Abstract
Background and objectives
Proteinuria is an independent predictor for IgA nephropathy (IgAN) progression. Urine albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio, and 24-hour urine protein excretion (UPE) are widely used for proteinuria evaluation in clinical practice. Here, we evaluated the association of these measurements with clinical and histologic findings of IgAN and explored which was the best predictor of IgAN prognosis.
Design, setting, participants, & measurements
Patients with IgAN were followed up for ≥12 months, were diagnosed between 2003 and 2012, and had urine samples available (438 patients). Spot urine ACR, protein-to-creatinine ratio, and 24-hour UPE at the time of renal biopsy were measured on a Hitachi Automatic Biochemical Analyzer 7180 (Hitachi, Yokohama, Japan).
Results
In our patients, ACR, protein-to-creatinine ratio, and 24-hour UPE were highly correlated (correlation coefficients: 0.71–0.87). They showed good relationships with acknowledged markers reflecting IgAN severity, including eGFR, hypertension, and the biopsy parameter (Oxford severity of tubular atrophy/interstitial fibrosis parameter). However, only ACR presented with positive association with the Oxford segmental glomerulosclerosis/adhesion parameter and extracapillary proliferation lesions. The follow-up time was 37.0 (22.0–58.0) months, with the last follow-up on April 18, 2014. In total, 124 patients reached the composite end point (30% eGFR decline, ESRD, or death). In univariate survival analysis, ACR consistently had better performance than protein-to-creatinine ratio and 24-hour UPE as represented by higher area under the curve using time–dependent survival analysis. When adjusted for well known risk factors for IgAN progression, ACR was most significantly associated with the composite end point (hazard ratio, 1.56 per 1-SD change of standard normalized square root–transformed ACR; 95% confidence interval, 1.29 to 1.89; P<0.001). Compared with protein-to-creatinine ratio and 24-hour UPE, addition of ACR to traditional risk factors resulted in more improvement in the predictive ability of IgAN progression (c statistic: ACR=0.70; protein-to-creatinine ratio =0.68; 24-hour UPE =0.69; Akaike information criterion: ACR=1217.85; protein-to-creatinine ratio =1229.28; 24-hour UPE =1234.96; P<0.001).
Conclusions
In IgAN, ACR, protein-to-creatinine ratio, and 24-hour UPE had comparable association with severe clinical and histologic findings. Compared with protein-to-creatinine ratio and 24-hour UPE, ACR showed slightly better performance in predicting IgAN progression.
Keywords: IgA nephropathy; albuminuria; proteinuria; creatinine; Follow-Up Studies; Glomerulonephritis, IGA; Humans; Kidney Failure, Chronic; Prognosis; Urinalysis
Introduction
IgA nephropathy (IgAN) represents a common type of primary GN characterized by mesangial IgA deposits (1,2). Patients with IgAN presented with variable clinical and pathologic findings ranging from asymptomatic microhematuria with mild mesangial proliferation to rapidly progressive GN with more than one half of glomerulus with crescentic lesion (3,4). As a highly heterogeneous disease, distinct progression was observed in IgAN. A small number of patients had rapid progression to chronic kidney failure, and about 30%–40% of patients progressed to ESRD in 10–20 years, whereas some kept stable renal function for many years (5).
Nephrologists and scientists have made a lot of effort in the past several years to get better predictions of IgAN prognosis. Population studies in IgAN widely proved proteinuria as one of the most important predictors for progression (6). Basic research suggested direct injury of proteinuria to renal tubulointerstitium (7). Using a rat model of kidney disease with protein overload, Shimizu et al. (8) revealed that urine protein plays a critical role in degradation and remodeling of the extracellular matrix as well as in infiltration of inflammatory cells, hence promoting the fibrosis process of renal tubulointerstitial.
Because proteinuria is a powerful and functional predictor of IgAN progression, it is essential to accurately evaluate the proteinuria levels for each patient. In current clinical practice, there are three measurements for proteinuria evaluation: urinary albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio, and 24-hour urine protein excretion (UPE). Although 24-hour UPE has been the most commonly used measurement for proteinuria in randomized, controlled clinical trials, 24-hour UPE has several limitations, including that it is inconvenient, cumbersome, and often imprecise because of errors in urine collection (9). Therefore, it is of vital importance to identify some simple, precise, and effective measurements to evaluate proteinuria. However, studies focused on comparison of these measurements are limited in regard to IgAN. Here, we evaluated the association of ACR, protein-to-creatinine ratio, and 24-hour UPE with clinical and histologic findings of IgAN and further explored the best measurement in predicting IgAN prognosis.
Materials and Methods
Study Population
In total, 438 patients with IgAN (238 men and 200 women) at Peking University First Hospital with regular follow-up of ≥12 months were recruited in this study. Diagnosis of IgAN was on the basis of the presence of dominant IgA deposition in mesangium by immunofluorescence, and it was confirmed by light microscopy and electronic microscopy. Patients with Henoch–Schönlein purpura, liver cirrhosis, and other secondary etiologies of IgAN were excluded after detailed clinical and laboratory examinations. During follow-up, patients received a therapy regimen according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (10).
The study protocol was reviewed and approved by the Ethics Committee of Peking University, and written informed consent was obtained from all participants.
