Abstract
Background and objectives
Unintended injuries or complications in hospitalized patients are common, potentially preventable, and associated with adverse consequences, including greater mortality and health care costs. Patients with CKD may be at higher risk of hospital-acquired complications (HACs).
Design, setting, participants, & measurements
Adults from a population-based cohort (Alberta Kidney Disease Network) who were hospitalized from April 1, 2003, to March 31, 2008, made up the study cohort. Kidney function was defined using outpatient eGFR and proteinuria (protein-to-creatinine ratio or dipstick) in the year before index hospitalization. Comorbid conditions were identified using validated algorithms applied to administrative data. A specific diagnostic indicator was used to identify HACs. Complications were classified into clinically homogeneous groups and subclassified as potentially preventable (p-HACs) or always preventable (a-HACs). Multivariable logistic regressions models were used to examine the association of CKD with HACs, accounting for confounders.
Results
Of 536,549 patients, 8.5% had CKD; those with CKD were older and more likely to be admitted for circulatory system diseases than those without CKD. In fully adjusted models, the odds ratio (OR) of any hospital complication in patients with CKD (reference: no CKD) was 1.19 (95% confidence interval [95% CI], 1.18 to 1.26); there was a graded relation between the risk of HACs and CKD severity, with an OR of 1.81 (95% CI, 1.51 to 2.17) in those with the most severe CKD (eGFR, 15–29 ml/min per 1.73 m2 and proteinuria, >30 mg/mmol). Findings were similar for p-HACs (OR, 1.20 [95% CI, 1.16 to 1.24] and 1.78 [95% CI, 1.43 to 2.11], respectively). The a-HACs had similar point estimates.
Conclusions
The presence of CKD and its severity are associated with a higher risk of HACs, including those considered preventable. Targeted strategies to reduce complications in patients with CKD admitted to the hospital should be considered.
Keywords: chronic kidney disease; hospitalization; hospital acquired complication; preventable hospital complications; renal insufficiency, chronic; patient safety; cohort studies; medical errors; health care costs; humans
Introduction
Hospital-acquired complications (HACs) are undesirable and unintended clinical conditions, distinct from the admitting diagnosis that may occur during a hospitalization episode. Specific diagnostic indicators in administrative hospital data by definition refer to new diagnoses or events that occur during hospitalization (“diagnosis type 2” in Canada, “not present on admission”) in the United States, and “condition-onset flag” in Australia). HACs are common and associated with adverse consequences including prolonged hospital stay, increased disability at discharge, and higher risk of death (1–4). Studies in the United Kingdom, New Zealand, and the United States report that HACs occur in 2.9%–11.7% of hospitalizations (5). In Canada, the proportion of hospital episodes with at least one reported HAC has been estimated to be between 7.5% (1) and 23.9% (6), and these episodes prolong length of stay by 4.7 days (1,6).
CKD is common and is associated with high risk of hospitalization and higher risk of complications, including bleeding, drug toxicity, drug dosing issues, and susceptibility to infection (7,8). To date, limited data are available on HACs in patients with CKD. An analysis of the Department of Veterans Affairs data for 2004–2005 showed that patients with CKD had a higher risk for several HACs than patients with normal kidney function (adjusted incidence rate ratio, 1.19; 95% confidence interval [95% CI], 1.13 to 1.25) (9). However, nonveteran patient populations with CKD have not been examined.
A significant proportion of HACs are deemed to be potentially preventable. The percentage of hospitalizations episodes with preventable hospital complications range from 2.8% (1) to 6% (10). Evidence suggests that these complications can be reduced. Two hospitals with relatively high potentially preventable HAC (p-HAC) rates (48.73 and 58.17 per 1000 discharges) reduced this rate to 32.36 and 48.15, respectively, by implementing various strategies by administrative and clinical staff (11). In a “pay-for-quality” initiative in 2005, Medicare decreased payments when a diagnosis-related group included particular complications that could reasonably have been prevented through the application of evidence-based guidelines; “no-payment” initiatives have been associated with reductions in the rate of two always preventable HACs (a-HACs): central line–associated blood stream infections and catheter-associated urinary tract infections (12).
