Abstract
Background and objectives
Roxadustat (FG-4592), an oral hypoxia–inducible factor prolyl hydroxylase inhibitor that stimulates erythropoiesis, regulates iron metabolism, and reduces hepcidin, was evaluated in this phase 2b study for safety, efficacy, optimal dose, and dose frequency in patients with nondialysis CKD.
Design, setting, participants, & measurements
The 145 patients with nondialysis CKD and hemoglobin ≤10.5 g/dl were randomized into one of six cohorts of approximately 24 patients each with varying roxadustat starting doses (tiered weight and fixed amounts) and frequencies (two and three times weekly) followed by hemoglobin maintenance with roxadustat one to three times weekly. Treatment duration was 16 or 24 weeks. Intravenous iron was prohibited. The primary end point was the proportion of patients achieving hemoglobin increase of ≥1.0 g/dl from baseline and hemoglobin of ≥11.0 g/dl by week 17 (16 weeks of treatment). Secondary analyses included mean hemoglobin change from baseline, iron utilization, and serum lipids. Safety was evaluated by frequency/severity of adverse events.
Results
Of the 145 patients enrolled, 143 were evaluable for efficacy. Overall, 92% of patients achieved hemoglobin response. Higher compared with lower starting doses led to earlier achievement of hemoglobin response. Roxadustat–induced hemoglobin increases were independent of baseline C–reactive protein levels and iron repletion status. Overall, over the first 16 treatment weeks, hepcidin levels decreased by 16.9% (P=0.004), reticulocyte hemoglobin content was maintained, and hemoglobin increased by a mean (±SD) of 1.83 (±0.09) g/dl (P<0.001). Overall mean total cholesterol level was reduced by a mean (±SD) of 26 (±30) mg/dl (P<0.001) after 8 weeks of therapy, independent of the use of statins or other lipid–lowering agents. No drug–related serious adverse events were reported.
Conclusions
In patients with nondialysis CKD who were anemic, various starting dose regimens of roxadustat were well tolerated and achieved anemia correction with reduced serum hepcidin levels. After anemia correction, hemoglobin was maintained by roxadustat at various dose frequencies without intravenous iron supplementation.
Keywords: chronic kidney disease; clinical trial; anemia; C-Reactive Protein; Erythropoiesis; Hemoglobins; Hepcidins; Humans; Iron; Renal Insufficiency, Chronic
Introduction
Anemia is a common CKD complication associated with increased morbidity and mortality (1,2). Recombinant erythropoiesis–stimulating agents (ESAs) provide improved quality of life in dialysis (1,3–5) and nondialysis (6–8) populations and reduce red blood cell (RBC) transfusions. However, anemia in a majority of patients with nondialysis CKD (NDD-CKD) stages 3b–5a is not treated and often delayed because of late nephrology referral and ESA reimbursement limitations (9). Between 2005 and 2010, only 28% of patients with CKD in the United States received ESA before dialysis initiation (10).
Additionally, safety of ESAs in CKD anemia treatment is being questioned after greater risks for death, cardiovascular events, and stroke were observed in ESA intervention trials targeting hemoglobin (Hb) >13 g/dl (11–13). Consequently, ESA use, even in patients with Hb<10 g/dl, decreased markedly after 2011 following US Food and Drug Administration warnings to reduce ESA utilization to transfusion avoidance only (14).
These developments may adversely affect outcomes in patients with CKD and Hb<10 g/dl who have higher risks of hospitalization, RBC transfusion (15), progression to dialysis, and mortality (16,17). Specifically, RBC transfusions may allosensitize patients with CKD, reducing likelihood to receive a transplant (18–20). These potential consequences of untreated/undertreated anemia coupled with ESA safety concerns call for safer, effective, and accessible treatment for CKD anemia (21).
Roxadustat (FG-4592) is a hypoxia–inducible factor prolyl hydroxylase inhibitor (HIF-PHI). Transient inhibition of prolyl hydroxylase by hypoxia or HIF-PHI activates several early response target genes, including the genes encoding erythropoietin (EPO) and EPO receptor, and proteins promoting iron absorption, iron transport (transferrin), and heme synthesis. In a placebo–controlled, phase 2a, 4-week treatment study, roxadustat increased Hb levels in a dose-dependent manner and improved iron homeostasis while modestly and transiently increasing endogenous EPO levels within or near physiologic range (22). We now report data from an open label, phase 2b study of roxadustat administered for 16–24 weeks to patients with NDD-CKD who were anemic, further exploring dose and frequency strategies to determine the optimal dose and regimen for phase 3 studies.
Materials and Methods
Study Design
This was a multicenter, open label, randomized study of roxadustat in patients with stage 3 or 4 NDD-CKD conducted at 36 sites in the United States (four sites in Puerto Rico). The study consisted of a screening period of up to 4 weeks, a treatment period of 16 or 24 weeks, and a 4-week follow-up period. It was registered at clinicaltrials.gov (NCT01244763), approved by all appropriate institutional review boards, and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients and Treatment
Eligible patients with NDD-CKD were 18−75 years of age with eGFR=15−59 ml/min per 1.73 m2, had mean screening Hb ≤10.5 g/dl, had a baseline ferritin >30 ng/ml, had transferrin saturation (TSAT) ≥5%, and had not received ESAs for the 12 weeks before enrollment (Supplemental Table 1).
In total, 145 patients were enrolled into six different dose cohorts (A–F) of 24 patients each in cohorts A–E and 25 patients in cohort F (Table 1). These cohorts were selected to determine which dose strategy (fixed versus tiered weight based) and which dose regimen (thrice weekly [TIW] or twice weekly [BIW]) produced optimal desired initial Hb response. During maintenance therapy, less frequent dosing options were assessed. Patients were initially randomized to cohorts A and B in parallel for 16 weeks of treatment. After their completion, two 24-week treatment cohorts, cohorts C and D, were then randomized in parallel. After completion of randomization cohorts A–D, the last two 24-week treatment cohorts, cohorts E and F, enrolled patients. Randomization was performed centrally across all sites using a predetermined randomization schedule with permuted block design. None of the sites had access to the master randomization schedule, and the sites received a treatment assignment for a patient only after the patient met the enrollment criteria.