Detection of ACR, Protein-to-Creatinine Ratio, and 24-Hour UPE
For detection of ACR and protein-to-creatinine ratio, early morning spot urine samples were used. On the day of renal biopsy, urine samples were collected and centrifuged immediately at 1800 rpm and 4°C for 5 minutes. Then, supernatants were aliquoted and stored at −80°C until assays were performed. Batch detection of urinary albumin, urinary protein, and urinary creatinine of these samples was performed on a Hitachi Automatic Biochemical Analyzer 7180 (Hitachi, Yokohama, Japan) using the immune transmission turbidity method, the pyrogallol red colorimetric method, and the reaction rate Jaffe method, respectively. ACR and protein-to-creatinine ratio were calculated by urinary creatinine divided by urinary albumin or urinary protein, respectively. For urine albumin, protein, and creatinine, the intra–assay coefficients of variation were 2.6%, 4.7%, and 2.2%, respectively, and interassay coefficients of variation were 3.4%, 5.8%, and 2.6%, respectively.
Information regarding 24-hour UPE was collected from medical records. During hospitalization, 24-hour urine collections were performed by standardized procedures. Briefly, the day before 24-hour urine collection, patients would be trained by nurses on how to collect urine between 7:00 a.m. on day 1 and 7:00 a.m. on day 2, uniformly mix 24-hour urine, and measure 24-hour urine volume using a standardized cylinder. After training, collection of urine samples was by patients. The protein concentration for calculating 24-hour UPE was also measured by a Hitachi Automatic Biochemical Analyzer 7180 using the pyrogallol red colorimetric method as described above; 24-hour UPE values were calculated by protein concentration times self-reported 24-hour urine volume.
Information on Clinical and Pathologic Manifestations
For enrolled patients, information and clinical manifestations, including age, sex, BP, serum creatinine, and 24-hour UPE, were collected from medical records. The eGFR was estimated according to the Chronic Kidney Disease Epidemiology Collaboration equation (11). Hypertension were defined as systolic BP (SBP) of ≥140 mmHg, diastolic BP of ≥90 mmHg, or use of antihypertensive drugs. Oxford classification was defined by four pathology features: mesangial hypercellularity score (M; M0≤0.5; M1>0.5), the presence of endocapillary proliferation (E; E0: absent; E1: present), segmental glomerulosclerosis/adhesion (S; S0: absent; S1: present), and severity of tubular atrophy/interstitial fibrosis (T; T0<25%; T1=26%–50%; T2>50%) (12), and they were used to evaluate pathologic lesions of renal biopsy samples, which were graded by a pathologist blinded to patients’ clinical data and outcomes. The prognostic significance of extracapillary proliferation (Ep) has been recently reported; therefore, evaluation of Ep was also included, in which Ep graded zero and one represented the absence and presence of active crescents, respectively (13).
For the analysis of time to event data, a composite end point, defined as 30% eGFR decline, ESRD, or death (whichever occurred first), was used (14,15). ESRD was defined as eGFR<15 ml/min per 1.73 m2 or need for RRT (including hemodialysis, peritoneal dialysis, or renal transplantation) for the purpose of this study.
Statistical Analyses
For data description, normally distributed quantitative variables were expressed as means±SDs. For non–normally distributed variables, medians and interquartile ranges were used. Categorical data were summarized as absolute frequencies and percentages. Furthermore, ACRs were extracted from the square root, whereas protein-to-creatinine ratio and 24-hour UPE were natural log transformed to make the transformed data resemble normal distribution. Then, they were scaled to meet standard normal distribution for the purpose of comparison. Correlation analysis and Kaplan–Meier survival analysis were performed with SPSS software (version 16.0; SPSS Inc., Chicago, IL). For survival analysis, Cox proportional hazards models were used. At first, ACR, protein-to-creatinine ratio, and 24-hour UPE were analyzed separately for their associations with the composite outcome. Then, some well known risk factors for IgAN progression, including SBP, oxford classification (M, E, S, and T), and Ep, were adjusted to evaluate the independent effects of ACR, protein-to-creatinine ratio, and 24-hour UPE on IgAN progression. For time–dependent survival analysis, survival package and survivalROC package in R (version 3.0.2) were used, respectively. A two–tailed P value <0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics of Patients with IgAN
Among 438 enrolled patients with IgAN, 238 (54.3%) were men, and 200 (45.7%) were women (Table 1). Their mean age at renal biopsy was 35.6±12.4 (range =14–83) years old. At the time of renal biopsy, patients presented with an average eGFR of 82.1±27.4 ml/min per 1.73 m2. The mean SBP was 124±16 mmHg, whereas diastolic BP was 79±12 mmHg. The ACR, protein-to-creatinine ratio, and 24-hour UPE levels were 460.1 (241.3–743.4) mg/g, 0.71 (0.29–1.59) g/g, and 1.60 (0.88–3.13) g/d, respectively. Regarding pathologic Oxford classifications of renal biopsy samples, M1, E1, and S1 were found in 82.3%, 49.2%, and 68.0% of patients, respectively. For Oxford T scores, 62.1%, 25.7%, and 12.2% patients had T0, T1, and T2 lesions, respectively. In these patients, 51.4% had Ep. All of the patients were regularly followed up, with the mean follow-up time of 37.0 (interquartile range, 22.0–58.0) months. During follow-up, 424 (96.8%) patients received angiotensin–converting enzyme inhibitors or angiotensin II receptor blocker therapy, whereas 175 (40.0%) received oral corticosteroids or other immunosuppressive agents. In total, 124 patients reached the composite end point (all of them reached 30% eGFR decline first; among them, 26 reached ESRD, and three died during follow-up).
Table 1.