The risk of HACs, including those that are potentially preventable, has not been determined in patients with CKD in a population-based cohort. Given the high hospitalization rate in patients with CKD, the potential for HACs in this high-risk group, and the potential to prevent some of these complications, we sought to determine the association of the presence of CKD and its severity with HACs (including preventable types of HACs) in a large population-based cohort of adults.
Materials and Methods
Study Population
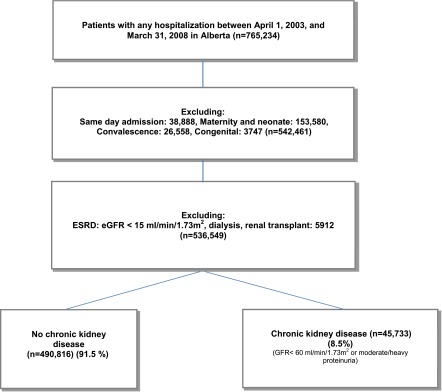
The health research ethics board of the University of Alberta and University of Calgary approved the study. The study cohort comprised all adults (age≥18 years) in Alberta hospitalized from April 1, 2003, to March 31, 2008 (Figure 1), from the population-based Alberta Kidney Disease Network (7). The first hospitalization was considered for each individual. Medical and surgical admissions with the exception of maternity/neonatal, congenital malformation, convalescence, and same-day admission were included. Patients with kidney failure (dialysis, renal transplant, eGFR<15 ml/min per 1.73 m2) were excluded. We used known designation case-mix groups to stratify admissions into medical or surgical when possible (because of data limitation, 25% could not be classified). Population attributable risk percentage was used to determine the proportion of hospitalization with at least one preventable HAC in the population (CKD and non-CKD) that may be attributable to CKD; Poisson regression was used to determine the adjusted risk ratio needed for this calculation.
Figure 1.
Study flowchart to construct cohort with CKD.
Assessment of Patients’ Characteristics
Kidney function was determined from outpatient serum creatinine measurement and urine studies. Average eGFR was estimated using the Modification of Diet in Renal Disease formula. The primary exposure variable of CKD was defined by eGFR<60 ml/min per 1.73 m2 and/or moderate to high proteinuria, defined as an albumin-to-creatinine ratio >3–30 mg/mmol, protein-to-creatinine ratio >15–50 mg/mmol, or >2+ protein dipstick in the year before index hospitalization. All outpatient eGFR measurements in the time frame from 365 days to 90 days before admission were considered; we excluded eGFR measurement within 3 months of admission to ensure that AKI did not affect CKD determination. CKD was further categorized using the Kidney Disease Improving Global Outcomes 2012 clinical practice guidelines.
We assumed that patients without any serum creatinine and proteinuria data had normal kidney function. Fifteen percent of patients had no laboratory data to present kidney function in the year before hospitalization. By excluding those patients, sensitivity analyses was tested and a very close odds ratio (OR) was obtained. Comorbid conditions, including cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes with complications, diabetes with no complications, HIV/AIDS, metastatic solid tumor, myocardial infarction, mild liver disease, moderate to severe liver disease, paraplegia or hemiplegia, peptic ulcer disease, peripheral vascular diseases, renal disease, and rheumatologic disease, were identified using validated algorithms applied to hospitalization discharge abstracts and physician claims data (13). The reason for hospitalizations was categorized into 16 homogeneous groups using the International Classification of Diseases (ICD), 10th revision, Canada (ICD-10-CA).
Hospital administrative data include “diagnosis type 2,” which indicates HACs. Using the ICD-10-CA, hospital coders record all clinical conditions and signs not present at hospital admission while reviewing patients’ charts. These ≥4000 ICD-10-CA diagnostic codes were mapped into ten groups, with 38 subgroups, according to clinical similarity (Supplemental Appendix A). We used published data to identify 63 potentially preventable HACs (10) by manually remapping the ICD, Ninth Revision, diagnostic codes to ICD-10-CA. Briefly, panels of clinicians (two general internists and one pediatrician supplemented by a surgical or obstetric specialist as needed) reviewed each of approximately 14,400 diagnosis values in the ICD, Ninth Revision, Clinical Modification coding scheme and classified 1562 codes as being p-HACs. We defined a-HACs on the basis of Medicare “never-event” diagnoses (14), and we manually remapped the US ICD-10 codes for these conditions to the Canadian version.
