Abstract
Background and objectives
We hypothesized that longitudinal changes in uric acid (UA) may have independent associations with changes in nutritional parameters over time and consequently, long-term survival of patients on maintenance hemodialysis.
Design, setting, participants, & measurements
We conducted a retrospective, longitudinal cohort study of a clinical database containing the medical records of patients on maintenance hemodialysis receiving dialysis between June of 1999 and December of 2012 in a single center; 200 patients (130 men and 70 women) with a median age of 69.0 (interquartile range, 59.3–77.0) years old were included in the study. Dietary intake, biochemical markers of nutrition, anthropometric measurements, and UA levels were recorded at 0, 6, 12, 18, 24, 30, and 36 months followed by 15 additional months of clinical observations. The patients were followed until January 31, 2015 (median follow-up was 38.0 [interquartile range, 30.0–46.8] months).
Results
In a linear mixed effects model adjusted for baseline demographics and clinical parameters, each 1.0-mg/dl longitudinal increase in UA was associated with a 13.4% slower rate of decline in geriatric nutritional risk index (GNRI) levels over 3 years of observation (95% confidence interval [95% CI], 0.11 to 0.39; P<0.001 for UA × time interaction). UA remained associated with the rate of change in GNRI, even after controlling for C-reactive protein. During the follow-up, 87 (43.5%) all-cause and 38 (19.0%) cardiovascular deaths were reported. For each 1.0-mg/dl increase in serum UA over time, the multivariate adjusted all–cause mortality hazard ratio using Cox models with the effect of time-varying risk was 0.83 (95% CI, 0.74 to 0.95; P<0.01), which continued to be significant, even after including the baseline GNRI levels in this model: 0.89 (95% CI, 0.79 to 0.98; P=0.02).
Conclusions
Longitudinal changes in serum UA seem to track with changes in nutritional status over time, and these changes are associated with survival of patients on maintenance hemodialysis. An increase in serum UA levels over time is accompanied by improvement of nutritional status and lower mortality rate.
Keywords: uric acid, hemodialysis, inflammation, nutrition, geriatric nutritional risk index, mortality, Cohort Studies, Humans, Longitudinal Studies, Nutritional Status
Introduction
The importance of serum uric acid (SUA) levels for patients on maintenance hemodialysis (MHD) has not been well established. Uric acid (UA) is a purine metabolite that is formed in the liver, and 65%–75% of its daily produced amount is excreted by the kidneys (1). When significant amounts of UA accumulate in patients with ESRD because of lack of renal clearance, the mean UA removal is approximately 1 g per hemodialysis (HD) session, even with high-flux hemodialysers (2). Consequently, hyperuricemia is common in patients with ESRD undergoing treatment by either HD (3,4) or peritoneal dialysis (PD) (5–7). In the general population, hyperuricemia has been shown to be associated with an increased risk of hypertension (8,9), prehypertension (10), peripheral arterial disease (11), diabetes mellitus (12), CKD (13), and cardiovascular disease (CVD) (8,10,14). Conversely, in the HD population, hyperuricemia has been shown as a good nutritional marker (15) that can also predict lower rates of upcoming hospitalizations (15) as well as lower all–cause and cardiovascular death risks (15,16). Although the data about the mortality predictive role of higher SUA levels in the HD population are somewhat controversial (3,4,15–18), most of the studies conducted on this topic presented low SUA as a mortality risk factor in patients on MHD (3,4,15,16,18).
However, to our knowledge, there is no information in the literature on the clinical effect of longitudinal changes in SUA levels on the prognosis of patients on HD. We hypothesized that longitudinal changes in SUA may have independent associations with changes in nutritional parameters over time and consequently, long-term survival in patients on MHD. This study was, therefore, designed to determine the association of changes in nutritional and inflammatory parameters over time on changes in SUA and subsequent clinical outcomes in patients on MHD.
Materials and Methods
Patients
We conducted a retrospective, longitudinal cohort study of a clinical database containing the medical records of patients with ESRD receiving dialysis treatment from June 7, 1999 to July 31, 2015 in a single dialysis center (Assaf Harofeh Medical Center, Zerifin, Israel). This study was approved by our Institutional Ethics Committee.