Baseline clinical and laboratory data of patients with IgA nephropathy
| Characteristics | Mean±SD or Median (IQR) |
|---|---|
| Baseline | |
| Age, yr | 35.6±12.4 |
| Sex, men/women | 238 (54.3%)/200 (45.7%) |
| 24-h UPE, g/d | 1.60 (0.88–3.13) |
| <0.3 | 19/438 (4.3%) |
| 0.3–0.99 | 110/438 (25.1%) |
| 1.0–2.99 | 194/438 (44.3%) |
| ≥3.0 | 115/438 (26.3%) |
| ACR, mg/g | 460.1 (241.3–743.4) |
| <30 | 14/438 (3.2%) |
| ≥30 | 424/438 (96.8%) |
| Protein-to-creatinine ratio, g/g | 0.71 (0.29–1.59) |
| <0.2 | 75/438 (17.1%) |
| ≥0.2 | 363/438 (82.9%) |
| eGFR, ml/min per 1.73 m2 | 82.1±27.4 |
| CKD stagesa | |
| 1 | 191 (43.6%) |
| 2 | 147 (33.6%) |
| 3 | 83 (18.9%) |
| 4 | 17 (3.9%) |
| SBP, mmHg | 124±16 |
| DBP, mmHg | 79±12 |
| Oxford classificationb | |
| M1 | 358 (82.3%) |
| E1 | 214 (49.2%) |
| S1 | 296 (68.0%) |
| T1/T2 | 112 (25.7%)/53 (12.2%) |
| Extracapillary proliferation | 225 (51.4%) |
| Follow-up | |
| Follow-up interval, mo | 37.0 (22.0–58.0) |
| Therapy | |
| ACE inhibitors or ARBs | 424 (96.8%) |
| Prednisone and any other immunosuppressive agents (cyclophosphamide, MMF, or others) | 175 (40.0%) |
IQR, interquartile range; UPE, urinary protein excretion; ACR, albumin-to-creatinine ratio; SBP, systolic BP; DBP, diastolic BP; M1, Oxford classification for mesangial hypercellularity >0.5; E1, Oxford classification for presence of endocapillary proliferation; S1, Oxford classification for presence of segmental glomerulosclerosis/adhesion; T1/T2, Oxford classification for severity of tubular atrophy/interstitial fibrosis of 26%–50% and >50%; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; MMF, mycophenolate mofetil.
CKD stages 1–4 were divided by eGFR≥90, =60–89, =30–59, and =15–29 ml/min per 1.73 m2, respectively, according to the Kidney Disease Outcomes Quality Initiative.
Oxford classification was developed by the Working Group of the International IgA Nephropathy Network and the Renal Pathology Society.
Correlation between ACR, Protein-to-Creatinine Ratio, and 24-Hour UPE
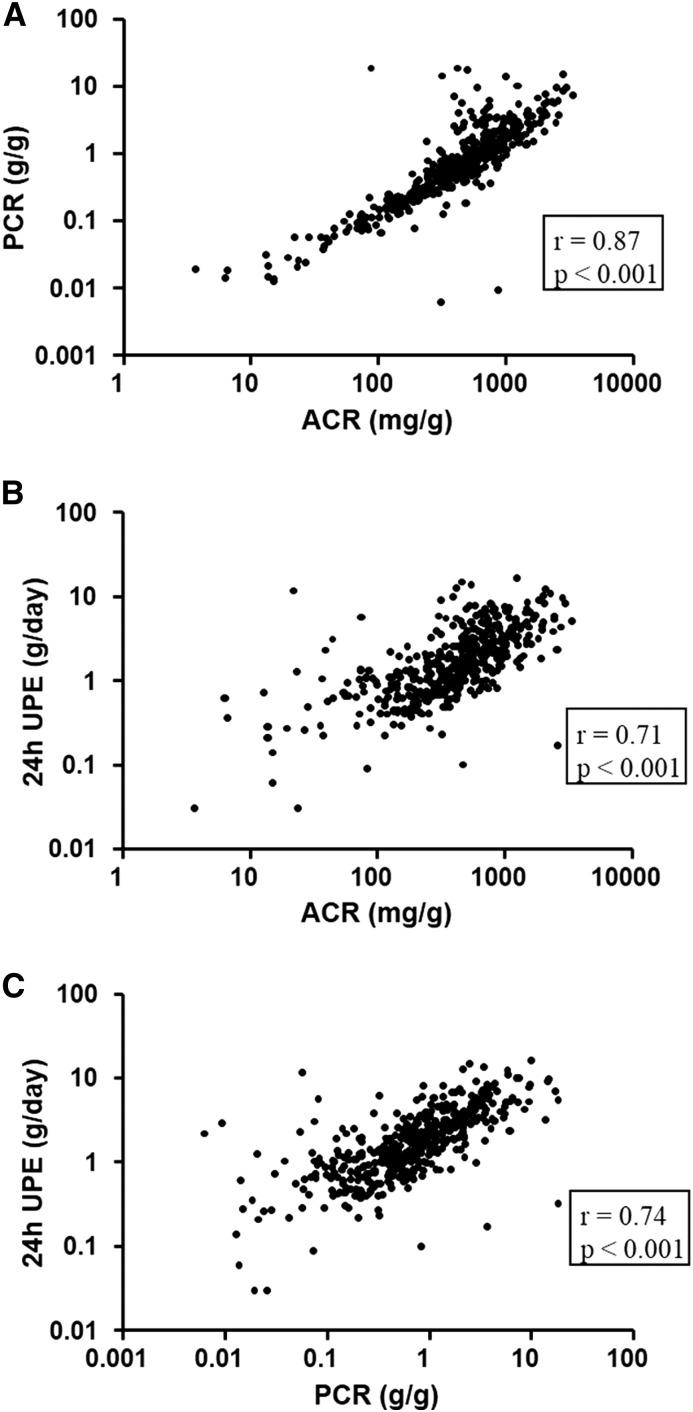
In patients with IgAN, ACR, protein-to-creatinine ratio, and 24-hour UPE showed high positive correlation (Figure 1). Among them, ACR and protein-to-creatinine ratio had the best correlation (r=0.87; P<0.001) (Figure 1A). Compared with higher ranges of proteinuria, ACR and protein-to-creatinine ratio correlated better at lower ranges of proteinuria (Figure 1A). On the contrary, for 24-hour UPE, its correlation with ACR or protein-to-creatinine ratio was worse at lower ranges of proteinuria (Figure 1, B and C).
