Abstract
Background and objectives
Patient self-management has been shown to improve health outcomes. We developed a smartphone-based system to boost self-care by patients with CKD and integrated its use into usual CKD care. We determined its acceptability and examined changes in several clinical parameters.
Design, setting, participants, & measurements
We recruited patients with stage 4 or 5 CKD attending outpatient renal clinics who responded to a general information newsletter about this 6-month proof-of-principle study. The smartphone application targeted four behavioral elements: monitoring BP, medication management, symptom assessment, and tracking laboratory results. Prebuilt customizable algorithms provided real–time personalized patient feedback and alerts to providers when predefined treatment thresholds were crossed or critical changes occurred. Those who died or started RRT within the first 2 months were replaced. Only participants followed for 6 months after recruitment were included in assessing changes in clinical measures.
Results
In total, 47 patients (26 men; mean age =59 years old; 33% were ≥65 years old) were enrolled; 60% had never used a smartphone. User adherence was high (>80% performed ≥80% of recommended assessments) and sustained. The mean reductions in home BP readings between baseline and exit were statistically significant (systolic BP, −3.4 mmHg; 95% confidence interval, −5.0 to −1.8 and diastolic BP, −2.1 mmHg; 95% confidence interval, −2.9 to −1.2); 27% with normal clinic BP readings had newly identified masked hypertension. One hundred twenty-seven medication discrepancies were identified; 59% were medication errors that required an intervention to prevent harm. In exit interviews, patients indicated feeling more confident and in control of their condition; clinicians perceived patients to be better informed and more engaged.
Conclusions
Integrating a smartphone–based self–management system into usual care of patients with advanced CKD proved feasible and acceptable, and it appeared to be clinically useful. The results provide a strong rationale for a randomized, controlled trial.
Keywords: chronic kidney disease; hypertension; self-management; smartphone; telemedicine; ambulatory care; blood pressure; Humans; Renal Insufficiency, Chronic; Self Care
Introduction
The delivery of optimal renal care to patients with advanced CKD is complex and resource intensive (1). Most are older adults, have several concomitant diseases, and see multiple physicians, thus placing them at risk for receiving contradictory medical advice. Self-management has been advocated as a way for patients with CKD to cope with the challenges of living with their condition and gain some measure of control over their own care (2). Our team has been developing and testing innovative mobile health systems to enhance self-management by patients with complex chronic diseases. In early versions, patients monitored their BP, blood sugar, or weight and in return, received tailored messages (3–5). In randomized, controlled trials (RCTs), the systems improved several health measures and achieved a high level of patient satisfaction across all age groups and levels of familiarity with mobile technology (5,6). However, these early systems tracked only one or two physiologic parameters and did not specifically engage health care professionals in promoting patient self-management.
Patients with CKD varyingly perform a spectrum of self-care activities, such as seeking out self-education programs, controlling dietary and medication interventions, and maintaining their own medical record (7). In the past, there has been a paucity of electronic self–management tools to support such health behaviors of patients with CKD (8). That situation is changing, with new reports documenting the benefits of mobile technology to improve CKD care (9,10). To address the limitations of earlier versions of our smartphone–based self–management system, we expanded the scope of the patient-directed activities tracked, developed a personalized feedback loop in real time on the basis of these activities, and enhanced remote surveillance using a tiered clinical alert system. We also added a web–based clinical dashboard application that gave providers immediate access to summarized health information on their patients. The objectives of this 6-month proof-of-principal study were to assess the acceptability of this enhanced smartphone system in managing patients with advanced CKD and examine changes in several clinical parameters.
Materials and Methods
Study Design and Participants
The study, approved by the local research ethics board (REB14–7638), was conducted in outpatient renal clinics in Toronto, Canada. The clinics provide care to approximately 440 patients and are staffed by a multidisciplinary team of nephrologists, nurses, pharmacists, dieticians, and social workers who oversee the management of patients with advanced (stage 4 or 5) CKD. The study was limited to a maximum convenience sample of 45 patients. English-speaking patients (n=216) were sent an information newsletter encouraging individuals to discuss participation at their next scheduled clinic visit. Participants were recruited from interested patients attending the clinic over the next several weeks on a first come, first served basis. Respondents were considered eligible if they were ≥18 years old, English speaking, and able and willing to provide informed consent. Patients were excluded if they were starting dialysis or having a kidney transplant (RRT) within the next 3 months, planning to travel out of province for >10 days during the study period, living in a long–term care or rehabilitation institution, taking less than two prescription medications, participating in another interventional study, or cognitively impaired.
Study participants were followed for 6 months after recruitment or until death or start of RRT. Those who left the study within the first 2 months were replaced. Baseline demographics and clinical data were extracted from the clinical charts. Informed patient consent to participate in the study and provider consent to be interviewed post study were obtained in accordance with the Declaration of Helsinki.
