The enrichment of DNA fragments was determined by quantitative PCR with specific primers.
Additional Information
How to cite this article: Xing, L. et al. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 6, 27177; doi: 10.1038/srep27177 (2016).
Supplementary Material
Acknowledgments
This work was supported by a grant from the US National Institutes of Health (R01GM059138) to J.-K.Z. and by the Chinese Academy of Sciences to J.-K.Z. and by a grant from Ministry of Science and Technology of China (2012CB114304) to C.X. and the National Nature Science Foundation of China (91417306 and 30830075) to C.X. We thank the Arabidopsis Biological Resource Center for providing the seeds of SAIL_1269_A02 and SALK_083621, Dr. J. Sheen for providing the ZmUBQ::GUS construct and Dr. S.R. Cutler for providing the pyr1pyl1/2/4 quadruple mutant.
Footnotes
Author Contributions L.X. conducted the phenotype analysis experiments and the yeast-two–hybrid experiments. Y.Z. and J.G. did the Arabidopsis transient transformation experiments. L.X., Y.Z., J.G., C.X. and J.-K.Z. designed the study. L.X. and Y.Z. analyzed the data. All authors reviewed the manuscript.
References
- Sabatini S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472 (1999). [DOI] [PubMed] [Google Scholar]
- Himanen K. et al. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14, 2339–51 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E. et al. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 115, 591–602 (2003). [DOI] [PubMed] [Google Scholar]
- Péret B. et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 14, 991–998 (2012). [DOI] [PubMed] [Google Scholar]
- Laskowski M., Biller S., Stanley K., Kajstura T. & Prusty R. Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol. 47, 788–92 (2006). [DOI] [PubMed] [Google Scholar]
- Lee H. W., Kim M.-J., Kim N. Y., Lee S. H. & Kim J. LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 73, 212–24 (2013). [DOI] [PubMed] [Google Scholar]
- Neuteboom L. W. et al. Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol. Biol. 39, 273–87 (1999). [DOI] [PubMed] [Google Scholar]
- De Smet I., Zhang H., Inzé D. & Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 11, 434–9 (2006). [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K. & Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803 (2006). [DOI] [PubMed] [Google Scholar]
- Tian L. & Brown D. C. W. Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Can. J. Plant Sci. 80, 271–276 (2000). [Google Scholar]
- Park S.-Y. et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science (80-.). 324, 1068–1071 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. et al. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science (80-.). 324, 1064–8 (2009). [DOI] [PubMed] [Google Scholar]
- Fujii H. et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature 462, 660–4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon F.-F. et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335, 85–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Verslues P. E. & Zhu J.-K. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19, 485–94 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I. et al. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 33, 543–555 (2003). [DOI] [PubMed] [Google Scholar]
- Ephritikhine G., Fellner M., Vannini C., Lapous D. & Barbier-Brygoo H. The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J. 18, 303–14 (1999). [DOI] [PubMed] [Google Scholar]
- Sharp R. E. et al. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 55, 2343–2351 (2004). [DOI] [PubMed] [Google Scholar]
- Cheng W.-H. A Unique Short-Chain Dehydrogenase/Reductase in Arabidopsis Glucose Signaling and Abscisic Acid Biosynthesis and Functions. Plant Cell Online 14, 2723–2743 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 64, 764–774 (2010). [DOI] [PubMed] [Google Scholar]
- Brady S. M., Sarkar S. F., Bonetta D. & McCourt P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 34, 67–75 (2003). [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D. & Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22, 3560–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. Plos Genet. 7, e1002172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni R. et al. PYL8 plays an important role for regulation of ABA signaling in root. Plant Physiol. 161, 931–941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. et al. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 7, ra53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 110, 15485–90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R. et al. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 19, 2440–53 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C. et al. MYB transcription factors in Arabidopsis. Trends Plant Sci. 15, 573–81 (2010). [DOI] [PubMed] [Google Scholar]
- Li D. et al. Arabidopsis ABA receptor RCAR1/PYL9 interacts with an R2R3-type MYB transcription factor, AtMYB44. Int. J. Mol. Sci. 15, 8473–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaradat M. R., Feurtado J. A., Huang D., Lu Y. & Cutler A. J. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 13, 192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. et al. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 146, 623–35 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. et al. The unique mode of action of a divergent member of the ABA-receptor protein family in ABA and stress signaling. Cell Res. 23, 1380–95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. et al. Identification and characterization of ABA receptors in Oryza sativa. PLoS One 9, e95246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Molecular basis for the selective and ABA-independent inhibition of PP2CA by PYL13. Cell Res. 23, 1369–79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q. et al. The molecular basis of ABA-independent inhibition of PP2Cs by a subclass of PYL proteins. Mol. Cell 42, 662–72 (2011). [DOI] [PubMed] [Google Scholar]
- Li Z. et al. ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J. Integr. Plant Biol. 54, 180–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M. et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24, 2483–96 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. 113, 1949–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L. et al. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25, 324–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K. & Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6, 251–264 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. et al. HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. J. Exp. Bot. 65, 4285–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak K. I. & Malamy J. Osmotic regulation of root system architecture. Plant J. 43, 17–28 (2005). [DOI] [PubMed] [Google Scholar]
- Kim S., Kang J.-Y., Cho D.-I., Park J. H. & Kim S. Y. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 40, 75–87 (2004). [DOI] [PubMed] [Google Scholar]
- Persak H. & Pitzschke A. Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signalling. PLoS One 8, e57547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P. AXR2 Encodes a Member of the Aux/IAA Protein Family. PLANT Physiol. 123, 563–574 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P. & Sheen J. Sugar and hormone connections. Trends Plant Sci. 8, 110–6 (2003). [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kao C.-Y., Cocciolone S. & McCarty D. R. Maize VP1 complements Arabidopsisabi3 and confers a novel ABA/auxin interaction in roots. Plant J. 28, 409–418 (2002). [DOI] [PubMed] [Google Scholar]
- Laskowski M. J., Williams M. E., Nusbaum H. C. & Sussex I. M. Formation of lateral root meristems is a two-stage process. Development 121, 3303–3310 (1995). [DOI] [PubMed] [Google Scholar]
- Belin C., Megies C., Hauserová E. & Lopez-Molina L. Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling. Plant Cell 21, 2253–68 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel A.-V., Lippman Z., Martienssen R. & Colot V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2, 213–8 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.