Figure 1.
Correlation among albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), and 24-hour urine protein excretion (UPE). Scatter plots show correlations (A) between ACR and PCR, (B) between ACR and 24-hour UPE, and (C) between PCR and 24-hour UPE.
Association of ACR, Protein-to-Creatinine Ratio, and 24-Hour UPE with the Severity of IgAN
A cross-section analysis regarding the correlation of urinary ACR, protein-to-creatinine ratio, and 24-hour UPE levels with clinical and histologic manifestations was performed at the time of renal biopsy. ACR, protein-to-creatinine ratio, and 24-hour UPE all showed good relationships with CKD stages, hypertension, and Oxford T scores. Patients with severe CKD stage, hypertension, and Oxford T2 score showed higher ACR, protein-to-creatinine ratio, and 24-hour UPE values compared with those with mild CKD stage without hypertension and without tubular and interstitial lesions, and their P values of ACR were smaller. In addition, ACR but not protein-to-creatinine ratio and 24-hour UPE showed positive association with Oxford S scores and Ep lesions. For other histologic lesions, including Oxford M and E scores, comparable levels of ACR, protein-to-creatinine ratio, and 24-hour UPE were observed among patients with different severities of these lesions (Table 2).
Table 2.
Correlation between albumin-to-creatinine ratio, protein-to-creatinine ratio, and 24-hour urinary protein excretion with severity of IgA nephropathy
| Characteristics | ACR | Protein-to-Creatinine Ratio | 24-h UPE | |||
|---|---|---|---|---|---|---|
| Level, mg/g | P Value | Level, g/g | P Value | Level, g/d | P Value | |
| CKD stagea | <0.001 | <0.001 | <0.001 | |||
| 1 | 321.5 (164.2, 544.9) | 0.50 (0.21, 0.99) | 1.18 (0.67, 2.33) | |||
| 2 | 472.2 (261.8, 730.2) | 0.71 (0.31, 1.59) | 1.44 (0.93, 2.91) | |||
| 3 | 703.1 (380.6, 1031.2) | 1.11 (0.57, 2.15) | 2.20 (1.44, 3.60) | |||
| 4 | 1218.4 (894.1, 1864.7) | 3.44 (1.43, 6.28) | 4.60 (3.03, 6.66) | |||
| Hypertension | <0.001 | <0.001 | <0.001 | |||
| Without | 398.5 (186.5, 668.8) | 0.56 (0.22, 1.17) | 1.38 (0.77, 2.60) | |||
| With | 612.5 (373.7, 955.2) | 1.08 (0.56, 2.16) | 2.37 (1.22, 4.06) | |||
| Oxford classification | ||||||
| M lesion | 0.13 | 0.65 | 0.16 | |||
| 0 | 415.3 (179.2, 693.9) | 0.64 (0.22, 1.59) | 1.30 (0.74, 3.08) | |||
| 1 | 470.6 (246.8, 767.2) | 0.71 (0.31, 1.60) | 1.69 (0.94, 3.14) | |||
| E lesion | 0.94 | 0.87 | 0.74 | |||
| 0 | 468.0 (226.5, 740.0) | 0.71 (0.30, 1.53) | 1.48 (0.88, 3.18) | |||
| 1 | 455.6 (243.7, 759.5) | 0.70 (0.29, 1.71) | 1.71 (0.84, 3.11) | |||
| S lesion | 0.03 | 0.67 | 0.92 | |||
| 0 | 370.7 (163.9, 768.4) | 0.69 (0.21, 1.81) | 1.53 (0.78, 3.72) | |||
| 1 | 484.3 (269.2, 744.4) | 0.71 (0.33, 1.50) | 1.67 (0.91, 3.02) | |||
| T lesion | <0.001 | <0.001 | <0.001 | |||
| 0 | 379.3 (176.8, 616.4) | 0.56 (0.22, 1.20) | 1.30 (0.74, 2.64) | |||
| 1 | 528.0 (334.0, 846.4) | 0.82 (0.41, 1.67) | 1.93 (1.11, 3.50) | |||
| 2 | 777.9 (592.7, 1277.6) | 1.64 (0.76, 2.89) | 2.89 (1.52, 4.70) | |||
| Extracapillary proliferation | ||||||
| 0 | 408.5 (192.2, 709.9) | 0.02 | 0.63 (0.23, 1.43) | 0.13 | 1.44 (0.76, 3.06) | 0.17 |
| 1 | 503.3 (264.4, 803.9) | 0.75 (0.35, 1.71) | 1.72 (0.97, 3.21) | |||
Values are shown in median (interquartile range). ACR, albumin-to-creatinine ratio; UPE, urinary protein excretion; M lesion, mesangial hypercellularity score; E lesion, presence of endocapillary proliferation; S lesion, segmental glomerulosclerosis/adhesion; T lesion, severity of tubular atrophy/interstitial fibrosis.