Table 1.
Cohort dosing characteristics
| Cohort (Sample Size) | Treatment Duration, wk | Starting Roxadustat Doses | Correction and Maintenance Dosing Frequency | ||
|---|---|---|---|---|---|
| Low Wt, 40–60 kg | Medium Wt, >60–90 kg | Heavy Wt, >90–140 kg | |||
| A (n=24) | 16 | 60 mg | 100 mg | 140 mg | TIW to TIW |
| B (n=24) | 16 | 60 mg | 100 mg | 140 mg | TIW to BIWa |
| C (n=24) | 24 | 50-mg fixed starting dose regardless of weight | TIW to TIW | ||
| D (n=24) | 24 | 100-mg fixed starting dose regardless of weight | TIW to TIW | ||
| E (n=24) | 24 | 70 mg | 100 mg | 150 mg | BIW to QWb |
| F (n=25) | 24 | 70-mg fixed starting dose regardless of weight | TIW to BIW to QWc | ||
Wt, weight; TIW, thrice weekly; BIW, twice weekly; QW, once weekly.
Dose frequency reduction from TIW to BIW when hemoglobin response was reached.
Dose frequency reduction from BIW to QW when hemoglobin response was reached.
Dose frequency reduction from TIW to BIW when hemoglobin response was reached; additional dose frequency reduction from BIW to QW when hemoglobin and dose had been stable for 8 weeks.
Cohorts A and B received starting roxadustat doses of 1.0–1.7 mg/kg (on the basis of phase 2a experience) (22) TIW in one of three doses tiered by body weight, whereas cohort E received tiered weight starting dose BIW (Table 1). Cohorts C, D, and F received three fixed starting doses of 50, 100, and 70 mg TIW (regardless of weight), respectively.
Hb was measured weekly, and Hb response was defined as an increase from baseline Hb (ΔHb) ≥1.0 g/dl and Hb level ≥11.0 g/dl. Doses could be titrated every 4 weeks. After an Hb response was achieved, the roxadustat dose frequencies that were used for Hb maintenance were that cohorts A, C, and D continued TIW, cohort B switched to BIW (from TIW), cohort E switched to once weekly (QW) from BIW, and cohort F first switched to BIW from TIW and, after stable Hb, to QW (Table 1).
ESAs, intravenous (iv) iron, androgens, and RBC transfusions were prohibited. Use of oral iron was allowed but not required.
Dose Modifications
Investigators titrated roxadustat doses on the basis of cohort–specific adjustment rules to correct and maintain Hb levels to prespecified Hb targets of 11.0–13.0 g/dl for cohorts A, B, E, and F and 10.5–12.0 g/dl for cohorts C and D (Supplemental Table 2). Maximum roxadustat dose was capped at 2.2 mg/kg per dose for cohorts A–D and 2.5 mg/kg for cohorts E and F.
Doses were held/decreased when Hb rate of rise was >1.5 g/dl within the first 3 weeks or ≥2.0 g/dl during any 2-week treatment period or if Hb level was ≥14.0 g/dl at any time (Supplemental Table 2).
Assessments
The primary end point was the cumulative proportion of patients in each cohort achieving Hb response by the end of week 16. To evaluate the full treatment effect in evaluable patients with 24 weeks of treatment, Hb response rates through end of treatment are also presented. The effect of roxadustat on Hb values was also assessed by mean Hb over time. The schedule of assessments and extent of missing data are provided in Supplemental Table 3.
Safety was assessed by physical examinations, electrocardiograms, laboratory tests, and adverse events (AEs; from onset of treatment through end of follow-up). Hepcidin, serum iron, transferrin, TSAT, and ferritin were measured (Supplemental Table 4). Lipid profiles were performed on stored serum samples at the end of the study.
Statistical Analyses
Twenty-four patients per cohort were enrolled to evaluate Hb responses to different dosing regimens. The efficacy-evaluable population consisted of patients receiving at least 2 weeks of study treatment with valid baseline and post–treatment Hb measurements.
ΔHb from baseline used the pooled variance from all groups (analysis of covariance model). Time to response was estimated (Kaplan–Meier test). Additional analyses included simple ANOVA, mixed model repeated measure ANOVA, and chi-squared test as appropriate. All analyses used SAS (v9.1.3; SAS Institute Inc., Cary, NC). Statistical significance was on the basis of P≤0.05 unadjusted for multiple comparisons. Results are presented as means±SDs (in the text and tables) and means±SEMs (in the figures). Missing data were imputed using last observation carried forward. Hb values for patients receiving blood transfusions were censored after transfusion.
Results
Patient Disposition
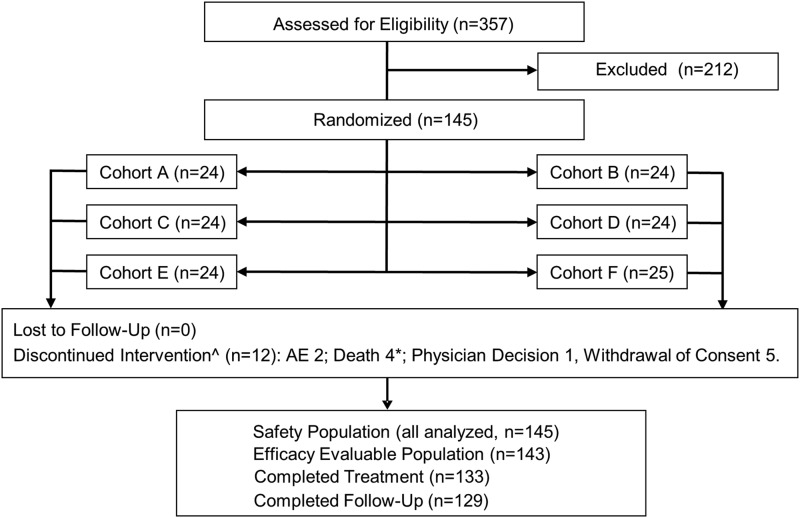
Of 357 patients screened, 145 were enrolled (safety population), of whom 143 were efficacy evaluable. Twenty-four patients were enrolled in each cohort (25 in cohort F). All randomized patients received roxadustat. Twelve patients (8.3%) discontinued treatment prematurely: consent withdrawal (n=5); AEs (n=6), four of which were fatal (see below); and noncompliance (n=1). The participant flow diagram is provided as Figure 1.