Statistical Analyses
Continuous variables were described using means and SDs or medians with 25th and 75th percentiles, as appropriate. The linearity assumption for age was satisfied. Categorical variables were described as proportions of the cohort with or without each condition or characteristic. A multivariable logistic regression analysis was used to determine the independent association of CKD and its severity with risk of developing at least one HAC, after controlling for potential confounders. In the primary analysis, all HACs were used to define the dependent variable; in secondary analyses, we considered p-HACs and a-HACs as the dependent variable. Purposeful-selection model building was used. The fully adjusted models included reason for admission, age, sex, admission type (urgent versus elective admission as defined in hospital administrative data), CKD, length of stay (LOS), and 17 comorbid conditions. We did a sensitivity analysis to assess the association of increasing number of HACs with the dependent variable of LOS. Multivariable regression analyses was used and adjusted to sex, age, admission type (elective versus urgent admission as defined in hospital administrative data), and 17 comorbid conditions. The analysis was done using Stata software, version 13 (Stata Corp., College Station, TX).
Results
Patient Characteristics
Of 765,234 adults hospitalized in Alberta during the study period, 536,549 (70.1%) met inclusion criteria (Figure 1). Mean age of patients with CKD was greater than that of patients without CKD. The median LOS was 4 days (25th–75th percentiles, 2–11 days) for patients with CKD and 3 days (25th–75th percentiles, 2–6 days) for patients without CKD. Cardiovascular diseases made up the largest "most responsible diagnosis" category in patients with CKD, accounting for 20% of admissions. Patients with CKD were also more likely to be admitted for cancer and endocrine disorders as the most responsible diagnoses and less likely to be admitted for respiratory or digestive system conditions compared with those without CKD. In the entire cohort, 6.7% and 2.6% of patients had eGFR<60 ml/min per 1.73 m2 and moderate to heavy proteinuria, respectively; 45,733 patients (8.5% of cohort) had CKD (Table 1).
Table 1.
Patient characteristics
| Characteristic | All Patients | Patients without CKD | Patients with CKD |
|---|---|---|---|
| Demographic | |||
| Patients, n (%) | 536,549 (100) | 490,816 (91.5) | 45,733 (8.5) |
| Mean age ±SD, yr | 52.4±22.8 | 50.6±22.5 | 72.1±15 |
| Men, % | 49.3 | 49.8 | 43.9 |
| Most responsible diagnosis category, % | |||
| Disease of digestive system | 13.9 | 14.2 | 11.1 |
| Injury, poisoning | 13.5 | 14 | 8.1 |
| Disease of circulatory system | 12.7 | 12 | 20.8 |
| Neoplasm | 9.5 | 9.4 | 11.4 |
| Disease of musculoskeletal system | 9.2 | 9.1 | 10.3 |
| Disease of genitourinary system | 9.2 | 9.1 | 9.6 |
| Disease of respiratory system | 8.8 | 8.9 | 7.6 |
| Mental behavioral | 6.9 | 7.2 | 3.1 |
| Symptom, signs of abnormal clinical and laboratory result | 5.8 | 5.8 | 5.8a |
| Endocrine | 3.4 | 3.2 | 5 |
| Disease of nervous system | 2.4 | 2.4 | 2.2 |
| Certain infectious and parasitic disease | 1.5 | 1.6 | 1.6a |
| Disease of skin and subcutaneous tissue | 1.2 | 1.2 | 1.1a |
| Disease of eye | 0.9 | 0.9 | 1a |
| Disease of blood and blood-forming organs | 0.7 | 0.7 | 1.9 |
| Disease of ear | 0.4 | 0.4 | 0.3 |