CKD stages 1–4 were divided by eGFR≥90, =60–89, =30–59, and =15–29 ml/min per 1.73 m2, respectively, according to the Kidney Disease Outcomes Quality Initiative.
ACR, Protein-to-Creatinine Ratio, and 24-Hour UPE in Predicting IgAN Progression
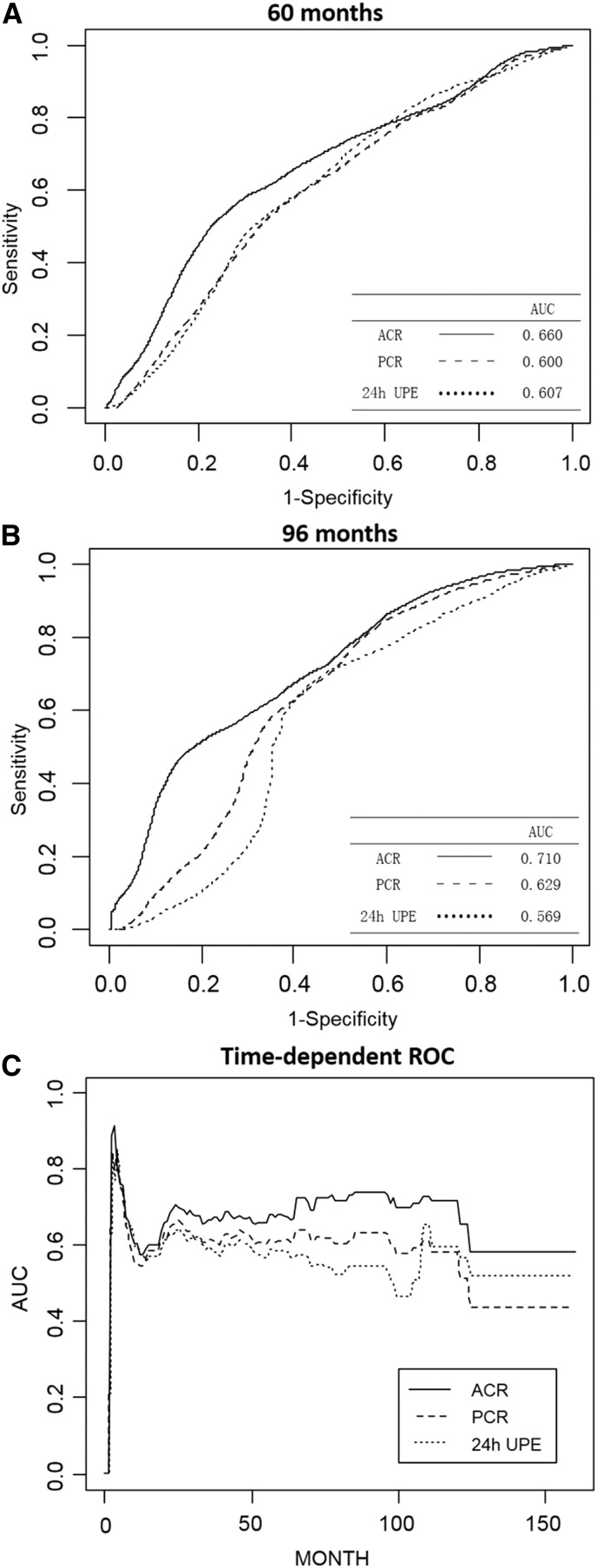
To evaluate the effect of ACR, protein-to-creatinine ratio, and 24-hour UPE on predicting IgAN progression, we first performed univariate survival analysis. We found that ACR, protein-to-creatinine ratio, and 24-hour UPE were associated with the composite end point (unadjusted analysis: ACR: hazard ratio [HR], 1.62; 95% confidence interval [95% CI], 1.36 to 1.93; P<0.001; protein-to-creatinine ratio: HR, 1.37; 95% CI, 1.14 to 1.64; P=0.001; 24-hour UPE: HR, 1.18; 95% CI, 0.99 to 1.41; P=0.06) (Table 3). Comparing the performances of ACR, protein-to-creatinine ratio, and 24-hour UPE in predicting IgAN prognosis, ACR had consistently better performance than the two other measurements as represented by higher values of area under the curve according to time–dependent sensitivity and specificity evaluation using time–dependent survival analysis (Figure 2). Furthermore, when we divided patients into four subgroups according to their respective quartiles of ACR, protein-to-creatinine ratio, and 24-hour UPE distribution, although all three measurements showed significant association with the composite end point (ACR, protein-to-creatinine ratio, and 24-hour UPE: P<0.001, P=0.002, and P<0.01, respectively), the Kaplan–Meier survival curves of the first and second quartiles of protein-to-creatinine ratio crossed. Meanwhile, first, second, and third quartiles of 24-hour UPE also crossed, and only the four survival curves of ACR subgroups showed no cross (Figure 3). Results from univariate survival analysis suggested ACR as a potential valuable predictor to IgAN progression.
Table 3.
