Abstract
Background and objectives
Indigenous Australians experience a heavy burden of CKD. To address this burden, the eGFR Follow-Up Study recruited and followed an Indigenous Australian cohort from regions of Australia with the greatest ESRD burden. We sought to better understand factors contributing to the progression of kidney disease. Specific objectives were to assess rates of progression of eGFR in Indigenous Australians with and without CKD and identify factors associated with a decline in eGFR.
Design, setting, participants, & measurements
This observational longitudinal study of Indigenous Australian adults was conducted in >20 sites. The baseline cohort was recruited from community and primary care clinic sites across five strata of health, diabetes status, and kidney function. Participants were then invited to follow up at 2–4 years; if unavailable, vital status, progression to RRT, and serum creatinine were obtained from medical records. Primary outcomes were annual eGFR change and combined renal outcome (first of ≥30% eGFR decline with follow-up eGFR<60 ml/min per 1.73 m2, progression to RRT, or renal death).
Results
Participants (n=550) were followed for a median of 3.0 years. Baseline and follow-up eGFR (geometric mean [95% confidence interval], 83.9 (80.7 to 87.3) and 70.1 (65.9 to 74.5) ml/min per 1.73 m2, respectively. Overall mean annual eGFR change was −3.1 (−3.6 to −2.5) ml/min per 1.73 m2. Stratified by baseline eGFR (≥90, 60–89, <60 ml/min per 1.73 m2), annual eGFR changes were −3.0 (−3.6 to −2.4), −1.9 (−3.3 to −0.5), and −5.0 (−6.5 to −3.6) ml/min per 1.73 m2. Across baseline eGFR categories, annual eGFR decline was greatest among adults with baseline albumin-to-creatinine ratio (ACR) >265 mg/g (30 mg/mmol). Baseline determinants of the combined renal outcome (experienced by 66 participants) were higher urine ACR, diabetes, lower measured GFR, and higher C-reactive protein.
Conclusions
The observed eGFR decline was three times higher than described in nonindigenous populations. ACR was confirmed as a powerful predictor for eGFR decline across diverse geographic regions.
Keywords: chronic kidney disease; albuminuria; indigenous Australian; end stage kidney disease; eGFR; Australia; humans; kidney failure, chronic; kidney function tests; longitudinal studies
Introduction
Rates of ESRD are 10–15 times higher among Indigenous Australians compared with the general Australian population, with rates highest in remote regions of Australia (1,2). Type 2 diabetes is common among Indigenous Australians with ESRD. Among Australians with new ESRD in 2010, 76% of Indigenous Australians had type 2 diabetes as a comorbidity compared with 39% of nonindigenous Australians (3). As with other chronic conditions, the age at onset of ESRD in Indigenous Australians is much earlier than in nonindigenous Australians. The greatest discrepancy in rates of onset of ESRD between Indigenous and nonindigenous Australians is for those aged 45–54 years (2).
Despite this significant burden of disease, there is a critical lack of longitudinal data on CKD, and therefore a significant evidence gap exists regarding rates or predictors of CKD progression among the high-risk Indigenous Australian population. The landmark study from one remote Aboriginal community by Hoy et al. reported that the strongest determinant of GFR loss and of albuminuria progression was the baseline urine albumin-to-creatinine ratio (ACR) (4,5). Macroalbuminuria was an identified risk factor for renal and all-cause death in this study; however, prospective cohort studies recruiting participants across multiple communities and geographic regions have not been reported. The important association of albuminuria with accelerated GFR loss is consistent with findings among Pima Indians with diabetes (6), the Prevention of Renal and Vascular End-stage Disease study among Europeans (7,8), a recent meta-analysis of available CKD cohorts (9), and recent changes in CKD classification to incorporate level of albuminuria (9).
The aim of the eGFR Follow-up Study was to assess progression of kidney disease in a cohort of >600 Indigenous Australian adults. The objectives of this analysis were to (1) assess rates of progression of eGFR in indigenous participants without any identifiable risk factors for CKD or with any one of albuminuria, diabetes, or impaired GFR (<90 ml/min per 1.73 m2) at baseline and (2) identify demographic, clinical, biochemical, and socioeconomic factors associated with a decline in eGFR.
Materials and Methods
The eGFR Study at baseline established a cohort of >600 Indigenous Australian participants across >20 sites in urban, regional, and remote Australia selected on the basis of five strata of health, diabetes status, and kidney function. Data at baseline included a reference measure of GFR (obtained using 4-hour plasma disappearance of iohexol) and detailed clinical, body composition, and biochemical assessment. The aim at baseline was to assess the accuracy of equations that estimate GFR in Indigenous Australians. We reported that the CKD-Epidemiology Collaboration (CKD-EPI) equation provides a reasonably accurate and unbiased estimate of GFR in this high-risk population (10).
The eGFR Follow-up Study was an observational longitudinal extension of the eGFR Study. The methods of the eGFR Baseline Study have been previously described in detail (11). The methods of follow-up are outlined below (details in the Supplemental Material). The eGFR Baseline and Follow-up Studies were approved by the joint Menzies School of Health Research–Northern Territory Department of Health Human Research Ethics Committee and ethics committees in all other relevant regions. Informed consent from the baseline study included consent for follow-up; participants completing a follow-up clinical assessment provided further consent at that assessment.
Participants
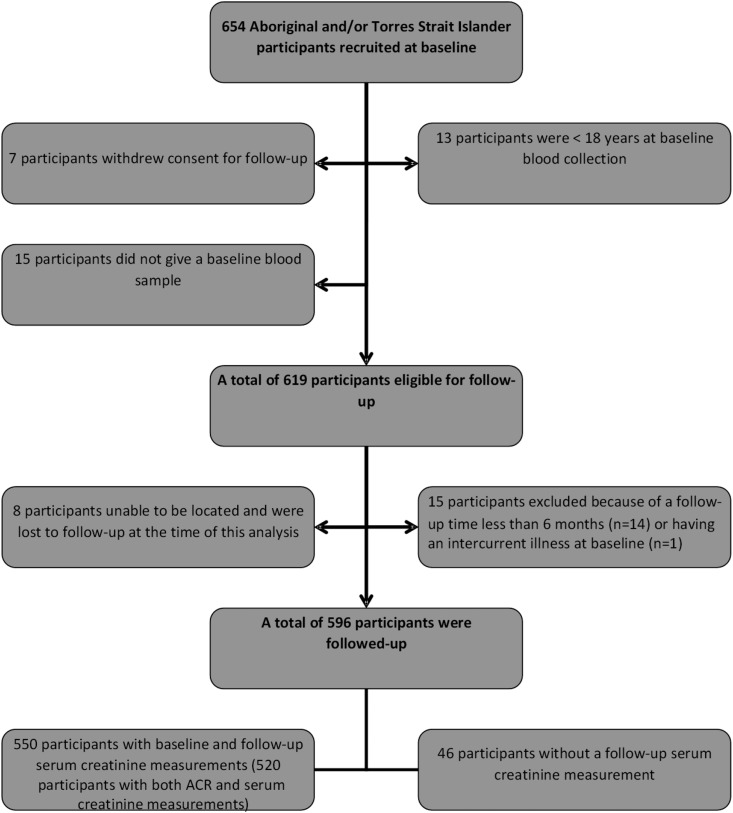
Participants in the eGFR Baseline Study were recruited from >20 community sites within four large geographic regions: Top End (of Northern Territory, Australia), Central Australia, Far North Queensland (North Queensland and the Torres Strait), and remote Western Australia (Kimberley and Goldfields regions). These regions were chosen because resident Indigenous Australians bear a disproportionately large burden of ESRD. Participants at baseline were recruited from participating Aboriginal Medical Services, primary care facilities, hospital specialist clinics, community networks, staff members of health services, word-of-mouth, and self-referral. Indigenous Australian participants in the eGFR Baseline Study were eligible for participation in the eGFR Follow-up Study if they were aged 18 years and older and had provided a blood sample for creatinine at baseline (Figure 1).