| Admissions | |||
| Urgent admission, % | 69.7 | 69.6 | 71 |
| Medical, n (%) | 228,450 (42.6) | 208,926 (42.6) | 19,524 (42.7) |
| Surgical, n (%) | 172,636 (32.2) | 156,361 (31.8) | 16.275 (35.6) |
| Other, n (%) | 135,463 (25.2) | 125,529 (25.6) | 9,934 (21.7) |
| Length of stay, db | 7.4 (3 [2–7]) | 7.1 (3 [2–6]) | 10.6 (4[ 2–11]) |
| HACs, n (%) | |||
| Any aHAC | 5419 (1) | 4711 (0.9) | 722 (1.6) |
| Any pHAC | 30851 (5.7) | 263,568 (5.4) | 4490 (9.8) |
| Any HAC | 42,036 (7.8) | 351,65 (7.2) | 5,911 (12.9) |
| Medical admission with any HAC | 16,194 (7.1) | 13,038 (6.7) | 2256 (11.6) |
| Surgical admission with any HAC | 16,540 (9.6) | 14,113 (9) | 2427 (14.9) |
| Other admission with any HAC | 9302 (6.9) | 8014 (6.4) | 1288 (13) |
| eGFR, n (%) | 500,199 (93.2) | 500,199 (93.2) | 0 |
| ≥60 ml/min per 1.73 m2 | |||
| 45–59 ml/min per 1.73 m2 | 22,736 (4.2) | 0 | 22,736 (4.2) |
| 30–44 ml/min per 1.73 m2 | 9402 (1.7) | 0 | 9402 (1.7) |
| 15–29 ml/min per 1.73 m2 | 4212 (0.8) | 0 | 4212 (0.8) |
| Proteinuria | 522,613 (97.4) | 522,613 (97.4) | 0 |
| None (<3 mg/mmol) | |||
| Moderate (3–30 mg/mmol) | 8341 (1.5) | 0 | 8341 (1.5) |
| Heavy (>30 mg/mmol) | 5595 (1) | 0 | 5595 (1) |
In patients with CKD, mean serum creatinine±SD) was 122.95±105 μmol/L and mean eGFR was 49.39±15.54 ml/min per 1.73 m2. HAC, hospital-acquired complication; aHAC, always preventable hospital-acquired complication; pHAC, potentially preventable hospital-acquired complication.
Not statistically significant.
Expressed as mean (median [25th–75th percentiles]).
Risk of HAC
In the entire cohort, 42,036 patients (7.8%) had at least one HAC, and the proportion of hospitalization episodes with complications was approximately two-fold higher in patients with CKD than in those without CKD (13% and 7%, respectively). The proportions of patients with at least one HAC were similar when stratified by medical or surgical admission. The proportion of hospital admissions with any HAC, p-HACs, and a-HACs in each year appeared numerically stable during the study period.
In a fully adjusted analysis, the OR of HACs in patients with CKD (reference: no CKD) was 1.19 (95% CI, 1.18 to 1.26) (Table 2). Every 5-ml/min per 1.73 m2 lower eGFR was associated with a 1% higher risk of HAC (OR, 1.01; 95% CI, 1.01 to 1.01). A graded association with severity of CKD was observed, with the most severe category of CKD associated with an OR of 1.81 (95% CI, 1.51 to 2.17) (Table 3).
Table 2.
Risk of hospital-acquired complications in patients with CKD
| Hospital-Acquired Complications | OR (95% CI) |
|---|---|
| Alla | 1.19 (1.15 to 1.23) |
| Potentially preventableb | 1.20 (1.16 to 1.24) |
| Always preventableb | 1.14 (1.05 to 1.24) |
OR, odds ratio; 95% CI, 95% confidence interval.
Fully adjusted for age, admission type (elective versus urgent), sex, length of stay, and 17 comorbid conditions.
Fully adjusted for age, admission type (elective versus urgent), sex, length of stay, and 17 comorbid conditions, except for reason for admission. Comorbid conditions were cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes with complications, diabetes with no complications, HIV/AIDS, metastatic solid tumor, myocardial infarction, mild liver disease, moderate or severe liver disease, paraplegia or hemiplegia, peptic ulcer disease, peripheral vascular diseases, renal disease, and rheumatologic disease. Normal or
Table 3.