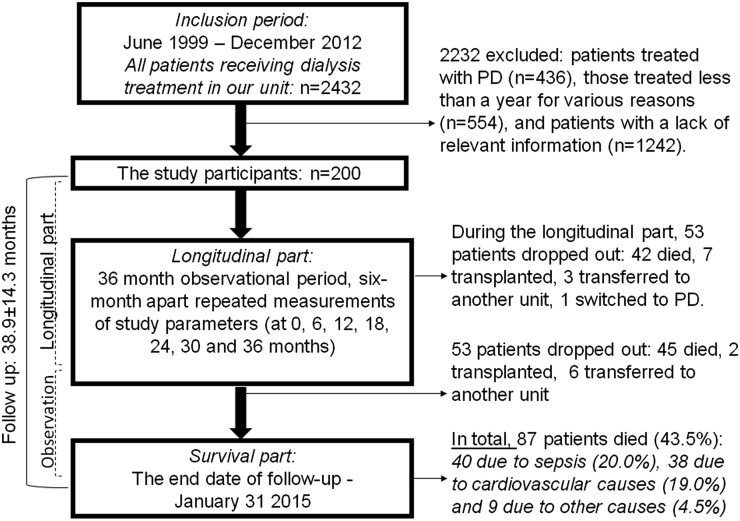
The database includes patient demographics, medications, clinical laboratory values, dialysis treatment records, records of dietitian consultations, and all comorbidities available to the dialysis unit. Patients included in the analysis were those ≥18 years old who received thrice weekly in–center HD for ≥1 year from June 7, 1999 to December 5, 2012. The first (baseline) visit for each patient was the calendar date on which the patient’s dialysis duration was >60 days. Study measurements were selected from the database at 0, 6, 12, 18, 24, 30, and 36 months followed by 15 additional months of clinical observations. Follow-up time began on the date of entry into the cohort. The end date of the follow-up was January 31, 2015. A flowchart of the study is presented in Figure 1. Patients were censored at the time of death, renal transplantation, or transfer to PD or another dialysis unit (loss of follow-up). From 2432 patients receiving dialysis during the cohort period, 990 were ineligible (436 patients treated with PD and 554 patients on MHD for <1 year were excluded). From 1442 potential participants, 1242 patients were excluded because of incomplete data (if we were unable to record SUA, C-reactive protein [CRP], dietary intake calculation, and anthropometric measurements every 6 months during the longitudinal part of the study) (Figure 1). However, a comparison of included patients versus patients excluded because of incomplete data (Table 1) showed that the two groups were almost identical with regard to age, comorbidity index, BP, Kt/V, dietary intake, anthropometric measurements, and UA, creatinine, cholesterol, triglyceride, transferrin, CRP, and geriatric nutritional risk index (GNRI) levels. Although statistically significant, the differences in sex, dialysis vintage, diabetes status, vascular access type, serum albumin, hemoglobin levels, mortality, and use of diuretics or allopurinol were of small magnitude. In total, 200 patients (130 men and 70 women) with a median age of 69.0 (interquartile range, 59.3–77.0) years old who had available UA and pertinent covariate data were included in the study. These patients contributed a total of 634 patient-years of at-risk time, and the median follow-up was 38.0 (interquartile range, 30.0–46.8) months. During the follow-up, in total, 87 (43.5%) all-cause and 38 (19.0%) cardiovascular deaths were reported. The causes of renal failure included diabetic kidney disease (50.0%), hypertension (32.0%), GN (4.0%), autosomal dominant polycystic kidney disease (6.0%), obstructive uropathy (4.0%), and other diseases or unknown causes (4.0%). All patients underwent regular dialysis via their vascular access for 4–5 hours three times per week at a blood flow rate of 250–300 ml/min and a dialysis solution flow rate of 500 ml/min. Dialysis treatments were performed with low–flux biocompatible dialyzer membranes with surface areas of 1.4–1.8 m2.
Figure 1.
Flow diagram of the study. PD, peritoneal dialysis.
Table 1.
Baseline characteristics of included patients (n=200) versus patients excluded because of missing data (n=1242)
| Variable | Included, n=200 | Excluded Because of Missing Data, n=1242 | P Value | Ra |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age, yr | 69.0 (59.3–77.0) | 67.0 (56.0–76.0) | 0.09 | — |
| Sex, men/women | 65/35 | 63/37 | 0.02 | — |
| Dialysis vintage, mo | 8.0 (3.0–44.0) | 12.0 (7.0–28.0) | <0.001 | — |
| DM, % | 51.5 | 53.8 | <0.001 | — |
| Comorbidity index | 4.0 (2.0–6.0) | 3.0 (2.0–6.0) | 0.40 | — |
| SBP, mmHg | 138.9±25.7 | 138.7±25.8 | 0.99 | — |
| DBP, mmHg | 68.6±13.4 | 68.2±13.9 | 0.76 | — |
| Kt/V | 1.34±0.28 | 1.32±0.29 | 0.35 | — |
| Access, fistula/graft/catheter | 55/10/35 | 65/15/20 | <0.001 | — |
| Use of allopurinol, % | 0.5 | 0.4 | <0.001 | — |
| Use of diuretics, % | 20.5 | 20.2 | 0.04 | — |
| Dietary intake | ||||
| Energy intake, kcal/kg per d | 23.9±7.01 | 23.2±6.27 | 0.82 | −0.01 |
| Protein intake, g/kg per d | 0.98±0.30 | 1.01±0.31 | 0.21 | 0.10 |
| nPCR, g/kg per d | 0.96±0.24 | 1.00±0.32 | 0.17 | 0.19b |
| Blood analysis | ||||
| Uric acid, mg/dl | 5.8±1.3 | 5.7±1.3 | 0.10 | — |
| Albumin, g/dl | 3.67±0.41 | 3.79±0.39 | 0.004 | 0.23c |
| Creatinine, mg/dl | 7.13±2.57 | 7.26±2.31 | 0.55 | 0.28c |
| Cholesterol, mg/dl | 147.7±37.2 | 150.3±37.1 | 0.38 | 0.03 |
| TG, mg/dl | 122.0 (89.0–193.0) | 126.5 (94.0–181.5) | 0.41 | 0.16b |
| Transferrin, mg/dl | 173.7±34.5 | 169.3±32.8 | 0.46 | 0.15b |
| Hemoglobin, g/dl | 10.78±1.44 | 11.36±1.30 | 0.001 | −0.01 |
| CRP, mg/L | 10.0 (4.8–24.1) | 8.29 (4.4–17.3) | 0.06 | −0.08 |
| Anthropometric measurements | ||||
| BMI, kg/m2 | 27.1±6.03 | 27.5±5.7 | 0.46 | 0.17b |
| TSF, mm | 15.8±6.62 | 16.4±6.79 | 0.27 | 0.15b |
| MAC, cm | 28.3±4.09 | 28.2±4.08 | 0.98 | 0.11 |
| MAMC, cm | 23.4±3.00 | 23.1±3.23 | 0.72 | 0.09 |
| Nutritional score | ||||
| GNRI | 107.7±13.1 | 108.0±14.6 | 0.80 | 0.19b |
| Outcome | ||||
| All-cause death | 43.5 | 39.1 | <0.001 | |
| CVD death | 14.5 | 16.1 | <0.001 |
Continuous variables are expressed as means (SDs) or medians (interquartile ranges) for non–normally distributed data, and categorical variables are expressed as percentages. Patients lacking repeated measurements every 6 months of serum uric acid, CRP, dietary intake calculation, and anthropometric data during the longitudinal part of the study were excluded. —, calculation is not applicable or of no interest; DM, diabetes mellitus; SBP, predialysis systolic BP; DBP, predialysis diastolic BP; nPCR, normalized protein catabolic rate; TG, triglyceride; CRP, C-reactive protein; BMI, body mass index; TSF, triceps skinfold thickness; MAC, midarm circumference; MAMC, midarm muscle circumference calculated; GNRI, geriatric nutritional risk index; CVD, cardiovascular disease.