Figure 1.
The eGFR Follow-up Study participant eligibility and follow-up process. ACR, albumin-to-creatinine ratio.
Data Collection
Two components of data collection were involved (participants who were unavailable or could not be contacted completed the second part only): (1) clinical assessment of available participants in-person on one occasion between 2 and 4 years after baseline assessment with collection of blood and urine samples, medical history, and medications recorded and (2) data collection from medical records and pathology companies.
Laboratory Methods
Blood samples were collected and transported on ice to be stored at −80°C. Serum creatinine levels were measured at Melbourne Pathology, Melbourne, on specimens that had been stored at −80°C. Creatinine was measured using an isotope-dilution mass spectrometry–aligned enzymatic method (Cobas c701, Roche Diagnostics, Mannheim, Germany).
Of the 550 participants followed up, 369 (67%) were assessed by the research team at follow-up and had both baseline and follow-up enzymatic creatinine measurements performed in the central laboratory. A total of 181(30.4%) participants had local pathology measurement of serum creatinine at baseline and follow-up. Participants who provided a baseline blood sample but no follow-up blood sample (n=46; Table 1) have not been included in this follow-up analysis.
Table 1.
Baseline participant characteristics stratified by baseline eGFR (n=550)
| Baseline factor | Observations, n | eGFR(CKD-EPI)<60 ml/min per 1.73 m2 (n=85) | eGFR (CKD-EPI) 60–89 ml/min per 1.73 m2 (n=125) | eGFR (CKD-EPI)≥90 ml/min per 1.73 m2 (n=340) | Participants with Follow-up Blood Samples (n=550) | Participants without Follow-up Blood Samples (n=46) | P Valuea |
|---|---|---|---|---|---|---|---|
| Age, yr | 550 | 60.1 (50.0–68.5) | 53.7 (44.1–63.5) | 39.6 (30.3–49.5) | 46.3 (34.8–56.5) | 32.4 (22.3–41.9) | <0.001 |
| Men | 550 | 36 (42.4) | 47 (37.6) | 117 (34.4) | 200 (36.4) | 21 (45.7) | 0.21 |
| Weight, kg | 548 | 76.4 (63.4–92.0) | 83.9 (71.6–98.0) | 82.7 (69.7–98.3) | 82.1 (68.8–97.4) | 73.6 (62.2–87.6) | 0.02 |
| BMI, kg/m2 | 546 | 27.8 (23.8–32.1) | 30.2 (26.6–34.5) | 30.0 (25.4–35.1) | 29.6 (25.3–34.8) | 25.7 (21.9–29.9) | 0.01 |
| Waist circumference, cm | 524 | 101.1 (93.5–112.9) | 103.3 (95.0–113.3) | 100.3 (88.4–111.8) | 101.6 (90.9–112.2) | 89.6 (77.5–101.7) | <0.001 |
| Waist-to-hip ratio | 520 | 1.00 (0.93–1.05) | 0.95 (0.90–1.03) | 0.93 (0.85–0.99) | 0.95 (0.87–1.01) | 0.87 (0.83–0.94) | <0.001 |
| Body surface area, m2b | 546 | 1.82 (1.68–2.00) | 1.93 (1.75–2.09) | 1.92 (1.75–2.08) | 1.90 (1.74–2.08) | 1.86 (1.72–2.00) | 0.10 |
| Diabetes | 550 | 57 (67.1) | 59 (47.2) | 125 (36.8) | 241 (43.8) | 6 (13.3) | <0.001 |
| Current smoker | 539 | 16 (19.8) | 32 (26.4) | 169 (50.1) | 217 (40.3) | 23 (51.1) | 0.16 |
| Systolic BP, mmHg | 542 | 120.0 (111.3–135.7) | 116.3 (106.0–129.7) | 114.2 (104.7–125.3) | 115.8 (106.0–128.0 | 108.8 (98.2–119.8) | 0.02 |
| Diastolic BP, mmHg | 542 | 73.7 (67.7–79.0) | 73.7 (67.3–80.3) | 74.5 (67.3–82.0) | 74.0 (67.3–81.3) | 69.8 (61.7–78.0) | 0.01 |
| HbA1c | |||||||
| Measured as mmol/mol)c | 536 | 47.5 (41.0–68.3) | 44.3 (39.9–57.4) | 42.1 (37.7–53.0) | 43.2 (37.7–56.3) | 38.3 (35.5–41.0) | <0.001 |
| Measured as percentage | 536 | 6.5 (5.9–8.4) | 6.2 (5.8–7.4) | 6.0 (5.6–7.0) | 6.1 (5.6–7.3) | 5.7 (5.4–5.9) | <0.001 |
| Cholesterol | |||||||
| Measured as mg/dl | 530 | 166.0 (142.8–189.1) | 173.7 (154.4–208.4) | 187.2 (162.1–216.2) | 181.4 (154.4–212.3) | 189.1 (158.3–220.0) | 0.10 |
| Measured as mmol/L | 530 | 4.3 (3.7–4.9) | 4.5 (4.0–5.4) | 4.9 (4.2–5.6) | 4.7 (4.0–5.5) | 4.9 (4.1–5.7) | 0.10 |
| HDL cholesterol | |||||||
| Measured as mg/dl | 519 | 34.7 (30.9–44.4) | 38.9 (34.7–46.3) | 38.6 (34.7–48.6) | 38.6 (33.6–46.3) | 46.3 (38.6–54.0) | 0.01 |
| Measured as mmol/L | 519 | 0.9 (0.8–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.3) | 1.0 (0.9–1.2) | 1.2 (1.0–1.4) | 0.01 |
| Cholesterol-to-HDL ratio | 519 | 4.2 (3.6–5.9) | 4.5 (3.7–5.7) | 4.6 (3.6–5.8) | 4.5 (3.6–5.8) | 4.2 (3.3–5.0) | 0.08 |
| Enzymatic creatinine | |||||||
| Measured as mg/dl | 532 | 1.70 (1.38–2.25) | 0.90 (0.81–1.05) | 0.68 (0.59–0.82) | 0.81 (0.64–0.99) | 0.79 (0.67–0.95) | 0.23 |
| Measured as µmol/La | 532 | 150 (122–199) | 80 (72–93) | 60 (52–73) | 72 (57–88) | 70 (59–84) | 0.23 |
| CKD-EPI eGFR, ml/min per 1.73 m2 | 544 | 37.7 (24.4–48.7) | 82.0 (74.5–90.7) | 107.6 (99.2–116.9) | 97.9 (79.3–111.7) | 111.8 (98.2–121.0) | <0.001 |
| Measured GFR, ml/min per 1.73 m2 | 516 | 46.2 (31.4–56.2) | 86.9 (73.8–98.4) | 114.2 (100.2–127.3) | 102.4 (82.6–120.8) | 112.5 (100.2–123.5 | 0.02 |