Adjusted odds ratios (95% confidence intervals) of hospital-acquired complications by GFR and albuminuria categories
| GFR Categories | Persistent Albuminuria Categories | ||
|---|---|---|---|
| Normal to Mildly Increased (<30 mg/g or <3 mg/mmol) | Moderately Increased (30–300 mg/g or 3–30 mg/mmol) | Severely Increased (>300 mg/g or >30 mg/mmol) | |
| HACs | |||
| Normal or high ≥90 ml/min per 1.73 m2 | Reference n=490,816 | 1.25 (1.15–1.35) n=6,195 | 1.33 (1.89–1.48) n=3,188 |
| Mildly decreased: 60–89 ml/min per 1.73 m2 | |||
| Mildly to moderately decreased: 45–59 ml/min per 1.73 m2 | 1.13 (1.08–1.18) n=20,735 | 1.14 (0.93–1.35) n=1,155 | 1.38 (1.14–1.6 n=846 |
| Moderately to severely decreased: 30–44 ml/min per 1.73 m2 | 1.23 (1.16–1.32) n=8,021 | 1.38 (1.11–1.70) n=655 | 1.29 (1.05–1.60) n=726 |
| Severely decreased: 15–29 ml/min per 1.73 m2 | 1.43 (1.29–1.58) n=3,041 | 1.29 (0.95–1.74) n=336 | 1.81 (1.51–2.17) n=835 |
| pHACs | |||
| Normal or high ≥90 ml/min per 1.73 m2 | Reference n=490,816 | 1.21 (1.07–1.36) n=6,195 | 1.36 (1.18–1.58) n=3,188 |
| Mildly decreased: 60–89 ml/min per 1.73 m2 | |||
| Mildly to moderately decreased: 45–59 ml/min per 1.73 m2 | 1.15 (1.05–1.25) n=20,735 | 1.05 (0.84–1.30) n=1,155 | 1.33 (1.05–1.69) n=846 |
| Moderately to severely decreased: 30–44 ml/min per 1.73 m2 | 1.21 (1.09–1.35) n=8,021 | 1.28(0.90–1.84) n=655 | 1.15 (0.88–1.49) n=726 |
| Severely decreased: 15–29 ml/min per 1.73 m2 | 1.41 (1.23–1.62) n=3,041 | 1.36 (1.18–1.58) n=336 | 1.78 (1.43–2.11) n=835 |
CKD was defined by GFR and albuminuria categories according to Kidney Diseae Improving Global Outcomes 2012 guidelines. All odds ratios fully adjusted for age, admission type (elective versus urgent), sex, length of stay, and 17 comorbid conditions, except for reason for admission. Comorbid conditions were cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes with complications, diabetes with no complications, HIV/AIDS, metastatic solid tumor, myocardial infarction, mild liver disease, moderate or severe liver disease, paraplegia or hemiplegia, peptic ulcer disease, peripheral vascular diseases, renal disease, and rheumatologic disease. HAC, hospital-acquired complication; p-HAC, potentially preventable hospital-acquired complication.
Risk of Potentially Preventable Complications
At least one p-HAC occurred in 9.8% of patients with CKD compared with 5.4% of those without CKD. Adjusted relative risk of a p-HAC was 1.17 (95% CI, 1.14 to 1.21) in patients with CKD, and the population attributable risk percentage of HAC that may be due to CKD was 1.2% (this value was 8.5% for the proportion of the cohort with CKD). Patients with CKD had a 20% higher risk of developing of p-HACs (OR, 1.20; 95% CI, 1.18 to 1.27) (Table 2). Patients with more severe kidney disease were also at higher risk of p-HACs. In the most severe CKD category, the OR was 1.78 (95% CI, 1.43 to 2.11) (Table 3). Postprocedural complications, including cardiovascular, respiratory, and other complications of surgical and medical care, were the most common p-HACs in patients with CKD. Anemia was the most common complications, acid-base, fluid, electrolyte balance metabolic disorders and infections were the second and third most common p-HACs (Supplemental Appendix B).