Univariate correlation with baseline uric acid concentrations of the study participants as assessed by Pearson correlation coefficients or Spearman rank order correlation coefficients in cases of skewed distribution of data.
Statistical significance at P<0.05.
Statistical significance at P<0.01.
Dietary Intake
Dietitian records on the basis of self–completed food diaries with continuous 3-day dietary histories (including a dialysis day, a weekend day, and a nondialysis day) were used to calculate the dietary intake. The dietary energy and protein intake were calculated and normalized for ideal body weight according to the European Best Practice Guidelines (19). Ideal weight in this study was calculated from the Lorentz equations differently for men and women. Dietary intake was calculated using computerized analysis (a Disk Operating System–based program MANA specially adapted for data entry and analysis of food intake records). This program was developed by the Israeli Food and Nutrition Administration on the basis of the Food Intake Analysis System of the US Department of Agriculture (20) and especially adapted to meet the needs of the Israeli population.
Dietary protein intake was also estimated by using the normalized protein catabolic rate (nPCR) calculation from the patient’s urea generation rate by urea kinetics modeling (21). Single–pool model urea kinetics were used to estimate the nPCR.
Anthropometric Measurements
Body mass index (BMI), triceps skinfold thickness (TSF), midarm circumference (MAC), and calculated midarm muscle circumference (MAMC) were measured as anthropometric variables. TSF was measured with a conventional skinfold caliper using standard techniques. MAC was measured with a plastic measuring tape. MAMC was estimated as follows: 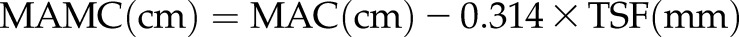 .
.
GNRI
GNRI was calculated from the patient’s serum albumin, body weight, and height by using the equation developed by Bouillanne et al. (22) and modifying the nutritional risk index for elderly patients.
Comorbidity Index and Clinical Outcomes
We determined the comorbidity index, which was recently developed by Liu et al. (23) and validated specifically for populations of patients on dialysis, as a measure of comorbid conditions.
Hospitalization data were obtained for all 200 patients on HD. Hospitalization was defined as any hospital admission that included at least one overnight stay in the hospital. The access-related hospitalizations were not included in the hospitalization data.
CVD was defined as myocardial infarction requiring coronary artery procedures, such as angioplasty or surgery, cerebrovascular accident, or peripheral vascular disease requiring angioplasty, bypass, or amputation. Cardiovascular mortality was defined as death resulting from coronary heart disease, sudden death, stroke, or complicated peripheral vascular disease.
Laboratory Evaluation
Blood samples were obtained from nonfasting patients on a midweek day predialysis, with the exception of postdialysis serum urea nitrogen to calculate urea kinetics. Albumin was measured using the bromocresol green method. All biochemical analyses, including UA, creatinine, urea, albumin, transferrin, complete blood count, triglycerides, and total cholesterol, were measured by an automatic analyzer. Additionally, serum high–sensitivity CRP was measured by a turbidimetric immunoassay.
Statistical Analyses
Data are expressed as means±SDs, medians and interquartile ranges (quartiles 1–3) for variables that did not follow a normal distribution, or frequencies as noted.
Longitudinal data were analyzed by using the Mixed model. Base models were adjusted for age, sex, diabetes status, dialysis vintage, comorbidity index, Kt/V, diuretic use, and vascular access type. F tests were used to assess the significance of the fixed effects, and P values of <0.05 were considered significant. To evaluate whether changes in time of UA associate the changes in various nutritional parameters, we included in each base model terms for individual UA by time interactions. We adjusted for baseline levels of UA when analyzing changes in nutritional parameters to minimize the likelihood of regression to the mean.
Normally distributed continuous variables were compared between the two groups using a two–sided t test, with chi-squared tests used for categorical variables and nonparametric Mann–Whitney U tests used for not normally distributed continuous variables. Associations between baseline SUA and nutritional parameters were assessed using Pearson correlation coefficients or Spearman rank order correlation coefficients in cases of skewed distribution of data. Survival analyses were examined in Cox models with UA as a time-varying predictor. Univariate and multivariate Cox regression analyses are presented as hazard ratios (HRs) and 95% confidence intervals (95% CIs).