Figure 1.
Patient disposition. AE, adverse event. *One patient died during the follow-up period. ^Four of the 12 discontinuations of treatment occurred in cohorts A and B (16 weeks of treatment) after 1.1, 4.7, 13.3, and 13.4 weeks of dosing. The remaining eight discontinuations occurred in cohorts C–F (24 weeks of treatment) after 1 day (single dose) and 2.6, 3.4, 8.1, 8.9, 11.1, 18.1, and 21.7 weeks of dosing.
Patient Characteristics at Baseline
Although minor differences existed among cohorts with regards to age, sex, and the presence of diabetes and hypertension, patient demographics and baseline characteristics were similar (Table 2). Mean baseline Hb (mean of three Hb values) was 9.7 g/dl (range =7.0–10.7 g/dl). Only 52.4% of patients were iron replete (ferritin >100 ng/ml and TSAT>20%); 45 of 145 patients were receiving oral iron at randomization, which was continued through end of study. Eight patients began oral iron during the treatment phase.
Table 2.
Patient demographics and baseline characteristics (safety population)
| Characteristic | Cohort | All Patients, n=145 | |||||
|---|---|---|---|---|---|---|---|
| A, n=24 | B, n=24 | C, n=24 | D, n=24 | E, n=24 | F, n=25 | ||
| Age, yr, mean (min, max) | 65.9 (44, 83) | 64.3 (32, 79) | 59.2 (25, 78) | 69.8 (48, 81) | 61.8 (32, 78) | 65.2 (41, 77) | 64.4 (25, 83) |
| Men, n (%) | 5 (20.8) | 7 (29.2) | 8 (33.3) | 13 (54.2) | 8 (33.3) | 12 (48.0) | 53 (36.6) |
| Weight, kg, mean (min, max) | 89.9 (53, 139) | 84.5 (55, 122) | 89.5 (48, 138) | 85.1 (48, 125) | 88.3 (56, 140) | 84.7 (48, 122) | 87.0 (48, 140) |
| Race, n (%) | |||||||
| White | 17 (70.8) | 14 (58.3) | 14 (58.3) | 17 (70.8) | 14 (58.3) | 17 (68.0) | 93 (64.1) |
| Black | 7 (29.2) | 7 (29.2) | 9 (37.5) | 5 (20.8) | 7 (29.2) | 7 (28.0) | 42 (29.0) |
| Other | 0 | 3 (12.5) | 1 (4.2) | 2 (8.3) | 3 (12.5) | 1 (4.0) | 10 (6.9) |
| Prevalence, n (%) | |||||||
| Diabetes | 16 (66.7) | 17 (70.8) | 18 (75.0) | 15 (62.5) | 13 (54.2) | 12 (48.0) | 91 (62.8) |
| Hypertension | 10 (41.7) | 13 (54.2) | 14 (58.3) | 11 (45.8) | 15 (62.5) | 16 (64.0) | 79 (54.5) |
| Hb, g/dl, mean (min, max) | 9.6 (7.4,10.6) | 9.7 (8.1, 10.5) | 9.8 (8.8, 10.6) | 9.7 (7.0, 10.6) | 9.9 (8.6, 10.7) | 9.7 (8.6, 10.5) | 9.7 (7.0, 10.7) |
| Ferritin, ng/ml, mean (min, max) | 322 (49, 1711) | 282 (42, 744) | 261 (33, 877) | 283 (38, 1289) | 207 (32, 803) | 306 (37, 689) | 277 (32, 1711) |
| >100, n (%) | 23 (95.8) | 19 (79.2) | 18 (75.0) | 17 (70.8) | 15 (62.5) | 20 (80.0) | 112 (77.2) |
| ≤100, n (%) | 1 (4.2) | 5 (20.8) | 6 (25.0) | 7 (29.2) | 9 (37.5) | 5 (20.0) | 33 (22.8) |
| TSAT, %, mean (min, max) | 24.5 (9, 48) | 19.9 (6, 41) | 21.0 (12, 37) | 24.4 (9, 41) | 20.9 (12, 36) | 21.5 (11, 39) | 22.0 (6, 48) |
| >20, n (%) | 18 (75.0) | 12 (50.0) | 12 (50.0) | 17 (70.8) | 12 (50.0) | 15 (60.0) | 86 (59.3) |
| ≤20, n (%) | 6 (25.0) | 12 (50.0) | 12 (50.0) | 7 (29.2) | 12 (50.0) | 10 (40.0) | 59 (40.7) |
| Iron replete,a n (%) | 18 (75.0) | 11 (45.8) | 11 (45.8) | 14 (58.3) | 9 (37.5) | 13 (52.0) | 76 (52.4) |
min, max, minimum, maximum; Hb, hemoglobin; TSAT, transferrin saturation.
Iron replete is defined as TSAT>20% and ferritin >100 ng/ml.