Risk of a-HACs
In a fully adjusted logistic regression analysis, patients with CKD were at higher risk of a-HAC (OR, 1.16; 95% CI, 1.07 to 1.26) (Table 2). A similar graded association of more severe CKD and larger OR was observed, although 95% CIs crossed unity. Surgical site infections, falls and trauma, and deep-vein thrombosis were the most common a-HACs in patients with CKD (Table 4).
Table 4.
Most common always preventable hospital-acquired complications in patients with CKD
| Complications | Patients, n (%) |
|---|---|
| Surgical site infection, including post-CABG, bariatric surgery, and orthopedic procedures | 2334 (39.1) |
| Falls and trauma, including fracture, dislocation, intracranial injury, crushing injury, other injuries | 1173 (19.7) |
| Deep-vein thrombosis and pulmonary embolism | 642 (10.8) |
| Postprocedural pneumothorax | 564 (9.5) |
| Others | 1207 (23.8) |
| Total | 5920 (100) |
CABG, coronary artery bypass grafting.
Sensitivity Analyses
Excluding patients with no eGFR measurement did not alter results. The ORs of HAC in medical or surgical patients with CKD were 1.14 (95% CI, 1.08 to 1.19) and 1.24 (95% CI, 1.17 to 1.30), respectively. After adjustment, a graded association of LOS with number of HACs was observed; patients with one HAC and three to five HACs stayed in the hospital 9.38 days (95% CI, 8.73 to 10.02) and 24.09 days (95% CI, 22.43 to 25.75) longer, respectively.
Discussion
In this large population-based cohort of hospitalized patients, we found that risk of HACs (including those considered potentially preventable or always preventable) were more likely in patients with CKD. The risk of these complications increases in a graded fashion with severity of CKD. We found that patients with CKD had a 19% higher risk of HACs and that the excess risk was as much as 81% higher in those with the most severe kidney impairment (eGFR of 15–29 ml/min per 1.73 m2 and proteinuria>30 mg/mmol). Because CKD is readily identifiable using routine laboratory tests that are commonly conducted in hospitalized patients, and because targeted strategies to prevent HACs are effective in some settings, patients with CKD may be an ideal high-risk population for whom to implement evidence-based strategies to reduce HACs. These strategies may subsequently improve patient and health care system outcomes.
Patients with CKD may be uniquely predisposed to complications during hospitalization because of known factors, such as impaired coagulation; altered renal handling of medications requiring drug dosing changes; and predisposition to drug toxicity, susceptibility to infection, and other complications. Underrecognition of CKD may contribute to the high frequency of HACs observed, particularly with milder severity of kidney impairment. CKD may also be a marker for sicker patients, as CKD often occurs in patients who are older and have multiple comorbid conditions; however, we attempted to control for potential confounders.
Our findings are consistent with those of previous studies that have examined hospital complication rates in patients with CKD. In a United States veterans population, the association of CKD with 13 HACs (patient safety indicators) showed a 19% higher risk for patients with CKD, as defined by eGFR alone. A similar linear trend was observed across varying CKD severity (9). Our results are congruent; however, we used both eGFR and proteinuria level and assessed outpatient values before hospitalization to define patients with CKD. Further, we studied a population-based cohort and considered all hospital complications and those deemed to be potentially or always preventable.
A large proportion of HACs are considered to be always or potentially preventable. Payment reform by the US Centers for Medicare & Medicaid Services altered the rate of central line–associated blood stream infections and catheter-associated urinary tract infections after hospitals implemented preventive strategies in response to these payment incentives (12).
We found that 9.8% of patients with CKD had at least one p-HAC compared with 5.3% of those without CKD. Patients with CKD had a 20% higher risk of developing P-HACs (OR, 1.20; 95% CI, 1.18 to 1.27). Postprocedural complications were the most frequent cause of HACs in people with underlying CKD compared with those without. The risk of a-HACs also increased with CKD and its severity; however, the graded association was not significant in some stages of kidney function. This finding may be due to lack of statistical power given the infrequent occurrence of these HACs. To highlight the importance of our findings, extrapolation of our data to 38 million admissions in North America in 2013 (15,16) suggest that 2.18 million patients had at least one potentially preventable complication (5.75%), and the excess number of admissions with at least one preventable HAC that may be attributable to CKD was 26,000 (based on the population attributable risk percentage of 1.2%).