All statistical analyses were performed using SPSS software, version 16.0 (IBM SPSS, Chicago, IL).
Results
The baseline characteristics of the cohort are shown in Table 1. The 200 patients on prevalent HD (median age, 69.0 years old; interquartile range, 59.3–77.0 years old) selected for this study included 35% women. One half of the participants (51.5%) had diabetes mellitus and a median dialysis vintage of 8.0 months. nPCR as a marker of daily protein intake, laboratory nutritional markers (albumin, creatinine, triglycerides, and transferrin), body composition parameters (BMI and TSF), and the overall nutritional status assessed by GNRI were positively correlated to SUA at baseline.
We examined the individual differences in the rate of change in GNRI using a linear mixed effects model controlling for age, sex, diabetes mellitus status, dialysis vintage, vascular access type, Kt/V, diuretic use, and comorbidity index. GNRI levels decreased by about 0.47 U per 3 years (95% CI, −0.65 to −0.29; P<0.001). We next added a term for UA and its interaction with time to the previous model (Table 2) to examine whether UA was associated with the rate of change in GNRI. In this model (model 2), both UA and GNRI levels decreased (UA by 0.38 mg/dl per 3 years; 95% CI, −0.77 to <0.01 and GNRI by 1.87 U per 3 years; 95% CI, −2.69 to −1.06), but each 1.0-mg/dl increase in UA at baseline was associated with a slower decrease in GNRI levels of 0.25 U per 3 years (P<0.001 for UA × time interaction). In practical terms, a person with UA 1 mg/dl above the mean at baseline would show about a 13.4% slower rate of decline in GNRI levels. Next, we examined whether controlling for CRP confounded the association of UA with the rate of change in GNRI over time. After adding CRP and its interaction with time to the previous model, UA remained associated with changes in GNRI over 3 years of observation. These results suggest that UA is independently associated with the rate of change in GNRI over time.
Table 2.
Associations of longitudinal uric acid changes with changes in geriatric nutritional risk index (slopes) over 36 months on the basis of a mixed effects model
| Variable | Model 1 Estimate (95% CI) | P Value | Model 2 Estimate (95% CI) | P Value | Model 3 Estimate (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Time, mo | −0.47 (−0.65 to −0.29) | <0.001 | −1.87 (−2.69 to −1.06) | <0.001 | −1.17 (−2.15 to −0.19) | 0.02 |
| Uric acid, mg/dl | −0.38 (−0.77 to <0.01) | 0.05 | 0.15 (−0.63 to 0.92) | 0.71 | ||
| Uric acid × time | 0.25 (0.11 to 0.39) | <0.001 | 0.17 (<0.01 to 0.34) | 0.04 | ||
| CRP, mg/L | <0.01 (−0.03 to 0.05) | 0.65 | ||||
| CRP × time | <−0.01 (−0.01 to <0.01) | 0.11 |
The table represents the three mixed models with geriatric nutritional risk index (GNRI) as a dependent variable and uric acid as an independent variable. The three models control for fixed factors, such as age, sex, diabetes mellitus status, dialysis vintage, vascular access type, Kt/V, diuretic use, and comorbidity index. Model 1 includes fixed factors and GNRI. In this model, GNRI decreased over 3 years of observations: linear estimate of −0.47. In model 2, we added terms for uric acid and its interaction with time. In model 3, we added terms for CRP and its interaction with time (these models take into account every measurement of GNRI, uric acid, and CRP at each time point for each patient separately). 95% CI, 95% confidence interval; CRP, C-reactive protein.
We also examined the associations of UA with longitudinal changes in nutritional parameters (slopes) over 36 months including fixed parameters, such as age, sex, Kt/V, diuretic use, diabetes status, dialysis vintage, vascular access type, and comorbidity index (Table 3). Longitudinally, SUA levels were associated with most of the nutritional markers studied at any given time point, controlling for demographic and clinical parameters. In addition, there was the significant association of the UA × time interaction on the slope of albumin, creatinine, transferrin, cholesterol, and triglycerides over 36 months of observation. Specifically, in a person with SUA 1 mg/dl above the mean at baseline, the decline in serum albumin was reduced by almost 15.7%, and the decline in serum creatinine was reduced by 18.9%. Additionally, UA levels were associated with the rates of change in BMI and TSF (slopes) over time as shown in Table 3. We did not observe any association between SUA and CRP levels over time.
Table 3.