Risks of the composite end point with standard normalization–transformed albumin-to-creatinine ratio, protein-to-creatinine ratio, and 24-hour urinary protein excretion
| Groups | Median (Range) | Unadjusted | Hazard Ratio (95% Confidence Interval) and P Value | ||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| Composite end point | 460.1 (3.7–3357.6) mg/g | 1.62 (1.36 to 1.93) | 1.61 (1.35 to 1.92) | 1.64 (1.36 to 1.96) | 1.56 (1.29 to 1.89) |
| Per 1 SD nACR | <0.001 | <0.001 | <0.001 | <0.001 | |
| Composite end point | 0.71 (0.01–18.54) g/g | 1.37 (1.14 to 1.64) | 1.37 (1.14 to 1.65) | 1.38 (1.14 to 1.69) | 1.35 (1.10 to 1.66) |
| Per 1 SD nprotein-to-creatinine ratio | 0.001 | 0.001 | 0.001 | 0.004 | |
| Composite end point | 1.60 (0.03–16.40) g/d | 1.18 (0.99 to 1.41) | 1.21 (1.01 to 1.45) | 1.19 (0.99 to 1.44) | 1.19 (0.97 to 1.45) |
| Per 1 SD n24-h UPE | 0.06 | 0.04 | 0.07 | 0.10 | |
The composite end point was defined as a 30% decline of eGFR, ESRD, or death. The unadjusted model analyzed nACR, nprotein-to-creatinine ratio, and n24-hour UPE as continuous data. nACR, square root–extracted and standard normalization–transformed albumin-to-creatinine ratio; nprotein-to-creatinine ratio, natural log– and standard normalization–transformed protein-to-creatinine ratio; n24-hour UPE, natural log– and standard normalization–transformed 24-hour urinary protein excretion.
Model 1 was adjusted for sex and age. Sex was analyzed as dichotomous data, and age was natural log transformed.
Model 2 was adjusted for the covariates in model 1 plus eGFR and systolic BP.
Model 3 was adjusted for the covariates in model 2 plus Oxford classification and extracapillary proliferation. The variables were analyzed as categorical data. Oxford classification includes mesangial hypercellularity score (>0.5), the presence of endocapillary proliferation, the presence of segmental glomerulosclerosis/adhesion, and the severity of tubular atrophy/interstitial fibrosis (T1=26%–50%; T2>50%).
Figure 2.
Time–dependent receiver operating characteristics (ROC) curves with composite outcome as the status variable. The areas under the receiver operating characteristics curve (AUCs) were compared among albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), and 24-hour urine protein excretion (UPE) at (A) 60 and (B) 96 months and (C) against each time during follow-up. Using composite end point as a status variable, ACR showed consistently higher AUCs at each survival time during follow-up compared with PCR and 24-hour UPE.
Figure 3.
Kaplan–Meier renal survival curves of patients with IgA nephropathy according to albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), and 24-hour urine protein excretion (UPE) levels. The patients were divided into four equal groups according to the quartiles of ACR, PCR, and 24-hour UPE distribution. Patients with IgA nephropathy in the first quartile group had the highest proteinuria, whereas those in the fourth quartile group had the lowest proteinuria. Kaplan–Meier survival analyses were performed to compare patients with the composite outcome in each group with different (A) ACR, (B) PCR, or (C) 24-hour UPE values.
Because ACR, protein-to-creatinine ratio, and 24-hour UPE had significant correlation with multiple well known risk factors for IgAN progression, we then applied an adjusted Cox proportional hazards model. When adjusted for eGFR, hypertension, and histologic lesions, ACR, protein-to-creatinine ratio, and 24-hour UPE still had significant association with poor renal outcome of IgAN (ACR: HR, 1.56; 95% CI, 1.29 to 1.89; P<0.001; protein-to-creatinine ratio: HR, 1.35; 95% CI, 1.10 to 1.66; P=0.004; 24-hour UPE: HR, 1.19; 95% CI, 0.97 to 1.45; P=0.10). Compared with protein-to-creatinine ratio and 24-hour UPE, ACR showed more robust association with poor renal outcome as represented by higher HR values. These results implied that ACR was an independent risk factor for IgAN progression.
For such a complex disease as IgAN, multiple risk factors proved to be involved in disease progression. Therefore, it is reasonable to develop a multivariable model for IgAN prognosis prediction. In survival analysis, compared with protein-to-creatinine ratio and 24-hour UPE, addition of ACR to the base prediction model (base model included age; sex; eGFR; SBP; Oxford M, E, S, and T scores; and Ep) resulted in more improvement in model fit as illustrated by an increase in c statistic from 0.68 to 0.70 and an Akaike information criterion decrease from 1235.78 to 1217.85 (Table 4).
Table 4.
Comparison of four models of survival analysis
| Model | c Statistic (95% CI) | AIC | Models Compared | P Value (LR Test) |
|---|---|---|---|---|
| 0: Base model of agea + sex + eGFR + SBP + M + E + S + T + Ep | 0.68 (0.64 to 0.72) | 1235.78 | — | |
| 1: Base model + nACR | 0.70 (0.67 to 0.74) | 1217.85 | 1 versus 0 | <0.001 |
| 2: Base model + nprotein-to-creatinine ratio | 0.68 (0.64 to 0.73) | 1229.28 | 2 versus 0 | 0.004 |
| 3: Base model + n24-h UPE | 0.69 (0.65 to 0.73) | 1234.96 | 3 versus 0 | 0.09 |
95% CI, 95% confidence interval; AIC, Akaike information criterion; LR, likelihood ratio; SBP, systolic BP; M, mesangial hypercellularity score; E, the presence of endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; Ep, extracapillary proliferation; nACR, square root–extracted and standard normalization–transformed albumin-to-creatinine ratio; nprotein-to-creatinine ratio, natural log– and standard normalization–transformed protein-to-creatinine ratio; n24-hour UPE, natural log– and standard normalization–transformed 24-hour urinary protein excretion.
Age was natural log transformed.