Efficacy
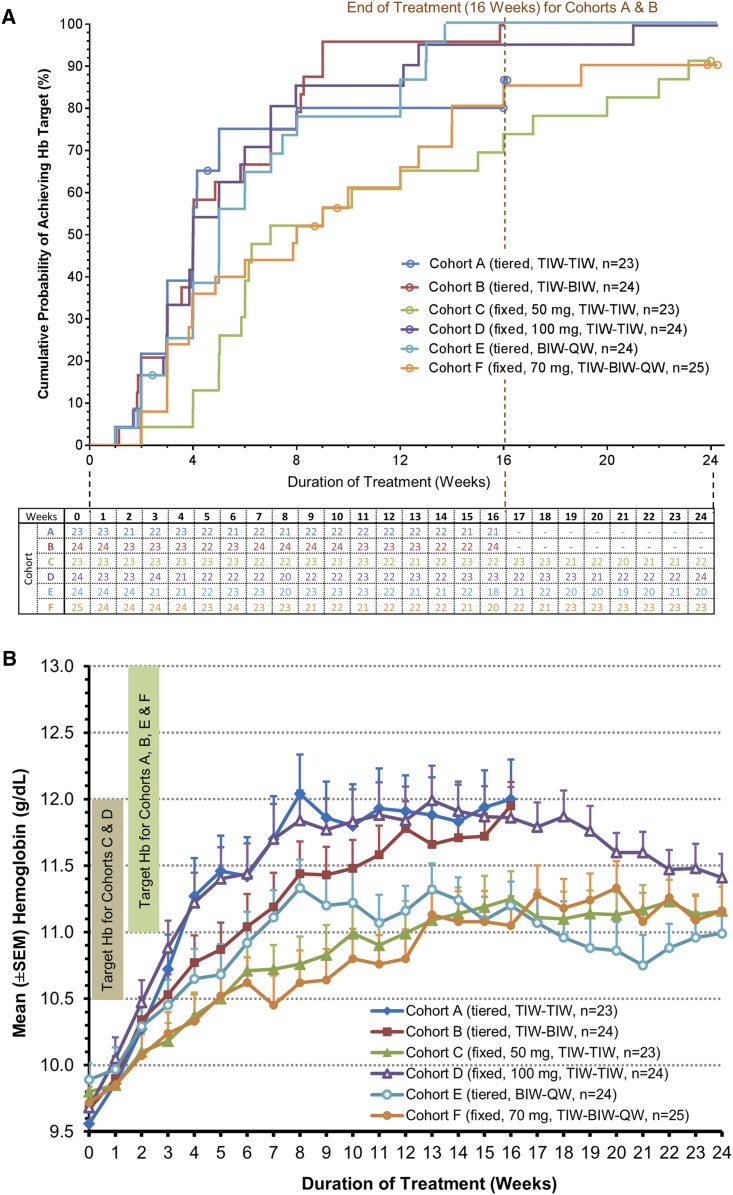
Overall cumulative Hb response rate (efficacy-evaluable patients across all cohorts; n=143) was 91.6% (Figure 2A, Table 3). Median times to Hb response were shorter in the cohorts with higher weekly starting dose than those with lower weekly starting dose: 28 days in cohorts A, B, and D versus 35 days in cohort E, 49 days in cohort C, and 56 days in cohort F.
Figure 2.
Roxadustat effectively corrects and maintains Hb in NDD-CKD patients. (A) Time to hemoglobin response in the efficacy-evaluable population by dosing cohort. Patients who terminated treatment before the full treatment period (112 days for cohorts A and B and 168 days for cohorts C–F) and did not achieve hemoglobin response during their time on treatment were censored. Each circle in the plot represents one censored patient at the time when the patient was censored. Therefore, although cohorts B, D, and E reached 100% response in this analysis, only in cohort B did 100% of the evaluable patients meet the primary end point. The table below the figure denotes the number of efficacy-evaluable patients with valid hemoglobin data on the first day after each study week by cohort. (B) Hemoglobin over time in the efficacy-evaluable population by cohort (last observation carried forward). BIW, twice weekly; Hb, hemoglobin; QW, once weekly; TIW, thrice weekly.
Table 3.
Proportion of patients experiencing a hemoglobin response at end of treatment and mean hemoglobin increase after 4 weeks of treatment by cohort
| Cohort | Responders Overall (EOT), n (%) | Mean (±SD) Hb Changes Overall, g/dl | Mean (±SD) Hb Changes by Category of Body Weight, g/dl | ||
|---|---|---|---|---|---|
| Lowest Tertile, ≤75.4 kg | Middle Tertile, 75.5–96.2 kg | Highest Tertile, ≥96.3 kg | |||
| A (n=23) | 19 (82.6)a | +1.71 (±0.21) | +1.80 (±0.98) | +1.72 (±1.10) | +1.65 (±1.10) |
| B (n=24) | 24 (100)a | +1.09 (±0.20) | +0.92 (±0.72) | +2.07 (±0.46) | +0.72 (±1.10) |
| C (n=23) | 21 (91.3)b | +0.57 (±0.21) | +0.90 (±0.45) | +0.40 (±0.62) | +0.60 (±0.80) |
| D (n=24) | 23 (95.8)b | +1.53 (±0.20) | +2.49 (±0.84) | +1.25 (±0.76) | +0.78 (±0.39) |
| E (n=24) | 23 (95.8)b | +0.77 (±0.20) | +0.61 (±0.88) | +0.95 (±0.83) | +0.77 (±0.75) |
| F (n=25) | 21 (84.0)b | +0.61 (±0.20) | +1.13 (±0.92) | +0.17 (±0.62) | +0.35 (±0.53) |
Hb, hemoglobin; EOT, end of treatment.
EOT 16 weeks.
EOT 24 weeks.
Correction Phase.
In the first 4 weeks of dosing, mean Hb increases in individual cohorts ranged from 0.6 to 1.7 g/dl. Higher starting dose cohorts A, B, and D achieved larger initial 4-week mean Hb increases of 1.7, 1.1, and 1.5 g/dl, respectively, than lower starting dose cohorts C, E, and F, with Hb increases of 0.57, 0.77, and 0.61 g/dl, respectively (Figure 2B).
In the first 4 weeks of therapy, initial mean Hb increases varied inversely with tertile of body weight in the fixed starting dose cohorts (cohorts C, D, and F) (Table 3), an effect absent in the three tiered weight dose cohorts.