Associations of uric acid with nutritional parameters and their rates of changes (slopes) over 36 months on the basis of a mixed effects model
| Variable | Uric Acid, mg/dl | Uric Acid × Time Interaction | ||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Dietary intake | ||||
| DEI, kcal/kg per d | −0.28 (−1.20 to 0.64) | 0.49 | 0.06 (−0.23 to 0.33) | 0.71 |
| DPI, g/kg per d | −0.13 (−3.23 to 2.97) | 0.93 | 0.06 (−0.49 to 0.60) | 0.84 |
| nPCR, g/kg per d | −0.02 (−0.05 to 0.02) | 0.35 | <0.01 (<−0.01 to 0.01) | 0.31 |
| Biochemical markers | ||||
| Albumin, g/L | −0.89 (−1.31 to −0.46) | <0.001 | 0.14 (0.06 to 0.21) | <0.001 |
| Transferrin, mg/dl | −0.06 (−0.09 to −0.02) | <0.001 | <0.01 (<0.01 to 0.01) | 0.03 |
| Creatinine, mg/dl | −0.37 (−0.56 to −0.18) | <0.001 | 0.07 (0.03 to 0.10) | <0.001 |
| Cholesterol, mg/dl | −5.37 (−9.86 to −0.88) | 0.02 | 0.73 (<0.01 to 1.51) | 0.04 |
| TG, mg/dl | −8.67 (−16.56 to −0.79) | 0.03 | 0.82 (<0.01 to 2.19) | 0.04 |
| CRP, mg/L | 0.17 (−3.91 to 4.24) | 0.94 | 0.21 (−0.50 to 0.92) | 0.56 |
| Anthropometric measurements | ||||
| BMI, kg/m2 | −0.61 (−0.83 to −0.38) | <0.001 | 0.07 (0.03 to 0.11) | 0.001 |
| TSF, mm | −1.18 (−1.82 to −0.55) | <0.001 | 0.12 (0.01 to 0.23) | 0.03 |
| MAC, cm | −0.54 (−0.94 to −0.15) | <0.01 | 0.03 (−0.04 to 0.10) | 0.45 |
| MAMC, cm | −0.17 (−0.54 to 0.20) | 0.37 | −0.01 (−0.07 to 0.05) | 0.76 |
All nutritional variables presented were modeled separately as dependent variables. Uric acid was included in these models as an independent variable together with fixed factors (such as age, sex, diabetes status, dialysis vintage, vascular access type, Kt/V, diuretic use, and comorbidity index). The models take into account every measurement of uric acid and present nutritional variables at each time point separately for each patient. Regression coefficients in column 2 indicate longitudinal associations of uric acid levels with nutritional markers at any given time point, while controlling for fixed factors (as mentioned above). Regression coefficients in column 3 indicate the rate of change in nutritional markers (slopes) over the study period by each 1-mg/dl increase in uric acid (uric acid by time interactions), controlling for the same fixed factors. 95% CI, 95% confidence interval; DEI, daily energy intake; DPI, daily protein intake; nPCR, normalized protein catabolic rate; TG, triglyceride; CRP, C-reactive protein; BMI, body mass index; TSF, triceps skinfold thickness; MAC, midarm circumference; MAMC, midarm muscle circumference calculated.
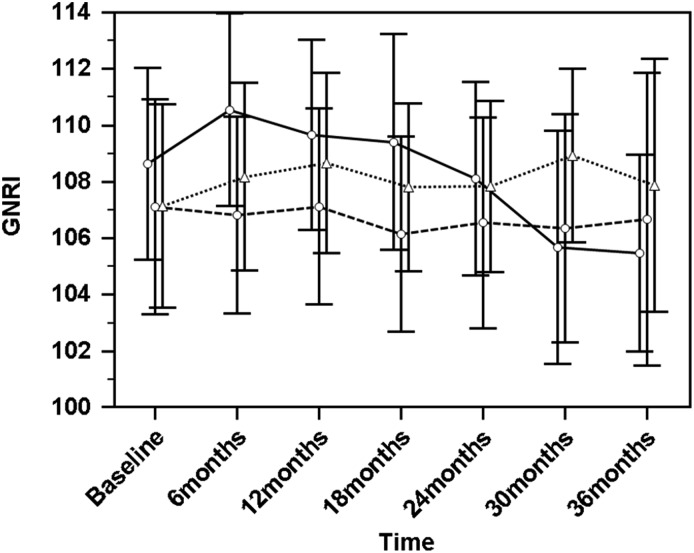
Next, we examined the trajectory of longitudinal changes in SUA and GNRI levels in groups of patients divided by tertiles of the absolute change in SUA (ΔSUA) from baseline to 36 months or from baseline to the final measurement of SUA for those dropped out during the longitudinal part of the study (Figure 2). GNRI in the first tertile of ΔSUA (the group of patients with a decrease in SUA to <−0.8 mg/dl, with a corresponding rate of change of −0.21; 95% CI, −0.25 to −0.16; P<0.001) exhibited a significant decline over time (−0.80; 95% CI, −1.11 to −0.48; P<0.001). However, in the second group (the group with SUA decline over time that was more gradual, with ΔSUA ranging from −0.8 to 0.2 mg/dl and a corresponding rate of change of −0.05; 95% CI, −0.08 to −0.02; P=0.002) and the third tertile of ΔSUA (the group with an increase in SUA to >0.2 mg/dl and a corresponding rate of change of 0.08; 95% CI, 0.03–0.13; P=0.002), GNRI did not decline significantly over the 36-month period.
Figure 2.
Comparison of changes in geriatric nutritional risk index (GNRI) levels over a period of 36 months in the tertiles of absolute changes in serum uric acid (ΔSUA) over the same period. The first tertile is uric acid decrease to <−0.8 mg/dl (circles and solid line). In this group, SUA declined significantly over time: the estimate was −0.21 (95% confidence interval, −0.25 to −0.16; P<0.001). The second tertile is change of SUA between −0.8 and 0.2 mg/dl (circles and dashed line). SUA decline was statistically significant but more gradual (−0.05; 95% confidence interval, −0.08 to −0.02; P=0.002). The third tertile is uric acid increase to >0.2 mg/dl (triangles and dotted line); in this group, SUA increased significantly over time (0.08; 95% confidence interval, 0.03 to 0.13; P=0.002). In the whole cohort, GNRI declined during the study period (−0.33; 95% confidence interval, −0.49 to −0.17; P<0.001; crude analysis), whereas a statistically significant decline was observed only in the first tertile of the ΔSUA over time (−0.80; 95% confidence interval, −1.11 to −0.48; P<0.001), and GNRI did not change in the second (−0.15; 95% confidence interval, −0.43 to 0.12; P=0.27) and third (−0.06; 95% confidence interval, −0.30; 0.19; P=0.66) tertiles of ΔSUA. Data are presented as means with 95% confidence intervals.