Intervention
The intervention, a smartphone–based self–management system, was designed and developed as an adjunct to usual CKD care. Its architecture consisted of a smartphone application for patients, a web–based clinical dashboard application for health care providers, and a data server for information management. The smartphone application targeted four patient behavioral elements (monitoring BP, managing medications, assessing symptoms, and tracking selected laboratory test results), and in real time, it generated personalized patient messages on the basis of prebuilt algorithms. A bidirectional interface relayed data to a secure server and pharmacy and laboratory databases. The data server allowed patients’ responses to be accessible to the kidney care team through a secure clinical dashboard, and it automatically sent email medical alerts when responses demanded more immediate attention. Through the smartphone application, patients had access to prevetted CKD care resources and the contact information of their health care providers. To comply with security and privacy regulations, the smartphone was password protected, and data were encrypted (11). Moreover, data stored on the smartphone could be deleted remotely using mobile device management software (Airwatch; VMware, Atlanta, GA).
Enrolled patients were provided with a smartphone (Motorola Moto G; Motorola, Schaumburg, IL) that had the self-management application preinstalled and a Bluetooth–enabled home BP monitoring device (Life Source UA-767; A&D Medical, San Jose, CA), which was paired to the smartphone for seamless transfer of BP readings. Patients were trained on the use of the application and taught how to measure their own BPs. Patients were asked to measure their BP twice in the morning and evening for 2 days every 2 weeks. Algorithm-based messaging increased the frequency if BP was uncontrolled. The medication component of the application provided patients with their current medication list through an active interface with the clinic’s pharmacy system. On a monthly basis, the system reminded patients to reconcile their medication list by tapping yes or no responses on the smartphone’s touchscreen to three questions about any new medication, changes in dose or frequency, and any adverse effects. Similarly, patients were reminded to assess (as none, mild, or moderate) five CKD-related symptoms (fatigue, nausea, loss of appetite, shortness of breath, and ankle swelling). The interval between symptom assessments varied according to a prespecified algorithm from monthly to every other day. Laboratory test results (hemoglobin, eGFR, serum potassium, and phosphate) were automatically uploaded to the smartphone application in real time.
After each BP reading, patients immediately received the current measurement result with a predefined comment on the control of their BP on the basis of a 2-month running average and an action message on values outside the target range. For symptoms and laboratory results, every interaction initiated an instructional response on the basis of customized clinical algorithms designed to boost self-management activity. For example, patients might be instructed to contact their health care team about worsening symptoms or from serum potassium values, make adjustments to their dietary potassium intake in accord with individualized prespecified threshold values. The patients’ answers to the medication and symptom assessments were sent automatically to the clinical dashboard with a flag signaling any changes or problems that might require attention by the kidney care team. Email messages were automatically sent when responses demanded more urgent action, and the recipients were determined by medical severity (e.g., nurse and/or pharmacist only or nurse, pharmacist, and physician). The threshold values for critical alerts were established for each patient by their physician and could be changed at any time.
At regularly scheduled clinic visits, clinicians were provided with an automated one–page report to avoid disrupting their usual work pattern. It contained the 60-day average of patients’ BP readings, the previous 60-day laboratory values, a list of new or worsening symptoms, and a list of medications that were new, changed, or causing problems. More detailed information was also immediately available on the password–protected clinical dashboard.
Outcome Measures
Acceptability.
The acceptability of the smartphone self–management application was assessed by determining its adoption, adherence to recommended scheduled use, user satisfaction, and feature usage. Adoption was measured by the total number of assessments performed by patients over the complete study period, the total number of critical and noncritical alerts sent to the clinical staff, and how the alerts were handled. Adherence was derived from the number of assessments actually performed divided by the number of assessments recommended. The performance of ≥80% of the recommended number of assessments for BP, medications, and symptoms individually indicated good adherence. User satisfaction was determined from semistructured interviews with patients and providers about their experience using the smartphone system. Google Analytics was used to assess the frequency of use of the application’s features over the course of the study (12).
Clinical Measures.
Preliminary data were collected on four clinical parameters to determine whether the intervention might be effective.
(1) BP. BP was assessed in the clinic and separately at home. Clinic readings were measured using the automated sphygmomanometer BpTRU (BpTRU Medical Devices, Coquitlam, BC, Canada) with patients sitting alone in a quiet examining room. The device automatically takes six readings and averages the last five readings. The average value was used as the baseline and the 6-month measure. At home, patients measured their BP using a validated Bluetooth–enabled home BP device twice in the morning and twice in the evening daily for 7 consecutive days. The second reading values of the two readings were averaged, and the mean of the 14 readings was used to compare baseline and exit values and assess mean changes. Six-month changes were also determined for participants with uncontrolled hypertension at baseline, which was defined as mean systolic BP of ≥135 mmHg or diastolic BP ≥85 mmHg either in the clinic from automated office BP measurements or at home, and those with normal BP readings (13,14).
(2) Medications. Identification of unintentional medication discrepancies was used to assess medication effectiveness. A medication discrepancy was defined as the difference between the medication history reported by the patient and the clinic medical record. Unintentional discrepancies were those differences where no clinical documentation or justification by the prescriber(s) was found (15). We report the proportion of patients with more than one unintentional medication discrepancy for all prescribed and CKD–relevant nonprescription medications identified by the renal pharmacist during regular clinic visits.