| CRP, mg/L | 527 | 5.0 (2.5–12.5) | 5.2 (3.0–10.0) | 6.0 (3.0–11.0) | 5.8 (3.0–11.0) | 5.0 (2.5–9.5) | 0.23 |
| Bilirubin | |||||||
| Measured as mg/dl ) | 543 | 0.29 (0.18–0.41) | 0.41 (0.29–0.53) | 0.35 (0.29–0.58) | 0.35 (0.23–0.53) | 0.47 (0.32–0.67) | 0.06 |
| Measured as µmol/L | 543 | 5.0 (3.0–7.0) | 7.0 (5.0–9.0) | 6.0 (5.0–10.0) | 6.0 (4.0–9.0) | 8.0 (5.5–11.5) | 0.06 |
| ACR | |||||||
| Measured as mg/g | 520 | 650.0 (146.9–1894.8) | 19.0 (7.1–122.1) | 12.4 (5.8–43.4) | 18.1 (6.2–151.8) | 4.4 (2.7–12.4) | <0.01 |
| Measured as mg/mmol | 520 | 73.5 (16.6–214.1) | 2.2 (0.8–13.8) | 1.4 (0.7–4.9) | 2.1 (0.7–17.2) | 0.5 (0.3–1.4) | <0.001 |
| ACR category | 520 | ||||||
| A1 | 6 (7.7) | 67 (56.8) | 224 (69.1) | 297 (57.1) | 34 (82.9) | 0.01 | |
| A2 | 21 (26.9) | 26 (22.0) | 67 (20.7) | 114 (21.9) | 4 (9.8) | ||
| A3 | 51 (65.4) | 25 (21.2) | 33 (10.2) | 109 (21.0) | 3 (7.3) | ||
| Use of ACE inhibitors | 550 | 51 (60.0) | 45 (36.0) | 69 (20.3) | 165 (30.0) | 2 (4.3) | <0.001 |
| Use of ARB | 550 | 23 (27.1) | 14 (11.2) | 19 (5.6) | 56 (10.2) | 3 (6.5) | 0.42 |
| Housing | |||||||
| Housing tenure | 541 | 0.66 | |||||
| Rent or other tenure | 77 (93.9) | 95 (77.9) | 296 (87.8) | 468 (86.5) | 4 (8.9) | ||
| Own/being purchased | 5 (6.1) | 27 (22.1) | 41 (12.2) | 73 (13.5) | 41 (91.1) | ||
| Education | |||||||
| Highest level of school completed | 534 | 0.04 | |||||
| Less than year 10/never went to school | 47 (58.8) | 50 (42.0) | 106 (31.6) | 203 (38.0) | 9 (20.0) | ||
| Year 10/equivalent | 19 (23.8) | 52 (43.7) | 142 (42.4) | 213 (39.9) | 21 (46.7) | ||
| Year 12/equivalent | 14 (17.5) | 17 (14.3) | 87 (26.0) | 118 (22.1) | 15 (33.3) |
Data are median (IQR) or n (%). CKD-EPI, CKD-Epidemiology Collaboration; BMI, body mass index; HbA1c, hemoglobin A1c; CRP, C-reactive protein; ACR, urine albumin-to-creatinine ratio; A1, albuminuria <27 mg/g (3 mg/mmol); A2, albuminuria, 27–265 mg/g (3–30 mg/mmol); A3, albuminuria>265 mg/g (30 mg/mmol); ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blockade.
The P value compares the group with follow-up blood samples to the group without follow-up blood samples and was calculated using ANOVA for continuous variables and chi-squared test between categorical variables. The difference between serum creatinine in the two participant groups was tested on the log serum creatinine level.
Calculated using the Dubois formula.
The difference between hemoglobin A1c (HbA1c) levels in the two participant groups was tested on the log HbA1c level.
For difference between participants with a follow-up blood sample and participants without a follow-up blood sample. Calculated using ANOVA for continuous variables and chi-squared test between categorical variables.
Outcome Definitions
The outcome of eGFR change (CKD-EPI, ml/min per 1.73 m2 per year) was calculated as (eGFR at follow-up − eGFR at baseline)/follow-up time. The follow-up time was the date between baseline and follow-up creatinine measurement (range, 0.52–5.75 years). There were 56 participants with follow-up time <2 years (including 11 who commenced RRT and seven who died); median follow-up time was 1.69 years (range, 0.52–1.97 years). Participants with follow-up time <6 months were excluded (Figure 1). Albuminuria categories were as follows: A1, <27 mg/g (3 mg/mmol); A2, 27–265 mg/g (3–30 mg/mmol); and A3, >265 mg/g (30 mg/mmol) (9). Urine ACR was also analyzed as a continuous independent variable (log-transformed). The combined renal endpoint was defined as the first of the following: an absolute 30% decline in eGFR (as proposed by the CKD Consortium) (12,13) with a follow-up eGFR<60 ml/min per 1.73 m2, death from renal causes, or initiation of RRT. All deaths occurring when eGFR declined to <15 ml/min per 1.73 m2 were defined as renal deaths. Participants were censored at the time the first endpoint was reached.
Statistical Analyses
Statistical analyses were performed using Stata software, version 14 (Stata Corp., College Station, TX), and SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). Associations between demographic, clinical, biochemical factors, and eGFR change were assessed using linear regression models adjusted for age and sex. A final multivariable model for the outcome of eGFR change within each eGFR strata was identified using covariates selected for entry into each linear regression model if crude P value was ≤0.1. Sensitivity analyses were performed: Participants with minimum follow-up duration of 2 years (n=439, according to the minimum follow-up time of 2 years proposed by Coresh et al. [12]) and participants with enzymatic creatinine results at baseline and follow-up (n=369).