Discussion
In the clinical practice of treating IgAN, accurate identification and quantification of proteinuria are core elements. ACR, protein-to-creatinine ratio, and 24-hour UPE are commonly used biomarkers for proteinuria evaluation in IgAN. However, there is uncertainty regarding the best measurement of urinary protein excretion, which is of clinical importance from practical and cost-effectiveness perspectives. Here, in an IgAN cohort, we showed that ACR, protein-to-creatinine ratio, and 24-hour UPE were highly correlated. In respect to prediction of IgAN prognosis, ACR showed the best correlation with the long–term renal outcome.
The most common assessment method of proteinuria is 24-hour UPE. Concerns with 24-hour UPE include inconvenience and patient forgetfulness, which often lead to incomplete collections and consequently, inaccurate assessment of proteinuria (9). Furthermore, handling and analysis of 24-hour urine samples open the assessment to error, which is greater than that in alternative approaches. Meanwhile, ACR and protein-to-creatinine ratio can be calculated from spot urine samples; they are both more precise and convenient than 24-hour samples, and a number of studies has shown the ability of both protein-to-creatinine ratio and ACR to rule out significant proteinuria (16) and predict renal outcomes and mortality (17,18) in patients with CKD. Towler et al. (19) suggested that protein-to-creatinine ratio and ACR were comparable with 24-hour UPE in terms of both agreement and repeatability. Thus, ACR and protein-to-creatinine ratio are quick and convenient alternatives to 24-hour UPE, and currently, they are advocated for by key guideline groups. In our patients with IgAN, both ACR and protein-to-creatinine ratio showed good positive correlation with 24-hour UPE, which is in agreement with previous studies that also supported their potential usage in clinical practice of IgAN. Nevertheless, currently, there is not a study for evaluation of whether ACR or protein-to-creatinine ratio is better for predicting severity and outcome of IgAN compared with 24-hour UPE.
As for CKD, studies comparing ACR and protein-to-creatinine ratio yielded conflicting results. Some prior studies suggested that ACR was better, because it was more specific, more sensitive, and better standardized than protein-to-creatinine ratio (20–23). However, some recent studies reported that protein-to-creatinine ratio was more sensitive when proteinuria was <0.5 and <1.0 g/d (24). Some other reports suggested that protein-to-creatinine ratio was comparable with ACR (17,25). Regarding proteinuria measurement, recommendations from different guidelines are also different. National Institute for Health and Clinical Excellence guidelines recommend ACR for proteinuria detection but accept protein-to-creatinine ratio as an alternative. Recently published KDIGO guidelines strongly advocate for ACR, partly because of substantial sample to sample and between-laboratory variations of protein-to-creatinine ratio resulting from different quantities and compositions of urinary proteins (10). In our IgAN cohort, ACR, protein-to-creatinine ratio, and 24-hour UPE showed good relationships, with acknowledged indicators reflecting disease severity, such as eGFR, hypertension, and Oxford T lesions. In addition, ACR was the only indicator associated with the presence of Oxford S lesions and Ep. Hence, as a predictor of IgAN severity, ACR seems slightly superior to protein-to-creatinine ratio and 24-hour UPE.
A limited number of studies has examined associations of ACR and protein-to-creatinine ratio with longitudinal outcomes in CKD. A meta-analysis conducted by the Chronic Kidney Disease Consortium concluded that there were no significant differences in the associations of protein-to-creatinine ratio or ACR with mortality or ESRD in patients with CKD (26). However, another study that enrolled 700 patients with diabetes found that ACR was superior in predicting doubling of creatinine or ESRD compared with proteinuria from 24-hour urine collections (18). To the best of our knowledge, our study is the first to compare different proteinuria measurements of patients with IgAN with regard to their predictive values in disease prognosis. Our results indicated that ACR was superior to 24-hour UPE and protein-to-creatinine ratio in predicting IgAN prognosis. In Kaplan–Meier survival curves, it is obvious that the survival rate could be distinguished clearly by quartiles of ACR, but for protein-to-creatinine ratio and 24-hour UPE, the quartile curves crossed. In additional evaluation by time–dependent receiver operating characteristics analysis, ACR performed consistently better than either of the other measurements. Additionally, in a Cox regression model, we showed that ACR could serve as a more robust independent prognostic factor for IgAN progression than protein-to-creatinine ratio and 24-hour UPE after adjustment for baseline risk factors. These results implied better performance of ACR over protein-to-creatinine ratio and 24-hour UPE for predictive value to IgAN progression. Moreover, when combined with other risk factors, such as age, sex, eGFR, SBP, Oxford classification, and Ep, the overall performance of the predictive model increased the most when adding ACR, which suggested that ACR was a valuable noninvasive biomarker in the construct of a multivariable, complex IgAN predictive model (better than protein-to-creatinine ratio and 24-hour UPE).
Other than for our survival analysis results, some other advantages were reported for ACR. Urinary measurement of protein-to-creatinine ratio includes albumin and other proteins; thus, measurement of ACR alone may be not comprehensive (27). However, nonalbumin proteins of various molecular weights in urinary proteins vary widely in pathologic states. For measurement, nonalbumin proteins are less well defined compared with albumin. Thus, we observed less inter- and intra-assay variation in albumin detection than in total protein detection, the same as was previously reported (22). Moreover, albuminuria was reported to be more sensitive than proteinuria in detecting CKD (27). In our patients with IgAN, 96.8% had elevated ACR, whereas 82.9% had abnormal protein-to-creatinine ratio. The proportion of patients with IgAN and proteinuria identified by ACR was higher than the proportion of patients with IgAN and proteinuria identified by protein-to-creatinine ratio, which suggested better sensitivity of ACR in IgAN. Additionally, previous comparison studies between spot and 24-hour urine tests suggested that spot urine detection was superior because of its strong association with poor outcomes (28,29). Although 24-hour urinary albumin excretion data were unavailable in our IgAN cohort, an analysis of the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan Study reported better performance of spot ACR to loss of GFR than 24-hour urinary albumin excretion (18). In our IgAN cohort, we indeed observed an association between low urinary creatinine values and our composite end point. We suspected that individuals with lower urinary creatinine showed relatively poor health, which then associated with poor renal outcomes. Therefore, low urine creatinine, in turn, increases ACR values and in part, contributes to the better predictive value of the spot urine ACR.