Strengths of this study include the use of a population-based cohort and inclusion of community, teaching, and specialized hospitals, which strengthen generalizability. Furthermore, we determined baseline kidney function before hospitalization using outpatient laboratory data to define both eGFR and proteinuria. Prior studies have focused largely on specific populations of hospitalized patients, such as those defined by age, diagnostic category (cardiac surgery, intensive care unit, and post–myocardial infarction) or treatment by a specific health care provider or institution, thereby limiting their generalizability (17–22).
Our study also had some limitations. Administrative data lack information regarding severity of comorbid conditions and most responsible diagnoses for admission. Access to certain clinical variables, such as BP control and lifestyle factors (smoking, exercise, and diet), is also limited. A second limitation is underestimation of HACs. Administrative data may not be sensitive for some types of HACs (23). As such, the number of HACs is likely to be underestimated; however, this is unlikely to invalidate results because incomplete ascertainment would be expected to occur in both patients with and without CKD. Third, it is possible that the association of CKD and HAC is mediated through other pathways, such as greater burden of illness or longer LOS (greater exposure to develop HAC), although our HAC analysis adjusted for available data on comorbidity as well as days in the hospital. Fourth, because of limitations of our source data, we were unable to obtain information on hospital-level factors, including hospital type, volume, and location. Fifth, we assumed that patients with no measure of proteinuria should be in the category of no proteinuria. However, this is a test ordered by providers according to clinical suspicion; therefore, the probability of significant proteinuria in patients for whom the test is not ordered is low. Sixth, in recent years increased efforts to improve hospital safety and quality of care have been implemented, which may modify the absolute risk of preventable HACs. Finally, our source data do not allow accurate classification of attribution, such as medication error causing a complication. Because patients with CKD may be uniquely predisposed to complications of medications, this should be a focus of future study using chart review or a prospective study.
In conclusion, the presence of CKD and its severity was associated with a higher risk for HACs, many of which may be preventable. Further investigations are needed to examine the effect of evidence-based strategies on the risk of p-HACs, with the goal of improving quality of care and outcomes for hospitalized patients with CKD.
Disclosures
None.
Acknowledgments
The Interdisciplinary Chronic Disease Collaboration is funded through a Collaborative Research and Innovation Opportunities Team grant from Alberta Innovates-Health Solution.
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the view of the government of Alberta or Alberta Health Services. The government of Alberta, Alberta Health, and Alberta Health Services express no opinion in relation to this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09450915/-/DCSupplemental.