Cox proportional hazards models were used to assess relationships with the dichotomous renal endpoint. Follow-up time for the Cox proportional hazards models was calculated from creatinine measurement to date of 30% decline in eGFR, death from renal causes, or RRT initiation (whichever event was earliest) or censor (date of follow-up visit). For the participants who did not reach the combined outcome, their censor date was the date of follow-up blood sample. Cox proportional hazards models were further adjusted for age, sex, and baseline ACR. A final model was assessed using covariates selected for entry into a Cox regression model for dichotomous outcome if the crude P value was ≤0.1. The following additional covariates were assessed in the final model: measured GFR, systolic BP, cholesterol-to-HDL ratio, C-reactive protein (CRP), and diabetes.
Results
Baseline characteristics for the 550 participants of the eGFR Follow-Up study are outlined in Table 1. The median follow-up time in years was 3.01 (interquartile range, 2.48–3.31), with a total follow-up time of 1771 person-years. The geometric mean eGFR for all participants was 83.9 (95% confidence interval [95% CI], 80.7 to 87.3) ml/min per 1.73 m2 at baseline and 70.1 (95% CI, 66.1 to 74.5) ml/min per 1.73 m2 at follow-up. Of the 550 participants, 23% identified coming from the Torres Strait, 24% from the Top End (Northern Territory), 21% from Central Australia, 4% from Northern Queensland, 16% from Kimberley (Western Australia), 5% from Gold Fields (Western Australia), and 5% from "other" regions. Compared with participants with both baseline and follow-up creatinine, participants who did not have a follow-up serum creatinine (n=46; Table 1) were younger and generally healthier (patients had lower body mass index, lower BP, and higher eGFR, and fewer of them had diabetes).
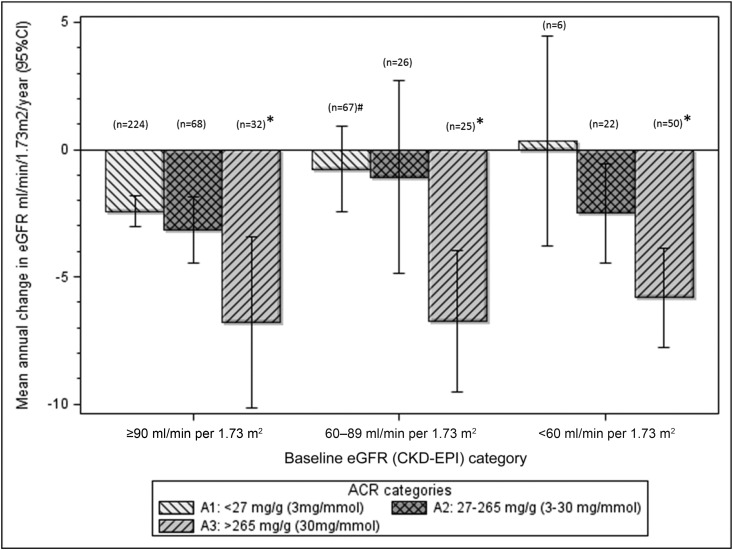
The mean annual change in eGFR (CKD-EPI) was −3.0 (95% CI, −3.6 to 2.5) ml/min per 1.73 m2 for the cohort, with annual change by baseline eGFR as follows: eGFR≥90, −3.0 (95% CI, −3.6 to −2.4) ml/min per 1.73 m2; eGFR 60–89, −1.9 (95% CI, −3.3 to −0.5) ml/min per 1.73 m2; eGFR<60, −5.0 (95% CI, −6.5 to −3.6) ml/min per 1.73 m2. Mean annual eGFR decline was greatest among participants with ACR>265 mg/g (30 mg/mmol) at baseline, across each of the three categories of baseline eGFR (Figure 2).
Figure 2.
Annual change in eGFR by baseline eGFR categories and albumin-to-creatinine ratio (ACR) categories. Significant change in eGFR in comparison with eGFR≥90 ml/min per 1.73 m2 in combination with an ACR<27 mg/g (3 mg/mmol). #P<0.05; *P≤0.001. 95% CI, 95% confidence interval; CKD-EPI, CKD-Epidemiology Collaboration.
Albuminuria was associated with eGFR decline on univariate analysis in participants with baseline eGFR<60 and 60–89 ml/min per 1.73 m2 (Table 2). In addition, significant relationships were seen with eGFR decline for the following variables (by baseline eGFR strata): eGFR<60 ml/min per 1.73 m2— female, younger age, diabetes, paying rent, or other housing tenure; eGFR 60–89 ml/min per 1.73 m2— higher CRP; eGFR≥90 ml/min per 1.73 m2—higher baseline eGFR and higher bilirubin (Table 2).
Table 2.
Relationship of annual absolute decline in eGFR with participant characteristics at baseline, adjusted for age and sex (n=550)
| Variable | eGFR (CKD-EPI)<60 ml/min per 1.73 m2 (n=85) | eGFR (CKD-EPI) 60–89 ml/min per 1.73 m2 (n=125) | eGFR (CKD-EPI)≥90 ml/min per 1.73 m2 (n=340) |
|---|---|---|---|
| Baseline factor | |||
| Age (per 5 yr) | 0.95 (−−0.40 to 1.45) | −0.315 (−0.86 to 0.20) | −0.14 (−0.38 to 0.09) |
| Female | −2.94 (−5.68 to −0.208) | 1.08 (−1.75 to 3.91) | 1.31 (0.091–2.52) |
| Weight (per 5 kg) | 0.09 (−0.24 to 0.42) | 0.15 (−0.20 to 0.49) | −0.0005 (−0.145 to 0.13) |
| BMI (kg/m2) | 0.027 (−0.16 to 0.22) | 0.099 (−0.10 to 0.30) | −0.008 (−0.075 to 0.092) |
| Waist circumference (2 cm) | 0.00 (−0.45 to 0.45) | 0.095 (−0.36 to 0.55) | 0.011 (−018 to 0.20) |
| Waist-to-hip ratio (0.1 unit) | −1.59 (−3.46 to 0.29) | −0.90 (−2.63 to 0.84) | 0.40 (−0.32 to 1.13) |
| Diabetes | −3.74 (−6.55 to −0.93) | 0.39 (−2.55 to 3.33) | −0.51 (−1.81 to 0.80) |
| Currently smoking | −2.48 (−6.02 to 1.06) | 2.16 (−1.28 to 5.59) | 0.22 (−0.95 to 1.40) |
| Systolic BP (per 5 mmHg) | −0.18 (−0.55 to 0.21) | −0.27 (−0.63 to 0.10) | 0.05 (−0.15 to 0.25) |
| Diastolic BP (per 5 mmHg) | 0.07 (−0.67 to 0.81) | −0.021 (−0.9 to 0.47) | 0.055 (−0.24 to 0.35) |
| HbA1c | |||
| Measured as mmol/mol | −0.021 (−0.091 to 0.049) | −0.028 (−0.11 to 0.048) | −0.022 (−0.053 to 0.008) |
| Measured as percentage | −0.23 (−1.00 to 0.53) | −0.31 (−1.16 to 0.54) | −0.24 (−0.58 to 0.094) |
| Cholesterol | |||
| Per 5 mg/dl | 0.093 (−0.075 to 0.26) | 0.17 (−0.025 to 0.36) | 0.065 (−0.005 to 0.14) |
| Measured as mmol/L | 0.72 (−0.57 to 2.01) | 1.28 (−0.20 to 2.75) | 0.51 (−0.043 to 1.06) |