Our study has a few limitations as well. First, all patients participating in our study were from a single center; therefore, our results should be validated in other independent IgAN cohorts before its application in clinical practice. Second, because IgAN is a complex disease with slow progression, <10% of patients in our cohort reached ESRD. Therefore, we adopted a composite end point in survival analysis. Future verification analysis to compare ACR and other proteinuira measurements should be performed by using a more convincing end point (such as ESRD alone). Third, in our cohort, there was a period of time between 24-hour urine collection for protein and kidney biopsy, which might lower the predictive ability of 24-hour urine results. However, considering the short period of time (average time interval was 4 days), the influence on survival analysis is limited.
In conclusion, we found that ACR, protein-to-creatinine ratio, and 24-hour UPE had comparable associations with severe clinical and histologic findings in IgAN. Compared with protein-to-creatinine ratio and 24-hour UPE, ACR showed slightly better performance in predicting IgAN progression.
Disclosures
None.
Acknowledgments
This work was supported by Major State Basic Research Development Program of China 973 Program grant 2012CB517700, Natural Science Foundation for Innovation Research Group of China grant 81321064, Capital of Clinical Characteristics and Applied Research Fund grant Z141107002514037, National Science Foundation of China grant 81470945, and Beijing Natural Science Foundation grant 7131016.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.D’Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Coppo R, Amore A: Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int 65: 1544–1547, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J: Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol 37: 1047–1056, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Schena FP: A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med 89: 209–215, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Reich HN, Troyanov S, Scholey JW, Cattran DC Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR: MCP-1 induces inflammatory activation of human tubular epithelial cells: Involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol 13: 1534–1547, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Shimizu H, Maruyama S, Yuzawa Y, Kato T, Miki Y, Suzuki S, Sato W, Morita Y, Maruyama H, Egashira K, Matsuo S: Anti-monocyte chemoattractant protein-1 gene therapy attenuates renal injury induced by protein-overload proteinuria. J Am Soc Nephrol 14: 1496–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Price CP, Newall RG, Boyd JC: Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin Chem 51: 1577–1586, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cattran DC, Feehally J, Cook HT, Liu ZH, Fervenza FC, Mezzano SA, Floege J, Nachman PH, Gipson DS, Praga M, Glassock RJ, Radhakrishnan J, Hodson EM, Rovin BH, Jha V, Troyanov S, Li PKT, Wetzels JFM: Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H: Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin J Am Soc Nephrol 6: 2806–2813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Guy M, Borzomato JK, Newall RG, Kalra PA, Price CP: Protein and albumin-to-creatinine ratios in random urines accurately predict 24 h protein and albumin loss in patients with kidney disease. Ann Clin Biochem 46: 468–476, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Methven S, MacGregor MS, Traynor JP, Hair M, O’Reilly DS, Deighan CJ: Comparison of urinary albumin and urinary total protein as predictors of patient outcomes in CKD. Am J Kidney Dis 57: 21–28, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Lambers Heerspink HJ, Gansevoort RT, Brenner BM, Cooper ME, Parving HH, Shahinfar S, de Zeeuw D: Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol 21: 1355–1360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towler JD, Dhaun N, MacDougall M, Melville V, Goddard J, Webb DJ: What is the best method of proteinuria measurement in clinical trials of endothelin receptor antagonists? Life Sci 91: 733–738, 2012 [DOI] [PubMed] [Google Scholar]
- 20.McIntyre NJ, Taal MW: How to measure proteinuria? Curr Opin Nephrol Hypertens 17: 600–603, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Guh JY: Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 15[Suppl 2]: 53–56, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Lamb EJ, MacKenzie F, Stevens PE: How should proteinuria be detected and measured? Ann Clin Biochem 46: 205–217, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ballantyne FC, Gibbons J, O’Reilly DS: Urine albumin should replace total protein for the assessment of glomerular proteinuria. Ann Clin Biochem 30: 101–103, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Methven S, MacGregor MS, Traynor JP, O’Reilly DS, Deighan CJ: Assessing proteinuria in chronic kidney disease: Protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant 25: 2991–2996, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Fisher H, Hsu CY, Vittinghoff E, Lin F, Bansal N: Comparison of associations of urine protein-creatinine ratio versus albumin-creatinine ratio with complications of CKD: A cross-sectional analysis. Am J Kidney Dis 62: 1102–1108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins RC, Briganti EM, Zimmet PZ, Chadban SJ: Association between albuminuria and proteinuria in the general population: The AusDiab Study. Nephrol Dial Transplant 18: 2170–2174, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Carter CE, Gansevoort RT, Scheven L, Heerspink HJ, Shlipak MG, de Jong PE, Ix JH: Influence of urine creatinine on the relationship between the albumin-to-creatinine ratio and cardiovascular events. Clin J Am Soc Nephrol 7: 595–603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]