Maintenance of Target Hb.
Among the cohorts achieving prompt Hb correction (cohorts A, B, and D), mean Hb levels were maintained in a vast majority of patients at end of treatment.
The two TIW tiered weight dose cohorts achieved initial Hb correction in 83% and 100% of patients, with durability of response (two consecutive Hb values between 10.5 and 13.0 g/dl over the final 8 weeks of treatment) in 70% and 96% of patients. Furthermore, 84% and 96% of initial responders were able to maintain Hb, despite 20%–30% dose reductions in mean weekly maintenance dose that included BIW frequency conversion in cohort B (from TIW). In the highest nonweight–based dose cohort receiving 100 mg TIW and the tiered weight 70–150 mg BIW cohort, 96% of patients achieved initial Hb correction, with durability of response in 92% and 75% of patients, respectively. Additionally, 96% and 78% of initial responders in the two highest nonweight–based dose cohorts (70 and 100 mg, respectively) were able to maintain Hb, despite 22%–25% reductions in mean weekly maintenance dose, including conversion from BIW to QW dosing.
Five patients (3.4%) received RBC transfusion. One patient each had gastrointestinal bleed, hemorrhage during scheduled surgery, and a low Hb after study treatment. Two patients underwent transfusion between days 9 and 15 before HIF-PHI treatment could meaningfully affect Hb.
Hb correction was independent of baseline C–reactive protein (CRP). Overall, mean maximum change in Hb (ΔHbmax) during the first 12 weeks of treatment was 1.76±1.14 g/dl. After 12 weeks of treatment, mean ΔHbmax values were similar between patients with baseline CRP ≤5 ng/ml (+1.82±1.24 g/dl; n=90) and those with baseline CRP >5 ng/ml (+1.52±1.14 g/dl; n=47; P=0.14).
Hb response rate and ΔHb were independent of baseline iron repletion status. Among iron-replete patients (baseline ferritin >100 ng/ml and TSAT>20%), mean ΔHb at the end of treatment was 1.56 (±1.03) g/dl, and 90.5% achieved Hb response (n=74). Among those not iron replete at baseline, mean ΔHb at the end of treatment was similar at 1.27 (±1.14) g/dl (P=0.17), and 92.8% achieved Hb response (n=69). No significant effect of oral iron administration on Hb levels was observed over the first 16 weeks of therapy.
Exploratory Measures
Baseline hepcidin correlated with baseline CRP (r=0.35; P<0.001). After 16 weeks of treatment, mean (±SD) hepcidin decreased by 27.7 (±107.2) ng/ml (16.9%) from baseline (P=0.004), whereas ferritin decreased by 85.9 (±112.6) ng/ml (30.9%; P<0.001) (Supplemental Table 4). Total iron-binding capacity increased by 40.4 (±41.0) μg/dl (15.3%; P<0.001). Although TSAT and ferritin declined during initial weeks of treatment, they were stable thereafter. The subgroup of patients with the highest baseline TSAT and ferritin experienced greater declines in these parameters in cohorts receiving TIW dosing (Supplemental Figure 1). Despite these changes in TSAT and ferritin, mean reticulocyte hemoglobin content (CHr) and mean corpuscular volume (MCV) remained constant or modestly increased during the study, despite active erythropoiesis. The above changes reverted toward or exceeded baseline values after therapy ended (Supplemental Table 4).
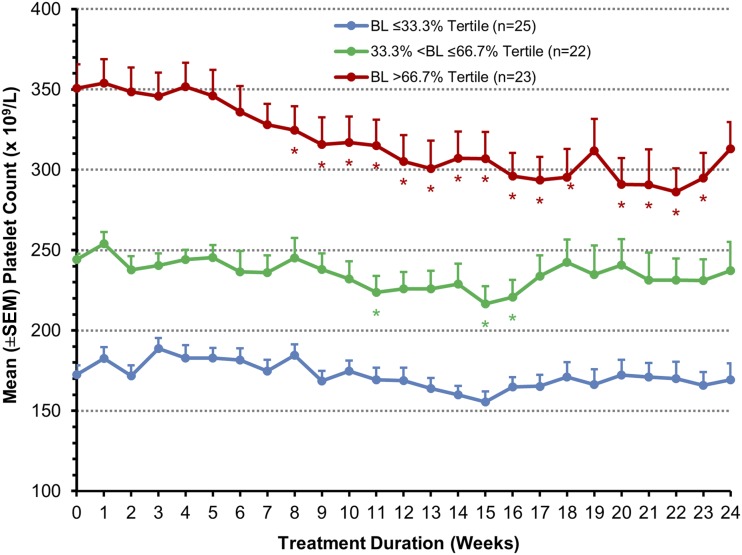
Mean overall platelet counts did not change significantly during treatment. In the high responder cohorts A, B, and D, the tertile of patients with the highest baseline platelet counts experienced a significant mean reduction in platelet count of 14% from baseline over the course of treatment (P<0.05) at all but two time points after treatment week 8. The platelet counts in the middle and lower tertiles did not change during treatment (Figure 3).
Figure 3.
Platelet levels over time among patients in cohorts A, C, and D stratified on baseline platelet level (efficacy-evaluable population with last observation carried forward). BL, baseline. *P value <0.05 (ANOVA model comparing change from baseline with zero using the pooled variance from all groups).