| HDL cholesterol | |||
| Measured per 5 mg/dl | −0.08 (−0.70 to 0.54) | 0.16 (−0.48 to 0.79) | −0.0085 (−0.23 to 0.21) |
| Measured as mmol/L | −0.63 (−5.40 to 4.14) | 1.19 (−3.69 to 6.07) | −0.064 (−1.76 to 1.64) |
| Cholesterol-to-HDL ratio | 0.52 (−0.44 to 1.47) | 0.38 (−0.64 to 1.40) | 0.22 (−0.17 to 0.60) |
| CKD-EPI eGFR (per 5 ml/min per 1.73 m2) | 0.33 (−0.16 to 0.81) | −0.05 (−0.99 to 0.9) | −0.65 (−0.95 to −0.34) |
| CRP (log mg/L) | 0.36(−0.94 to 1.66) | −1.50 (−2.95 to −0.054) | 0.48 (−0.13 to 1.08) |
| Bilirubin | |||
| Measured as mg/dl | 3.32 (−4.73 to 11.38) | −1.69 (−8.09 to 4.71) | −3.46 (−5.40 to −1.51) |
| Measured as μmol/L | 0.19 (−0.27 to 0.66) | −0.10 (−0.47 to 0.28) | −0.20 (−0.32 to −0.089) |
| Urine ACR (log mg/g) | −1.03 (−1.76 to −0.31) | −1.03 (−1.71 to −0.36) | −0.53 (−0.89 to −0.18) |
| ACR categories | |||
| A2 versus A1 | −3.95 (−9.17 to 1.27) | 0.63 (−2.89 to 4.14) | −0.22 (−1.73 to 1.30) |
| A3 versus A1 | −6.02 (−10.74 to −1.31) | −5.86 (−9.32 to −2.39) | −4.20 (−6.09 to −2.31) |
| ACE inhibitor or ARB use | −1.66 (−4.57 to 1.24) | −2.28 (−5.18 to 0.63) | −1.01 (−2.61 to 0.59) |
| Rent or other tenure versus owning/being purchased | −6.46 (−12.1 to −0.80) | −0.77 (−4.23 to 2.67) | 0.014 (−1.80 to 1.82) |
| Highest level of school completed | |||
| Never went to school versus year 12/equivalent | 1.93 (−1.63 to 5.48) | −1.28 (−4.59 to 2.02) | 1.13 (−0.27 to 2.53) |
| Less than year 10 versus year 12/equivalent | 2.01 (−1.95 to 5.97) | −0.78 (−5.43 to 3.87) | 0.91 (−0.71 to 2.54) |
Linear regression models, adjusted for age and sex. Values are expressed as ml/min per 1.73 m2, with 95% confidence intervals in parentheses. CKD-EPI, CKD-Epidemiology Collaboration; BMI, body mass index; HbA1c, hemoglobin A1c; CRP, C-reactive protein; ACR, urine albumin-to-creatinine ratio; A2, albuminuria, 27–265 mg/g (3–30 mg/mmol); A1, albuminuria<27 mg/g (3 mg/mmol); A3, albuminuria>265 mg/g (30 mg/mmol); ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blockade.
On multivariable linear regression of eGFR change (Table 3), ACR was significantly associated with the outcome in each baseline eGFR stratum. Results of the sensitivity analyses that restricted participants to those with a minimum follow-up of 2 years or to those with enzymatic creatinine revealed similar variables associated with eGFR decline (data not shown).
Table 3.
Multivariable linear regression model of the annual decline in eGFR stratified by baseline eGFR
| Baseline Factor | eGFR (CKD-EPI)<60 ml/min per 1.73 m2 (n=78) | eGFR (CKD-EPI) 60–89 ml/min per 1.73 m2 (n=118) | eGFR (CKD-EPI)≥90 ml/min per 1.73 m2 (n=324) | |||
|---|---|---|---|---|---|---|
| β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | |
| Age (per 5 yr) | 0.65 (0.09–1.22) | 0.02 | −0.25 (−0.79 to 0.29) | 0.36 | −0.38 (−0.70 to −0.09) | 0.01 |
| Female | −1.81 (−4.44 to 0.81) | 0.17 | 0.83 (−2.01 to 3.67) | 0.56 | 1.76 (0.51– to 3.01) | 0.01 |
| Baseline CKD-EPI (per 5–ml/min per 1.73 m2 increase) | −0.11 (−0.18 to −0.05) | 0.001 | ||||
| Diabetes | −2.49 (−5.27 to 0.30) | 0.08 | ||||
| Urine ACR (log mg/g) | −0.92 (−1.65 to −0.20) | 0.01 | −1.03 (−1.71 to −0.35) | 0.003 | −0.41 (−0.76 to −0.06) | 0.02 |
| Model R2, % | 23.5 | 8.8 | 7.6 | |||
Final model (n=520). A final multivariable model was identified using covariates selected for entry into a linear regression model. All the variables described in Table 2 were included in the selection. The following covariates were entered into the final model: eGFR<60 ml/min per 1.73 m2: sex, age, urine albumin-to-creatinine ratio (ACR), diabetes; eGFR 60–89 ml/min per 1.73 m2: sex, age, urine ACR; eGFR≥90 ml/min per 1.73 m2: sex, age, urine ACR, baseline eGFR (calculated with CKD-Epidemiology Collaboration formula). CKD-EPI, CKD-Epidemiology Collaboration; 95% CI, 95% confidence interval; ACR, albumin-to-creatinine ratio.
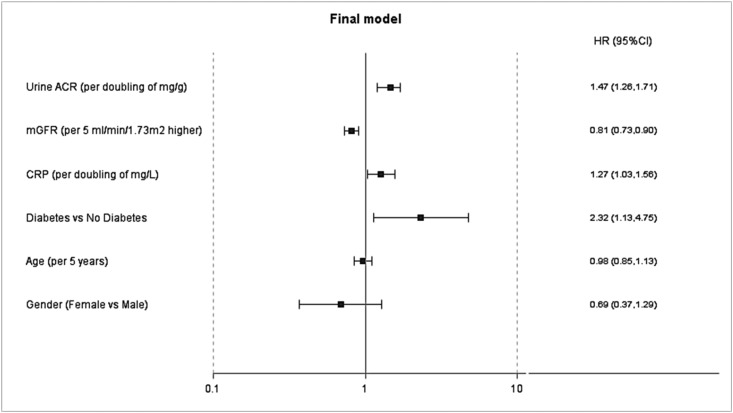
There were a total of 66 incident cases of the combined renal outcome of absolute 30% decline in eGFR among participants who had a follow-up eGFR<60 ml/min per/ 1.73 m2 (n=64 reached this component first, including 4 who later died of renal causes without RRT commencement and 19 who later commenced RRT) died of renal causes (n=2 not included in eGFR decline component), or commenced RRT (all n=19 included in eGFR decline component). The overall crude incidence rate of the combined outcome was 37.3 per 1000 person-years (95% CI, 28.3 to 46.3) (Supplemental Table 1). Higher ACR (hazard ratio, 1.47 [95% CI, 1.26 to −1.71] per doubling of mg/g), diabetes (2.32 [95% CI, 1.13 to 4.75], higher CRP (1.27 [95% CI, 1.03 to 1.56] per doubling of mg/L), and lower measured GFR (0.81 [95% CI, 0.73 to 0.90]) per 5 ml/min per 1.73 m2 were independently associated with the combined renal endpoint of absolute 30% decline in eGFR, death from renal causes, or initiation of RRT (Figure 3, Table 4).