Overall, for each 1.0-mg/dl increase in SUA, the crude all–cause mortality (87 of 200 participants died during the study period) HR using Cox models with the effect of time-varying risk was 0.91 (95% CI, 0.83 to 1.01; P=0.06), whereas after multivariate adjustments for all above–mentioned demographic and clinical covariates, HR was 0.83 (95% CI, 0.74 to 0.95; P<0.01), which continued to be significant even after including the baseline GNRI levels in this model (0.89; 95% CI, 0.79 to 0.98; P=0.02). Time-varying UA did not show an association with CVD-related hospitalization (75 cardiovascular events were reported during the follow-up) and mortality (38 cardiovascular deaths were reported) (Table 4).
Table 4.
Hazard ratios (95% confidence intervals) of all-cause mortality, cardiovascular disease–related mortality, and hospitalization associated with time–varying serum uric acid levels in unadjusted and multivariable–adjusted Cox models
| Model | All-Cause Mortality | P Value | CVD Mortality | P Value | CVD Hospitalization | P Value |
|---|---|---|---|---|---|---|
| n (%) of eventsa | 87 (43.5) | 38 (19.0) | 75 (37.5) | |||
| Univariate HR (95% CI) | 0.91 (0.83 to 1.01) | 0.06 | 0.96 (0.82 to 1.12) | 0.57 | 0.98 (0.86 to 1.10) | 0.70 |
| Model 1 (adjustedb) HR (95% CI) | 0.83 (0.74 to 0.95) | <0.01 | 1.01 (0.84 to 1.22) | 0.93 | 0.96 (0.83 to 1.11) | 0.62 |
| Model 2 (model 1 and GNRI) HR (95% CI) | 0.89 (0.79 to 0.98) | 0.02 | 0.87 (0.72 to 1.07) | 0.18 | 0.99 (0.83 to 1.18) | 0.91 |
CVD, cardiovascular disease; HR, hazard ratio; 95% CI, 95% confidence interval; GNRI, geriatric nutritional risk index.
Numbers of events are shown as absolute numbers and percentages.
Adjusted for age, sex, diabetes status, dialysis vintage, comorbidity index, Kt/V, diuretic use, and vascular access type.
Discussion
In this study, we wished to determine whether changes of SUA levels are associated with parallel changes in nutritional parameters over time in a cohort of patients on MHD. We provide novel evidence that changes in SUA levels independently reflect changes in nutritional status in our population. In addition, these changes can predict long-term survival in patients on MHD.
Regarding the effects of longitudinal associations, we found that SUA, most of the studied nutritional parameters, and nutritional status as a whole expressed by GNRI declined simultaneously over 36 months of observation. Only a few existing longitudinal studies have investigated changes in SUA levels in patients on MHD. In a 6-month longitudinal study with three 3-month repeated blood samplings, no statistically significant differences among the UA levels were found in 48 patients on HD (24). However, in agreement to our findings, in a 2-year study, on the basis of four 6-month blood samples, UA significantly decreased over the study period in 117 patients on HD (25). With regard to nutritional status, most of studies have shown that MHD associates with a significant decline in certain laboratory nutritional parameters, including albumin (26,27), as well as anthropometric measurements (27,28). GNRI, an objective and simple nutritional index, has also been reported to decrease significantly with time in patients on MHD in a recent prospective longitudinal study (29). The longitudinal behavior of SUA may be explained as follows: it is a proposed nutritional marker in the MHD population as reported in a recently published prospective observational study (15), and changes in SUA levels over time may simply follow changes in nutritional status. In addition, overproduction of UA is usually associated with visceral fat accumulation (30,31) as part of the metabolic syndrome, the prevalence of which is high in the ESRD population (32). Therefore, higher and stable over time SUA levels may express a proportion of metabolic syndrome in our cohort. Although not all markers of the metabolic syndrome were measured in this study, the significant associations observed between SUA changes over time and the rates of change (slopes) in BMI, TSF (as a surrogate of fat mass), and triglycerides (statistically significant SUA × time interactions) may support this assumption. Metabolic syndrome is associated with better nutritional status (expressed as better Subjective Global Assessment scores) in patients on MHD as reported by Xie et al. (33).
One must also consider whether changes in inflammatory markers observed in our study were relevant to the association with SUA changes and in parallel to GNRI changes over time. Although there is in vitro and experimental evidence of the proinflammatory effects of UA in human vascular smooth muscle and endothelial cells (34,35), the relationship of SUA with the inflammatory response in the HD population has not been sufficiently studied and is still controversial. SUA was positively associated with serum CRP levels in an incident HD population (4). Conversely, no significant association of SUA with CRP was found in the same population by another study (17). Of interest, monocytes from patients with ESRD purified from peripheral blood mononuclear cells and incubated with monosodium urate crystals showed a reduced secretion of proinflammatory cytokines, including IL-6, compared with healthy controls (36). We found no longitudinal study in the literature regarding the relationship between SUA and inflammation. We showed that SUA changes independently associate with the rate of change in GNRI levels over time, even after controlling for such covariates as CRP. We suggest, therefore, that changes in SUA over time associated with longitudinal changes in GNRI are independent from inflammation. However, because IL-6, the best inflammatory biomarker in the MHD population (37), was not measured in this study, we cannot completely rule out the confounding role of inflammation in the association between SUA and nutritional status.