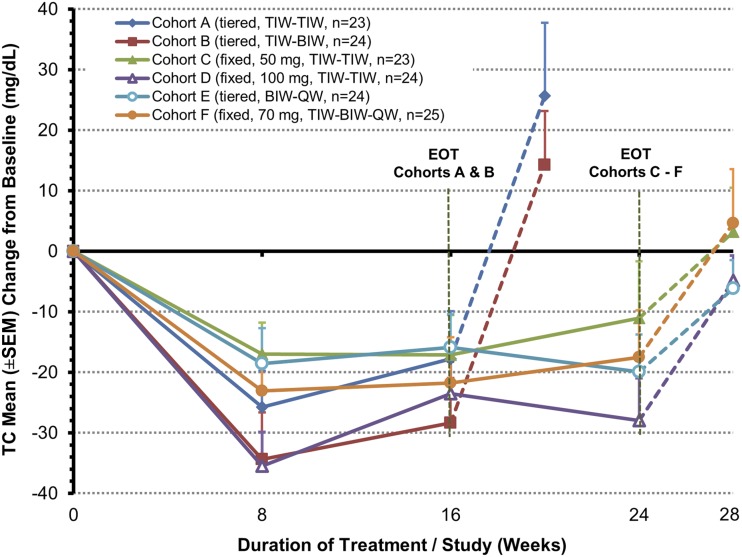
Mean (±SD) baseline total cholesterol of 171 (±45) mg/dl (n=143 efficacy-evaluable patients) was reduced by 26 (±30) mg/dl overall and 17–36 mg/dl (P<0.001 to P=0.02) in all cohorts after 8 weeks of treatment (Figure 4). These reductions persisted: 21 (±32) mg/dl (P<0.001) after 16 weeks and 19 (±37) mg/dl (P<0.001) after 24 weeks (cohorts C–F only); thereafter, total cholesterol levels returned toward baseline. Reduction in mean total cholesterol was greater for patients with higher (≥200 mg/dl) versus lower (<200 mg/dl) baseline total cholesterol levels at 8 weeks (39.6 versus 13.6 mg/dl, respectively) and 24 weeks (39.6 versus 13.6 mg/dl, respectively). Subgroup analysis showed similar reductions in LDL cholesterol and increases in HDL-to-LDL ratio, and magnitudes of changes in total and LDL cholesterol were independent of patient use of statins (Supplemental Figure 2).
Figure 4.
Change from baseline in total cholesterol over time (efficacy-evaluable population with last observation carried forward). Note that the vertical dashed lines show post-treatment levels. BIW, twice weekly; EOT, end of treatment; QW, once weekly; TC, total cholesterol; TIW, thrice weekly.
Safety
Treatment-emergent AEs were reported by 116 (80%) of 145 patients enrolled (Table 4). Fifty-seven serious adverse events (SAEs) were reported in 35 (24.1%) patients (Supplemental Table 5). Six patients were discontinued from treatment because of an AE or an SAE. Four of these were fatal: pulmonary embolism, cardiopulmonary arrest (2), and an unwitnessed death (Supplemental Table 3). The two nonfatal AEs leading to treatment discontinuation were worsening congestive heart failure with peripheral edema in one patient and insomnia in another patient. Twelve serious cardiovascular events were reported in 11 patients, two of which were reported after completion of roxadustat treatment, and the other nine serious cardiovascular events were reported before achieving Hb response. Five SAEs resulted in deaths, all in patients with a history of significant cardiovascular disease (Supplemental Table 6): four during treatment (described above) and one (cerebral infarction and pulmonary edema) during follow-up. None of the deaths were attributed to study medication, and none of the SAEs was considered related to study drug.
Table 4.
Most frequent treatment emergent adverse events (reported by >5% of patients) and serious adverse events (reported by >2% of patients)
| System Organ Class and Adverse Event/Serious Adverse Event | n (%) |
|---|---|
| Most frequent treatment emergent adverse events (reported by >5% of patients) | |
| Gastrointestinal disorders | |
| Nausea | 14 (9.7) |
| Diarrhea | 12 (8.3) |
| Constipation | 9 (6.2) |
| Vomiting | 8 (5.5) |
| General disorders and administrative site conditions | |
| Peripheral edema | 18 (12.4) |
| Infections and infestations | |
| Urinary tract infection | 14 (9.7) |
| Nasopharyngitis | 13 (9.0) |
| Sinusitis | 8 (5.5) |
| Nervous system disorders | |
| Dizziness | 9 (6.2) |
| Headache | 8 (5.5) |
| Vascular disorders | |
| Hypertension | 11 (7.6) |
| Most frequent serious adverse eventsa (reported by >2% of patients) | |
| Cardiac disorders | |
| Cardiac failure, congestive | 5 (3.4) |
| Gastrointestinal disorders | |
| Pancreatitis | 3 (2.1) |
| Infections and infestations | |
| Cellulitis | 3 (2.1) |
| Metabolism and nutrition disorders | |
| Hyponatremia | 3 (2.1) |
| Renal and urinary disordersb | |
| Renal failure, acute | 4 (2.8) |
| Renal failure, chronic | 3 (2.1) |
Regarding serious cardiovascular events, all patients had been in the study for <10 weeks, and none had achieved target hemoglobin of 11 g/dl before the onset of the serious adverse event. During their treatment period, patients had hemoglobin <10 g/dl for 18% of the exposure time. Six of nine (67%) of serious cardiovascular events occurred when hemoglobin <10 g/dl. The remainder of the serious cardiovascular events were experienced while patient hemoglobin was 10–11 g/dl. No serious cardiovascular events were reported when hemoglobin was ≥11 g/dl during the study, although this hemoglobin range accounted for 52% of total patient exposure.
Renal serious adverse events and dialysis initiation. Of four patients who reported acute renal serious adverse event, three fully recovered from the events, and one progressed to dialysis. The three patients reporting chronic renal serious adverse event initiated dialysis. Clinical scenarios consistent with volume depletion were judged to be a major contributing factor in a majority of the renal serious adverse events. Another patient initiated hemodialysis without reporting a serious adverse event. Of the five patients who initiated dialysis during the study, two had stage 5 CKD with eGFR<15 ml/min per 1.73 m2 at baseline, and the other three patients had borderline stage 4/5 CKD with baseline eGFR values of 15 or 16 ml/min per 1.73 m2.