Figure 3.
Forest plot of the final model of the Cox regression model of combined renal endpoint. Final model assessed age, sex, albumin-to-creatinine ratio (ACR) (log2-transformed), diabetes, systolic BP, cholesterol-to-HDL ratio, measured GFR, and C-reactive protein (CRP) (log2-transformed); variables with P<0.1, age, and sex were retained in the model. In the final model, there were 48 events versus 427 nonevents. 95% CI, 95% confidence interval; HR, hazard ratio; mGFR, measured GFR.
Table 4.
Cox regression model of combined renal endpoint
| Baseline Factor | HR (95% CI) | ||
|---|---|---|---|
| Crude (66 Events/n=550a | Adjusted for Age and Sex (66 Events/n=550)a | Adjusted for Age, Sex, and ACR (60 Events/n=520)b | |
| Age (per 5 r) | 0.97 (0.88–1.06) | ||
| Female | 1.05 (0.64–1.75) | ||
| Weight (5 kg) | 0.95 (0.90–1.05) | 0.95 (0.91–1.06) | 1.00 (0.90–1.05) |
| BMI (kg/m2) | 0.98 (0.94–1.01) | 0.98 (0.95–1.02) | 0.97 (0.94–1.01) |
| Waist circumference (2 cm) | 1.00 (0.96–1.02) | 1.00 (0.96–1.02) | 1.00 (0.96–1.02) |
| Waist-to-hip ratio (0.1 unit) | 1.21 (0.90–1.63) | 1.36 (0.99–1.87) | 1.18 (0.85–1.65) |
| Diabetes | 2.27 (1.32–3.92) | 2.56 (1.44–4.54) | 1.41 (0.77–2.59b |
| Currently smoking | 1.19 (0.66–2.13) | 1.20 (0.66–2.18) | 0.85 (0.45–1.63) |
| Systolic BP (per 5 mmHg) | 1.05 (1.00–1.16) | 1.10 (1.00–1.16) | 0.95 (0.90–1.05) |
| Diastolic BP (per 5 mmHg) | 1.10 (1.00–1.22) | 1.10 (1.00–1.28) | 1.00 (0.90–1.16) |
| HbA1c | |||
| Measured as mmol/mol (log) | 1.82 (0.92–3.57) | 1.99 (1.00–3.96) | 1.00 (0.43–2.34) |
| Measured as percentage | 1.09 (0.98–1.22 | 1.10 (0.98–1.23) | 1.01 (0.87–1.17) |
| Cholesterol | |||
| Measured as mmol/L | 0.97 (0.75–1.24) | 0.95 (0.73–1.23) | 0.95 (0.70–1.30) |
| Measured per 5 mg/dl | 0.99 (0.96–1.03) | 0.99 (0.96–1.03) | 0.99 (0.95–1.03) |
| HDL cholesterol | |||
| Measured as mmol/L | 0.87 (0.38–1.95) | 0.83 (0.37–1.88) | 0.79 (0.33–1.89) |
| Measured per 5 mg/dl | 0.98 (0.88–1.09) | 0.98 (0.88–1.09) | 0.97 (0.87–1.09) |
| Cholesterol-to-HDL ratio | 1.04 (0.88–1.23) | 1.04 (0.88–1.24) | 1.05 (0.87–1.27) |
| Measured GFR (per 5 ml/min per 1.73 m2 higher) | 0.80 (0.73–0.87) | 0.78 (0.71–0.86) | 0.82 (0.75–0.91) |
| CRP (mg/L) | 1.22 (1.03–1.46) | 1.24 (1.04–1.49) | 1.22 (1.01–1.47) |
| Urine ACR (log 2 mg/g)c | 1.51 (1.34–1.69) | 1.57 (1.38–1.78) | |
| ACR categoriesc | |||
| A2 versus A1 | 6.22 (1.69–22.92) | 5.89 (1.59–21.81) | |
| A3 versus A1 | 21.92 (6.45–74.45) | 22.20 (6.53–75.52) | |
| Taking ACE inhibitor or ARB versus not taking ACE inhibitor or ARB | 2.49 (1.40–4.42) | 2.90 (1.58–5.34) | 1.83 (1.00–3.38) |
| Highest level of school completed | |||
| Less than year 10/never went to school versus year 12/equivalent | 1.56 (0.74–3.28) | 1.84 (0.85–3.98) | 1.02 (0.45–2.31) |
| Year 10/equivalent versus year 12/equivalent | 1.06 (0.47–2.39) | 1.11 (0.49–2.53) | 0.66 (0.27–1.62) |
All models are stratified by baseline eGFR category. HR, hazard ratio; 95% CI, 95% confidence interval; ACR, albumin-to-creatinine ratio; BMI, body mass index; HbA1c, hemoglobin A1c; CRP, C-reactive protein; A2, albuminuria 27–265 mg/g (3–30 mg/mmol); A1, albuminuria<27 mg/g (3 mg/mmol); A3, albuminuria>265 mg/g (30 mg/mmol); ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockade.
Events versus all participants in the model.
Analyses presented for each variable: unadjusted (crude); adjusted for age and sex; adjusted for age, sex, and ACR.
Fewer participants are included for ACR and ACR categories because of missing data for ACR.
Discussion
In the eGFR Follow-up Study, we assessed clinical, biochemical, anthropometric, and socioeconomic factors associated with eGFR loss in a cohort of indigenous Australians from >20 sites across urban, regional, and remote regions of Australia. We reported rates of eGFR decline in indigenous Australians to be approximately three times higher than found in studies from other populations. We confirmed the powerful predictive value of ACR in eGFR decline across diverse regions of Australia. Because ACR is a key modifiable risk factor associated with GFR decline, CKD management programs in this high-risk population should include regular ACR screening and targeted management approaches.