References
- 1.Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, Etchells E, Ghali WA, Hébert P, Majumdar SR, O'Beirne M, Palacios-Derflingher L, Reid RJ, Sheps S, Tamblyn R: The Canadian Adverse Events Study: The incidence of adverse events among hospital patients in Canada. CMAJ 170: 1678–1686, 2004 [DOI] [PMC free article] [PubMed]
- 2.Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA: Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 21: 177–180, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paradis AR, Stewart VT, Bayley KB, Brown A, Bennett AJ: Excess cost and length of stay associated with voluntary patient safety event reports in hospitals. Am J Med Qual 24: 53–60, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Zhan C, Miller MR: Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290: 1868–1874, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Baker GR, Norton PG: Adverse events and patient safety in Canadian health care. CMAJ 170: 353–354, 2004 [PMC free article] [PubMed]
- 6.Jackson T, Fong A, Liu MF, Murray K, Walz L, Houston C, Walker K, Dean S: Incremental costs of hospital-acquired complications in Alberta, Canada. Presented at the 2011 Patient Classification Systems International Conference,Montreal, Quebec, Canada, October 19–22, 2011 [Google Scholar]
- 7.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed]
- 8.Chapter 1: Definition and classification of CKD. Kidney Int Suppl 3: 19–62, 2013 [DOI] [PMC free article] [PubMed]
- 9.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC: Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol 19: 2414–2419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Texas Health and Human Services Commission: Potentially preventable complications in the Texas Medicaid population: State fiscal year 2012. November 2013. Available at: http://www.hhsc.state.tx.us/reports/2013/PPC-Report.pdf. Accessed November 21, 2014
- 11.Lagoe RJ, Westert GP, Czyz AM, Johnson PE: Reducing potentially preventable complications at the multi hospital level. BMC Res Notes 4: 271, 2011 [DOI] [PMC free article] [PubMed]
- 12.Waters TM, Daniels MJ, Bazzoli GJ, Perencevich E, Dunton N, Staggs VS, Potter C, Fareed N, Liu M, Shorr RI: Effect of Medicare’s nonpayment for hospital-acquired conditions: Lessons for future policy. JAMA Intern Med 175: 347–354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H; Alberta Kidney Disease Network: Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak 15:31, 2015 [DOI] [PMC free article] [PubMed]
- 14.Centers for Medicare & Medicaid Services. Provider-preventable conditions. Medicaid.gov. Available at: http://www.medicaid.gov/medicaid-chip-program-information/by-topics/financing-and-reimbursement/provider-preventable-conditions.html. Accessed December 14, 2015
- 15.Canadian Institute for Health Information (CIHI): QuickStats: Inpatient hospitalizations: Volumes, length of stay, and standardization rates. Available at: https://www.google.ca/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=0ahUKEwip37O0kLnKAhVM9WMKHYiXA4gQFgggMAE&url=https%3A%2F%2Fapps.cihi.ca%2Fmstrapp%2Fasp%2FMain.aspx%3FServer%3Dapmstrextprd_i%26project%3DQuick%2BStats%26uid%3Dpce_pub_en%26pwd%3D%26evt%3D2048001%26visualizationMode%3D0%26documentID%3DC6F8B4144B03958E3AE3CAB5DD440EA7&usg=AFQjCNF3TxSzrj92EFP8sTgbx5XUBa_-Og. Accessed November 12, 2015
- 16.US Department of Health and Human Services, Agency for Healthcare Research and Quality: HCUPnet. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=29B51F2E87B4209B&Form=DispTab&JS=Y&Action=Accept. Accessed November 17, 2015
- 17.Rowell D, Nghiem HS, Jorm C, Jackson TJ: How different are complications that affect the older adult inpatient? BMJ Qual Saf Health Care 19: e34, 2010 [DOI] [PubMed]
- 18.Sikdar KC, Alaghehbandan R, Macdonald D, Barrett B, Collins KD, Gadag V. Adverse drug events among children presenting to a hospital emergency department in Newfoundland and Labrador, Canada. Pharmacoepidemiol Drug Saf 19: 132–140, 2010 [DOI] [PubMed]
- 19.Wong RY, Miller WC: Adverse outcomes following hospitalization in acutely ill older patients. BMC Geriatr 8: 10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanzel KR, Jamieson CG, Bohnen JM. Complications on a general surgery service: Incidence and reporting. Can J Surg 43: 113–117, 2000 [PMC free article] [PubMed]
- 21.Florea A, Caughey SS, Westland J, Berckmans M, Kennelly C, Beach C, Dyer A, Forster AJ, Oppenheimer LW: The Ottawa hospital quality incident notification system for capturing adverse events in obstetrics. J Obstet Gynaecol Can 32: 657–662, 2010 [DOI] [PubMed]
- 22.Rahim SA, Mody A, Pickering J, Devereaux PJ, Yusuf S: Iatrogenic adverse events in the coronary care unit. Circ Cardiovasc Qual Outcomes 2: 437–442, 2009 [DOI] [PubMed]
- 23.Quan H, Parsons GA, Ghali WA: Assessing accuracy of diagnosis-type indicators for flagging complications in administrative data. J Clin Epidemiol 57: 366–372, 2004 [DOI] [PubMed] [Google Scholar]