During treatment, 14 patients (9.7%) had at least one alanine aminotransferase (ALT) value between one and two times the upper limit of normal (ULN), and four patients (2.8%) had ALT greater than three times the ULN. During the follow-up period, three patients had at least one ALT greater than the ULN, with a similar pattern for aspartate aminotransferase. No patient had total bilirubin greater than the ULN at baseline or during follow-up, but four (2.8%) patients had total bilirubin up to two times the ULN during treatment. Liver function abnormalities were transient only, and no patient met Hy's Law.
Discussion
Roxadustat, an orally bioavailable HIF-PHI, is being developed to treat CKD-associated anemia. In this phase 2b study, various roxadustat dose regimens were evaluated for 16 and 24 weeks in patients with NDD-CKD. Both fixed and weight-based dosing corrected Hb levels, providing a range of Hb increases and median correction times to be considered for phase 3 testing. These data show the flexibility in dosing frequency (TIW, BIW, and QW) during maintenance therapy.
The package label for conventional ESA therapy requires iron repletion and often, iv iron supplementation (23). In contrast, roxadustat corrected anemia and maintained Hb without iv iron, despite baseline iron depletion in almost one half of the patients. Although TSAT and ferritin decreased during the initial Hb response, these parameters subsequently stabilized, permitting the continued maintenance of Hb levels at TSAT and ferritin thresholds lower than those required during ESA treatment. The ability to maintain this correction for longer periods of time without iv iron will be tested in phase 3 studies. Furthermore, CHr levels were maintained, despite robust erythropoiesis, indicating absence of functional iron deficiency during roxadustat treatment. Iron used in erythropoiesis in these patients could have come from mobilization of internal stores as well as from oral iron supplementation, but the latter was given to only 37% of patients. Hb response in patients who were not iron replete and not on oral iron at baseline was as good as in those who were iron replete and on oral iron.
Hepcidin levels decreased with roxadustat treatment. This negative regulator of iron absorption and mobilization is elevated with inflammation and sequesters iron impeding erythropoiesis. Hypoxia-inducible factor (HIF) acts as an iron sensor (24); HIF stabilization is associated with hepcidin suppression, increased intestinal iron absorption (25), and increases in iron transport enzymes. In HIF-2α knockout mice, intestinal HIF-2α induces iron absorption genes and increases serum iron necessary for effective erythropoiesis (26). Hepcidin likely mediates part of the mechanism by which inflammation results in hyporesponsiveness to ESAs (27,28). CRP, an inflammatory marker, is elevated in approximately 25% of patients with CKD (29). In contrast to ESAs, where lower Hb responses are reported in patients with higher CRP who were inflamed (30), responses to roxadustat were independent of the degree of baseline inflammation reflected by CRP levels. Thus, hepcidin reduction by roxadustat potentially enables coordinated erythropoiesis, regardless of inflammation or exogenous iron supplementation.
Treatment with roxadustat had different effects on platelet levels and MCV of RBCs than that described with ESAs. ESA treatment can increase platelet count and decrease MCV (31,32), both possibly related to functional iron deficiency. In contrast, treatment with roxadustat was associated with stable platelet counts and even decreasing platelet counts in those in the highest baseline tertile. We hypothesize that patients with the most elevated baseline platelet counts had some degree of reactive thrombocytosis because of iron deficiency (functional or otherwise) ameliorated by roxadustat through improved iron transport and metabolism, producing a decline in platelets. Evidence for this improved iron metabolic state during roxadustat treatment is maintenance of CHr levels and MCV, despite robust erythropoiesis (a state incompatible with functional iron deficiency), perhaps fostered by higher total iron-binding capacity (31% increase) and thus, improved transferrin transport of iron from tissue stores or oral absorption of dietary iron. Although these effects may reflect better iron delivery/utilization, the precise mechanism, the extent to which they minimize iron deficiency–mediated reactive thrombocytosis, and the risk for thromboembolism require additional exploration.
Mean total and LDL–associated cholesterol levels fell with roxadustat treatment. Cholesterol reduction occurs during high-altitude exposure (33). The potential cholesterol-lowering effect of roxadustat may be mediated, in part, by the effects of HIF on degradation of the rate-limiting enzyme, 3-hydroxy-3-methylglutaryl-CoA reductase (34). Although a reduction in total and LDL cholesterol may be a benefit, because dyslipidemia and hypertension are risk factors for cardiovascular disease in patients with CKD, the potential for this benefit requires assessment in larger future trials.
Roxadustat was well tolerated with no drug-related SAEs reported in this study. All serious cardiovascular events reported during the treatment period occurred before achieving anemia correction to Hb≥11.0 g/dl; Hb≥11.0 g/dl was maintained during a major portion of the roxadustat treatment period in this study. In contrast to the hypertensive risks of ESAs, no SAEs of hypertension were reported in this study. The reported rate of hypertensive AEs (7.6% of treated patients) was below what has been reported for similar patient populations treated with ESAs (16%–32%) (35,36) and comparable with placebo rates seen in other similar studies in this patient population (37). A recent CKD patient cohort study estimated 7.7 deaths and 8.0 progression to dialysis initiation events per 100 patient-years for those with stage 4 CKD and 41.4 deaths and 9.4 progression to dialysis initiation events per 100 patient-years for those with stage 5 CKD (38). With 145 such patients enrolled and followed for approximately one half of a year, the occurrence of five deaths and the initiation of dialysis in seven patients were not unexpected. These data need to be interpreted in the setting of the open label study design, in which no comparator group was included, and factor in the patients’ background comorbidities and risk factors.
This study shows that roxadustat can potentially correct anemia, regardless of inflammation or initial iron repletion status, while reducing hepcidin and cholesterol levels in patients with NDD-CKD. Roxadustat has a potentially acceptable safety profile. We did not find ESA–associated side effects, and the hepcidin- and cholesterol-lowering activity of roxadustat has not been reported with ESAs. Risks from iv iron supplementation may be obviated. Taken together, these results informed the design of phase 3 studies investigating roxadustat as a novel oral therapeutic and a potentially safer and more accessible alternative to parenteral ESAs in the treatment of anemia secondary to CKD.