We observed a spectrum of rates of eGFR decline in this cohort, from age-related GFR loss of 1 ml/min per 1.73 m2 per year (as has been described in Europeans without diabetes aged >40 years [14]) to the more rapid GFR loss of 6 ml/min per 1.73 m2 per year in participants with ACR>265 mg/g (30 mg/mmol), regardless of eGFR strata. Hoy et al. described an even greater loss of 12 ml/min per 1.73 m2 per year in one remote Aboriginal community, associated with baseline albuminuria (ACR≥1770 mg/g [200 mg/mmol]); however, that was in a category of ACR higher than we have reported in the current study (5). Although studies from the United States consistently report higher incidence of ESRD among ethnic minorities, data on more rapid progression of CKD in ethnic minorities in the United States are conflicting (15). Data on CKD progression rates among Canadian First Nation populations are limited, with recent findings suggesting faster progression of kidney dysfunction leading to high rates of ESRD in that population (16).
Albuminuria was strongly associated with eGFR loss in both continuous and dichotomous outcome models. Diabetes, ACR, and lower GFR were strong independent factors associated with the combined renal outcome. These findings are consistent with previous work of Hoy et al. in a remote Australian community (4), studies among Pima Indians (6), and more broadly among high-risk population cohorts (where ACR and low GFR were the key factors) (17). Our findings of the important role of albuminuria are consistent with those of the Prevention of Renal and Vascular End-stage Disease study among Europeans (7,8) and those of international CKD cohorts (9).
We described a rapid eGFR loss of 6 ml/min per 1.73 m2 per year in participants with ACR>265 mg/g (30 mg/mmol) and eGFR≥90 ml/min per 1.73 m2. We are unable to comment on whether the eGFR decline in this group is related to possible normalization of hyperfiltration or commencement of decline related to CKD. Because the decline was greater in patients with high eGFR and ACR>30 mg/mmol than in those with high eGFR and normoalbuminuria, the decline may be related to CKD progression or to other factors, such as commencement of medications to block the renin-angiotensin system. We recently reported that the prevalence of hyperfiltration among indigenous Australians with normal renal function ranged from 7% to 27%, depending on the definition of hyperfiltration used (18); longer-term follow-up data are required to explore this further. The decline may be related to CKD progression, as it has been reported that during 8–12 years of follow-up, kidney function decline progressed in one third of patients with type 1 diabetes and microalbuminuria with normal or elevated baseline GFR (19).
We reported that the absolute annual eGFR decline of 3.0 ml/min per 1.73 m2 in participants with eGFR≥90 ml/min per 1.73 m2 was three times higher in this “healthy,” relatively young group than reported in studies from other populations (14,20). The relatively high rate of eGFR decline in the current study is consistent with higher rates of ESRD among indigenous Australians, with likely multiple contributory factors ranging from socioeconomic disadvantage, early life insults (low birthweight, post-streptococcal GN, recurrent infections), high rates of cigarette smoking, and high rates of metabolic syndrome and diabetes among this high-risk population (21–24).
High CRP was an additional factor associated with the combined renal outcome. Elevated CRP has been previously reported to be strongly associated with central obesity in remote Aboriginal populations (25), which in turn may be associated with hyperfiltration and thus eGFR decline. The potential role of central obesity is supported by a recent report that percentage body fat (rather than weight) was independently associated with eGFR decline in 615 healthy Koreans (26). Elevated CRP is a marker of inflammation and was independently associated with eGFR decline in the Multi-Ethnic Study of Atherosclerosis (27). Other traditional risk factors, such as BP, were not independently associated with kidney disease progression in our cohort, but BP values were within normal range; thus, the lack of association of BP with eGFR decline may be related to the relatively large group of “healthy” participants in this cohort.
A key strength of our study is that of a high follow-up rate that compares favorably with that seen in other studies. Longitudinal follow-up is a substantial challenge in indigenous health research. In a recent 6-year follow-up study of 1814 Indigenous Australian adults across 19 remote communities, 554 (30.5%) completed the follow-up health check (27). The eGFR Study team successfully followed up 66% of participants in person, 91% with a follow-up serum creatinine result, and 99% for vital status. The eGFR Study team includes indigenous researchers and indigenous research assistants and has formed strong relationships with communities and health services, working closely with these partners to optimize cohort follow-up. Other strengths are that we used standardized measurements (isotope-dilution mass spectrometry–aligned creatinine) and known accuracy of eGFR to reference GFR in this population (10). In addition, universal health care in Australia reduces the potential bias in this study compared with other studies of ethnic disparities in CKD progression (15); follow-up may be more thorough than in countries where disadvantaged populations may have reduced access to primary care. Thus, we are more likely to accurately estimate true rates of renal function decline and predictors. However, access to health care may be poorer for Indigenous than nonindigenous Australians in some settings despite universal health care (29).
We have used a minimum follow-up time of 6 months rather than the 2-year minimum proposed by Coresh et al. (12) because participants are from a population at high risk for ESRD and a high proportion progressed to an endpoint with <2 years of follow-up. We assessed participants with at least 6 months of follow-up duration and also performed a sensitivity analysis for those with at least 2 years of follow-up, which revealed similar results. A strength of our analysis is that a continuous outcome (eGFR decline) and a time-to-event combined renal endpoint were used, with results consistent between the two outcomes.
Limitations of the current study include that serum creatinine results from local laboratories were used in addition to centralized enzymatic creatinine results (to maximize follow-up numbers), but sensitivity analysis of those with enzymatic creatinine results was consistent. In addition, the small group without creatinine at follow-up was younger and generally healthier than patients with creatinine results at follow-up, which may limit the generalizability of our findings. Results for only two time points were available in the current analysis; this does not allow for assessment of nonlinear trends in eGFR decline, but trends have generally been reported to be linear in previous studies assessing multiple time points (19). Participants were volunteers recruited from clinics and the community, and as such there may have been selection bias in that those who volunteer to participate in studies such as this may be healthier and have attended a primary care clinic. A further limitation is that inclusion of a comparison group in the current study was not feasible.
Implications of our findings for CKD management programs in this high-risk population are to include regular urine ACR screening for all Indigenous Australians aged 18 years and older and targeted management approaches with renoprotective agents and monitoring to assess treatment adherence and response. Management of diabetes and hypertension remains a crucial part of CKD management programs. Other community-based approaches have been successful, such as the integrated model of care using community health workers for Maori and Pacific Islander participants with CKD and hypertension that resulted in improved BP control and albuminuria; these are key to developing targeted management approaches (30). In addition, incorporation of the rate of eGFR decline into the CKD definition may help identify high-risk patients (31). Longer-term follow-up of this cohort will further inform these clinical implications.
In conclusion, the results of the eGFR Follow-up Study, performed across >20 sites in urban, regional, and remote regions of Australia, confirm that among high-risk Indigenous Australian populations there is a rapid rate of decline in renal function and that ACR, diabetes, and level of kidney function are strong markers of progression of kidney damage.
Disclosures
G.R.D.J. has received travel and accommodation support from Roche Diagnostics to present at a scientific meeting.