In the context of associating SUA with prognosis, our study expands the cross-sectional observations (15,16) showing that changes of SUA over time may predict outcomes in patients on MHD. We did not find use of a longitudinal design in the few studies that we did find providing data about the association of SUA and clinical outcome in patients on MHD (3,4,15–18). Instead, a relatively large number of studies has used longitudinal data to analyze the association of changes in albumin (38), BMI (39), and TSF (28) over time with prognosis. This probably supports our data given the estimates of the regression coefficients in Table 3 expressing associations of SUA and the above–mentioned nutritional parameter changes over time. Thus, in our study, we have shown that the longitudinal increase in SUA over time is correlated with a more gradual decrease in GNRI and consequently, better all–cause mortality but not correlated with CVD mortality and first CVD event. Regarding the association between changes of SUA over time and CVD morbidity and mortality, prospective observational but not longitudinal studies present strong evidence on the prognostic power of SUA (8–12,10,14), even in patients on MHD (15,16). Therefore, this question should be examined in larger and prospective longitudinal studies.
Finally, multivariable adjustments in Cox models with the effect of time-varying risk, including baseline GNRI levels, did not abolish the prognostic value of longitudinal SUA changes on mortality. This suggests that, together with being associated with nutritional status, additional mechanisms contribute to the prognostic significance. In this context, the antioxidant properties of SUA (40) may play a role. Oxidative stress is highly linked to inflammation (41) and poor survival (42) in patients on MHD.
In interpreting the results of our study, we should be concerned with whether regression to the mean is relevant to the association of longitudinal SUA changes on changes in GNRI levels (slopes) over time. The linear mixed model used in our study to analyze longitudinal data models the covariance matrix, which gives a high statistical power and adjusts each subject’s follow-up measurement according to the baseline measurement. The use of analysis of covariance in such a way is accepted as one of the practical ways to deal with regression to the mean in statistical analysis (43).
A number of limitations inherent to observational research applies to our analysis. First, this study only used an observational approach without manipulating exposure factors, and therefore, no definitive cause and effect relationship can be derived for any of the risk factors analyzed. Second, selection bias is typical for retrospective studies. A difference in incidence of the outcome of interest between those who participated and those who did not would give biased results. Despite the similarity between the study sample and excluded patients (Table 1), selection bias can still affect the generalizability of our findings to the wider MHD population. Third, because of the retrospective design, we could not obtain a number of parameters of interest (e.g., residual renal function, waist circumference, hydration status, IL-6, and glutathione). Fourth, the overall nutritional status was assessed by GNRI, which may not be the ideal nutritional marker in the MHD population. Despite the observed significant associations of GNRI with dietary intake, laboratory markers of nutrition, body composition, and prognosis (29,44), the only validation study of GNRI as a nutritional marker in the MHD population was conducted in Japanese patients on HD (45). Fifth, the proportion of cardiovascular deaths may be underestimated, because the causes of death were extracted from patient records and were not confirmed by autopsies. Despite these limitations, the wide array of nutritional parameters, which included anthropometrics, biochemical markers, and dietary intake, longitudinal design, and long–term follow-up used in this study strengthens the results herein.
In summary, our study shows that longitudinal changes in SUA seem to track changes in the nutritional status over time and that these changes are associated with survival of patients on MHD. An increase in SUA levels over time is accompanied by improvement of nutritional status and lower mortality rate.
Disclosures
None.