Disclosures
A.B., T.L., R.L., B.K.R., S.H., L.A.S., P.Y., and T.B.N. are employees of FibroGen, Inc. and hold stock and/or stock options in FibroGen, Inc. At the time that the study was performed, R.P. was affiliated with St. John Hospital and Medical Center and Wayne State University School of Medicine. He currently is an employee and holds stock in DaVita Healthcare Partners. Also, at the time that the study was performed, A.B. was affiliated with the Henry Ford Hospital and Wayne State University School of Medicine.
Acknowledgments
The data reported here have been disclosed in part in abstract form (Besarab et al., J Am Soc Nephrol 22: 196A, 2011).
FibroGen, Inc. was the study sponsor that designed the study in consultation with the principal investigators (R.P. and A.B.). All authors except those employed by the sponsor but including A.B. contributed patients to the study. FibroGen, Inc. was responsible for data collection and analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06890615/-/DCSupplemental.
References
- 1.Collins AJ, Ma JZ, Xia A, Ebben J: Trends in anemia treatment with erythropoietin usage and patient outcomes. Am J Kidney Dis 32[Suppl 4]: S133–S141, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW: Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Muirhead N: A rationale for an individualized haemoglobin target. Nephrol Dial Transplant 17[Suppl 6]: 2–7, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA, Brown RE, Feeny DH, Henry D, Teehan BP, Rudnick MR, Benz RL: Health-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. Am J Kidney Dis 25: 548–554, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Collins AJ: Anaemia management prior to dialysis: Cardiovascular and cost-benefit observations. Nephrol Dial Transplant 18[Suppl 2]: ii2–ii6, 2003 [PubMed] [Google Scholar]
- 8.Benz R, Schmidt R, Kelly K, Wolfson M: Epoetin alfa once every 2 weeks is effective for initiation of treatment of anemia of chronic kidney disease. Clin J Am Soc Nephrol 2: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System: 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. Available at: http://www.usrds.org/atlas.htm. Accessed October 1, 2014
- 10.Yan G, Cheung AK, Ma JZ, Yu AJ, Greene T, Oliver MN, Yu W, Norris KC: The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol 8: 610–618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Amgen, Inc.: Epogen Package Insert. Available at: http://pi.amgen.com/united_states/epogen/epogen_pi_hcp_english.pdf. Accessed April 1, 2015
- 15.Seliger S, Fox KM, Gandra SR, Bradbury B, Hsu VD, Walker L, Chiou CF, Fink JC: Timing of erythropoiesis-stimulating agent initiation and adverse outcomes in nondialysis CKD: A propensity-matched observational study. Clin J Am Soc Nephrol 5: 882–888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin A: The treatment of anemia in chronic kidney disease: Understandings in 2006. Curr Opin Nephrol Hypertens 16: 267–271, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD: Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int 66: 1131–1138, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Karpinski M, Pochinco D, Dembinski I, Laidlaw W, Zacharias J, Nickerson P: Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol 15: 818–824, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Amgen, Inc.: ESAs in the Treatment of Anemia in Chronic Kidney Disease Patients, 2010. Available at: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads//0716ofmancomment.pdf. Accessed May 13, 2015
- 20.Obrador GT, Macdougall IC: Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol 8: 852–860, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Macdougall IC: Recent advances in erythropoietic agents in renal anemia. Semin Nephrol 26: 313–318, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KHP, Neff TB: Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30: 1665–1673, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE: A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 50: 1694–1699, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS: Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 117: 1926–1932, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ: Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 9: 152–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson ER, Xue X, Shah YM: Intestinal hypoxia-inducible factor-2α (HIF-2α) is critical for efficient erythropoiesis. J Biol Chem 286: 19533–19540, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdougall IC, Cooper AC: Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. Eur J Clin Invest 35[Suppl 3]: 32–35, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Adamson JW: Hyporesponsiveness to erythropoiesis stimulating agents in chronic kidney disease: The many faces of inflammation. Adv Chronic Kidney Dis 16: 76–82, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Marcovina SM, Levey AS, Sarnak MJ: Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis 42: 44–52, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Inrig JK, Bryskin SK, Patel UD, Arcasoy M, Szczech LA: Association between high-dose erythropoiesis-stimulating agents, inflammatory biomarkers, and soluble erythropoietin receptors. BMC Nephrol 12: 67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, Korbet S, Krantz SB, Lundin AB, Nissenson AR, Ogden DA, Paganini EP, Rader B, Rutsky EA, Stivelman J, Stone WJ, Teschan P, Van Stone JC, Van Wyck DB, Zuckerman K, Adamson JW: Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz AB, Orquiza CS: The effects of recombinant human erythropoietin on mean corpuscular volume in patients with the anemia of chronic renal failure. Clin Nephrol 43: 256–259, 1995 [PubMed] [Google Scholar]
- 33.Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA: Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 282: 27436–27446, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Férézou J, Richalet JP, Coste T, Rathat C: Changes in plasma lipids and lipoprotein cholesterol during a high altitude mountaineering expedition (4800 m). Eur J Appl Physiol Occup Physiol 57: 740–745, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Roger SD, Locatelli F, Woitas RP, Laville M, Tobe SW, Provenzano R, Golper TA, Ruangkanchanasetr P, Lee HY, Wu KD, Nowicki M, Ladanyi A, Martínez-Castelao A, Beyer U, Dougherty FC: C.E.R.A. once every 4 weeks corrects anaemia and maintains haemoglobin in patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant 26: 3980–3986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locatelli F, Olivares J, Walker R, Wilkie M, Jenkins B, Dewey C, Gray SJ European/Australian NESP 980202 Study Group : Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int 60: 741–747, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Krapf R, Hulter HN: Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol 4: 470–480, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Sud M, Tangri N, Levin A, Pintilie M, Levey AS, Naimark DM: CKD stage at nephrology referral and factors influencing the risks of ESRD and death. Am J Kidney Dis 63: 928–936, 2014 [DOI] [PubMed] [Google Scholar]