Acknowledgments
The authors gratefully acknowledge the support of the eGFR Study participants, study staff, and partner organizations. We thank Dr. Kevin Warr and Dr. William Majoni for facilitating participant recruitment and follow-up at the sites of their employing organization and Loyla Leysley, Sian Graham, Mary Ward, and Joseph Fitz for assistance with follow-up in their communities. The authors also thank Melbourne Pathology for providing the technical support in the enzymatic creatinine analysis and Roche Diagnostics for supplying the enzymatic creatinine reagent kit for the baseline study.
The eGFR Study was funded by the Australian National Health and Medical Research Council (NHMRC; project grants #545202 and 1021460). The eGFR Study received additional support from NHMRC program grant #631947, Kidney Health Australia, Colonial Foundation, Rebecca L Cooper Foundation and SeaSwift, Thursday Island. L.J.M.-B. was supported by NHMRC fellowship #605837 and NHMRC practitioner fellowship #1078477, J.T.H. by NHMRC scholarship #490348, P.D.L. by NHMRC scholarship #1038529, A.C. by NHMRC principal research fellowship #1027204, R.R. by a fellowship with NHMRC program grant #631947, W.H. by NHMRC Australia fellowship #511081, and A.B. by a Charles and Sylvia Viertel senior medical research fellowship.
The views expressed in this publication are those of the authors and do not reflect the views of the NHMRC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09770915/-/DCSupplemental.
References
- 1.Cass A, Cunningham J, Wang Z, Hoy W: Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust 175: 24–27, 2001 [DOI] [PubMed] [Google Scholar]
- 2.McDonald S: Incidence and treatment of ESRD among indigenous peoples of Australasia. Clin Nephrol 74[Suppl 1]: S28–S31, 2010 [DOI] [PubMed] [Google Scholar]
- 3.McDonald S, Hurst K, eds: Thirty-fourth annual report. Australia and New Zealand Dialysis and Transplant Registry. Available at http://www.anzdata.org.au/v1/report_2011.html. Accessed June 1, 2015 [Google Scholar]
- 4.Hoy WE, Wang Z, VanBuynder P, Baker PR, McDonald SM, Mathews JD: The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int 60: 249–256, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Hoy WE, Wang Z, VanBuynder P, Baker PR, Mathews JD: The natural history of renal disease in Australian Aborigines. Part 1. Changes in albuminuria and glomerular filtration rate over time. Kidney Int 60: 243–248, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD Diabetic Renal Disease Study Group : Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 335: 1636–1642, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Halbesma N, Kuiken DS, Brantsma AH, Bakker SJ, Wetzels JF, De Zeeuw D, De Jong PE, Gansevoort RT: Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol 17: 2582–2590, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT PREVEND Study Group : Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 23: 3851–3858, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Maple-Brown LJ, Hughes JT, Lawton PD, Jones GR, Ellis AG, Drabsch K, Brown AD, Cass A, Hoy WE, MacIsaac RJ, O’Dea K, Jerums G: Accurate assessment of kidney function in indigenous Australians: The estimated GFR study. Am J Kidney Dis 60: 680–682, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Maple-Brown LJ, Lawton PD, Hughes JT, Sharma SK, Jones GR, Ellis AG, Hoy W, Cass A, Macisaac RJ, Sinha AK, Thomas MA, Piers LS, Ward LC, Drabsch K, Panagiotopoulos S, McDermott R, Warr K, Cherian S, Brown A, Jerums G, O’Dea K: Study Protocol--accurate assessment of kidney function in Indigenous Australians: Aims and methods of the eGFR study. BMC Public Health 10: 80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebholz CM, Grams ME, Matsushita K, Selvin E, Coresh J: Change in novel filtration markers and risk of ESRD. Am J Kidney Dis 66: 47–54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rius F, Pizaro E, Salinas I, Lucas A, Sanmarti A, Romero R: Age as a determinant of glomerular filtration rate in non-insulin-dependent diabetes mellitus. Nephrol Dial Transplant 10: 1644–1647, 1995 [PubMed] [Google Scholar]
- 15.Barbour SJ, Schachter M, Er L, Djurdjev O, Levin A: A systematic review of ethnic differences in the rate of renal progression in CKD patients. Nephrol Dial Transplant 25: 2422–2430, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Manns BJ, Culleton BF, Tonelli M, Quan H, Crowshoe L, Ghali WA, Svenson LW, Hemmelgarn BR Alberta Kidney Disease Network : Prevalence of chronic kidney disease and survival among aboriginal people. J Am Soc Nephrol 18: 2953–2959, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekinci EI, Hughes JT, Chatfield MD, Lawton PD, Jones GR, Ellis AG, Cass A, Thomas M, MacIsaac RJ, O’Dea K, Jerums G, Maple-Brown LJ: Hyperfiltration in Indigenous Australians with and without diabetes. Nephrol Dial Transplant 30: 1877–1884, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 18: 1353–1361, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, Krause I: A longitudinal assessment of the natural rate of decline in renal function with age [published online ahead of print March 19, 2014]. J Nephrol [DOI] [PubMed] [Google Scholar]
- 21.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hoy WE, Kincaid-Smith P, Hughson MD, Fogo AB, Sinniah R, Dowling J, Samuel T, Mott SA, Douglas-Denton RN, Bertram JF: CKD in Aboriginal Australians. Am J Kidney Dis 56: 983–993, 2010 [DOI] [PubMed] [Google Scholar]
- 23.White AV, Hoy WE, McCredie DA: Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Med J Aust 174: 492–496, 2001 [DOI] [PubMed] [Google Scholar]
- 24.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Shemesh T, Rowley KG, Jenkins A, Brimblecombe J, Best JD, O’Dea K: Differential association of C-reactive protein with adiposity in men and women in an Aboriginal community in northeast Arnhem Land of Australia. Int J Obes 31: 103–108, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kim JK, Song YR, Kwon YJ, Kim HJ, Kim SG, Ju YS: Increased body fat rather than body weight has harmful effects on 4-year changes of renal function in the general elderly population with a normal or mildly impaired renal function. Clin Interv Aging 9: 1277–1286, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiramoto JS, Katz R, Peralta CA, Ix JH, Fried L, Cushman M, Siscovick D, Palmas W, Sarnak M, Shlipak MG: Inflammation and coagulation markers and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 225–232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott RA, Li M, Campbell SK: Incidence of type 2 diabetes in two Indigenous Australian populations: A 6-year follow-up study. Med J Aust 192: 562–565, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Ong MA, Weeramanthri TS: Delay times and management of acute myocardial infarction in indigenous and non-indigenous people in the Northern Territory. Med J Aust 173: 201–204, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Hotu C, Bagg W, Collins J, Harwood L, Whalley G, Doughty R, Gamble G, Braatvedt G DEFEND Investigators : A community-based model of care improves blood pressure control and delays progression of proteinuria, left ventricular hypertrophy and diastolic dysfunction in Maori and Pacific patients with type 2 diabetes and chronic kidney disease: A randomized controlled trial. Nephrol Dial Transplant 25: 3260–3266, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]