Acknowledgments
The authors thank Dr. Mechael Kanovsky for his language editing, which has greatly improved the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Oliveira EP, Burini RC: High plasma uric acid concentration: Causes and consequences. Diabetol Metab Syndr 4: 12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sombolos K, Tsitamidou Z, Kyriazis G, Karagianni A, Kantaropoulou M, Progia E: Clinical evaluation of four different high-flux hemodialyzers under conventional conditions in vivo. Am J Nephrol 17: 406–412, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Hsu SP, Pai MF, Peng YS, Chiang CK, Ho TI, Hung KY: Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 19: 457–462, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Suliman ME, Johnson RJ, García-López E, Qureshi AR, Molinaei H, Carrero JJ, Heimbürger O, Bárány P, Axelsson J, Lindholm B, Stenvinkel P: J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 48: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Feng S, Jiang L, Shi Y, Shen H, Shi X, Jin D, Zeng Y, Wang Z: Uric acid levels and all-cause mortality in peritoneal dialysis patients. Kidney Blood Press Res 37: 181–189, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Dong J, Han QF, Zhu TY, Ren YP, Chen JH, Zhao HP, Chen MH, Xu R, Wang Y, Hao CM, Zhang R, Zhang XH, Wang M, Tian N, Wang HY: The associations of uric acid, cardiovascular and all-cause mortality in peritoneal dialysis patients. PLoS One 9: e82342, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia X, He F, Wu X, Peng F, Huang F, Yu X: Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis 64: 257–264, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med 359: 1811–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alper AB Jr., Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL: Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension 45: 34–38, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Syamala S, Li J, Shankar A: Association between serum uric acid and prehypertension among US adults. J Hypertens 25: 1583–1589, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Shankar A, Klein BE, Nieto FJ, Klein R: Association between serum uric acid level and peripheral arterial disease. Atherosclerosis 196: 749–755, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bandaru P, Shankar A: Association between serum uric acid levels and diabetes mellitus. Int J Endocrinol 2011: 604715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feig DI: Uric acid: A novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens 18: 526–530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P: Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 36: 1072–1078, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Beberashvili I, Sinuani I, Azar A, Shapiro G, Feldman L, Stav K, Sandbank J, Averbukh Z: Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition 31: 138–147, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Latif W, Karaboyas A, Tong L, Winchester JF, Arrington CJ, Pisoni RL, Marshall MR, Kleophas W, Levin NW, Sen A, Robinson BM, Saran R: Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol 6: 2470–2477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon JS, Chung SH, Han DC, Noh H, Kwon SH, Lindholm B, Lee HB: Mortality predictive role of serum uric acid in diabetic hemodialysis patients. J Ren Nutr 24: 336–342, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Lee AL, Winters TJ, Tam E, Jaleel M, Stenvinkel P, Johnson RJ: Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol 29: 79–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, Haage P, Konner K, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Tordoir J, Vanholder R: EBPG guideline on nutrition. Nephrol Dial Transplant 22[Suppl 2]: ii45–ii87, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Human Nutrition Center: University of Texas, Houston, School of Public Health, Human Nutrition Center. Available at: http://www.sph.uth.edu.tmc:8052/hnc/software/soft.htm. Accessed June 9, 2004
- 21.Depner TA, Daugirdas JT: Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol 7: 780–785, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C: Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82: 777–783, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Alarcon M, Reyes-Pérez A, Lopez-Garcia H, Palomares-Bayo M, Olalla-Herrera M, Lopez-Martinez MC: Longitudinal study of serum zinc and copper levels in hemodialysis patients and their relation to biochemical markers. Biol Trace Elem Res 113: 209–222, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Martí del Moral L, Agil A, Navarro-Alarcón M, López-Ga de la Serrana H, Palomares-Bayo M, Oliveras-López MJ: Altered serum selenium and uric acid levels and dyslipidemia in hemodialysis patients could be associated with enhanced cardiovascular risk. Biol Trace Elem Res 144: 496–503, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG: Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int 57: 1176–1181, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, Kusek JW, Leung J, Burrowes JD, McLeroy SL, Poole D, Uhlin L HEMO Study Group : The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2321–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Su CT, Yabes J, Pike F, Weiner DE, Beddhu S, Burrowes JD, Rocco MV, Unruh ML Su CT1 : Changes in anthropometry and mortality in maintenance hemodialysis patients in the HEMO Study. Am J Kidney Dis 62: 1141–1150, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Beberashvili I, Azar A, Sinuani I, Kadoshi H, Shapiro G, Feldman L, Averbukh Z, Weissgarten J: Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin J Am Soc Nephrol 8: 443–451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y: Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 47: 929–933, 1998 [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira EP, Moreto F, Silveira LV, Burini RC: Dietary, anthropometric, and biochemical determinants of uric acid in free-living adults. Nutr J 12: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young DO, Lund RJ, Haynatzki G, Dunlay RW: Prevalence of the metabolic syndrome in an incident dialysis population. Hemodial Int 11: 86–95, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Xie Q, Zhang AH, Chen SY, Lai X, Zhang F, He L, Zhuang Z, Zhu N, Fan MH, Wang T: Metabolic syndrome is associated with better nutritional status, but not with cardiovascular disease or all-cause mortality in patients on haemodialysis. Arch Cardiovasc Dis 105: 211–217, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Kang DH, Park SK, Lee IK, Johnson RJ: Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16: 3553–3562, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ: Hyperuricemia induces endothelial dysfunction. Kidney Int 67: 1739–1742, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Schreiner O, Wandel E, Himmelsbach F, Galle PR, Märker-Hermann E: Reduced secretion of proinflammatory cytokines of monosodium urate crystal-stimulated monocytes in chronic renal failure: An explanation for infrequent gout episodes in chronic renal failure patients? Nephrol Dial Transplant 15: 644–649, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Panichi V, Maggiore U, Taccola D, Migliori M, Rizza GM, Consani C, Bertini A, Sposini S, Perez-Garcia R, Rindi P, Palla R, Tetta C: Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant 19: 1154–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H Jr., Kopple JD, Greenland S: Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20: 1880–1888, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Simie MG, Jovanovic SV: Antioxidation mechanisms of uric acid. J Am Chem Soc 111: 5778–5782, 1989 [Google Scholar]
- 41.Mezzano D, Pais EO, Aranda E, Panes O, Downey P, Ortiz M, Tagle R, González F, Quiroga T, Caceres MS, Leighton F, Pereira J: Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int 60: 1844–1850, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Brennan ML, Hazen SL: Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis 48: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Barnett AG, van der Pols JC, Dobson AJ: Regression to the mean: What it is and how to deal with it. Int J Epidemiol 34: 215–220, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, Mori K, Inaba M, Nishizawa Y: Geriatric nutritional risk index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant 25: 3361–3365, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H: Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr 87: 106–113, 2008 [DOI] [PubMed] [Google Scholar]