(3) CKD-related symptoms. The effectiveness of symptom assessment was determined by the number of alerts sent to the kidney care team and the proportion that required clinical intervention. The latter was defined as an instruction to change a behavior (diet, drug, or attendance at a clinic or hospital emergency room) or a request for additional laboratory testing.
(4) CKD–specific laboratory tests. The proportion of patients in the target range for each laboratory parameter (hemoglobin, potassium, and phosphate) was compared for each individual at the beginning and end of the study.
Data Analyses
The primary purpose of the pilot study was to gather prospective data to better inform the design of an RCT; therefore, no sample size was computed. Mean values (±SDs) or medians and quartiles as appropriate for continuous variables and percentages for categorical variables were calculated for collected variables using R software (16). Generalized linear mixed effects models were used to assess BP change and trends in adherence, whereas changes in laboratory parameters were assessed using paired t tests and McNemar tests (for proportions of patients in range).
Results
Study Participants (Patients and Health Care Providers)
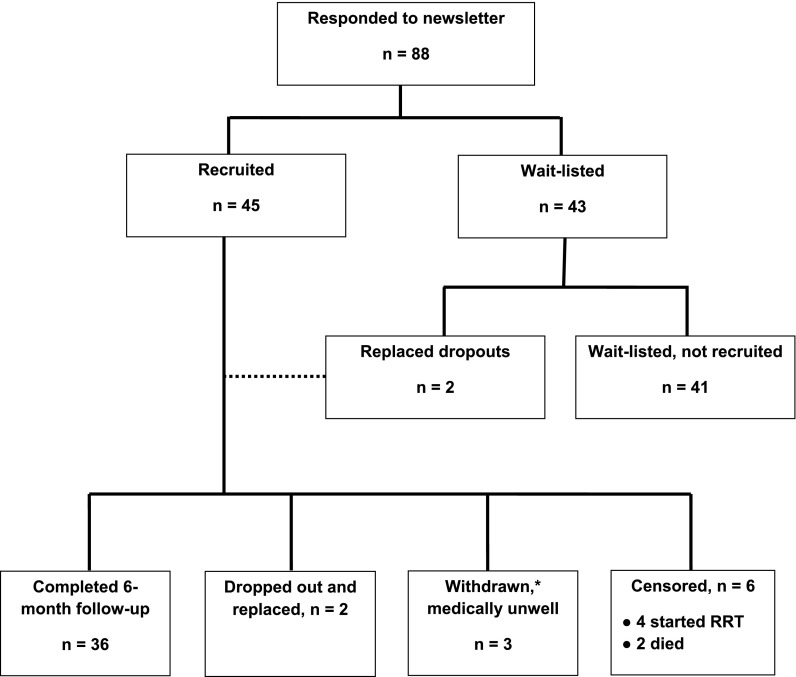
The 45 available study slots were filled within 2 weeks (Figure 1). Additional patients who expressed an interest in participating in the study were placed on a waitlist. Two patients who left the study within the first 2 months were replaced.
Figure 1.
Flow diagram of patient recruitment and involvement in study. *Three patients had active medical problems and were advised to discontinue self-management activities to allow more intense inpatient and outpatient medical management.
Patient characteristics are shown in Table 1; 33% were age ≥65 years old, and 50% had a college/university education. Sixty percent had not previously used a smartphone, and 27% had not previously used a conventional cell phone. Patient confidence in using technological devices and experience with home BP monitoring are outlined in Supplemental Table 1.
Table 1.
Patient characteristics
| Variable | Study Group, n=47 |
|---|---|
| Mean age, yr | 59.4±14 |
| Age in year categories, n (%) | |
| ≤44 | 6 (13) |
| 45–54 | 10 (21) |
| 55–64 | 13 (28) |
| 65–74 | 11 (23) |
| ≥75 | 7 (15) |
| Men, n (%) | 26 (55) |
| Ethnicity, n (%) | |
| White/European | 33 (70) |
| Asian | 13 (27) |
| Black/West Indian | 1 (3) |
| Education, n (%) | |
| Not available | 1 (2) |
| Primary school | 4 (9) |
| High school | 12 (25) |
| College/university (undergraduate) | 24 (51) |
| Graduate school | 6 (13) |
| Days followed in RMC | |
| Median (quartiles 1, 3) | 919 (463, 1370) |
| Use of technology devices,a n (%) | |
| Personal computer (desktop/laptop) | 37 (82) |
| Smartphone | 18 (40) |
| Cellphone | 33 (73) |
| Tablet | 26 (58) |
| Home BP monitor | 33 (73) |
| Mean BP at baseline, mmHg | |
| Systolic BP | 130.6±17.2 |
| Diastolic BP | 78.9±10.9 |
| Patients with uncontrolled hypertension, n (%) | 17 (36) |
| Mean no. of medications ±SD | 9.5±4.2 |
| Median no. of medications (quartiles 1, 3) | 10 (7, 12.5) |
| Primary cause of CKD, n (%) | |
| Diabetes | 7 (15) |
| Hypertension | 5 (11) |
| Glomerular/vasculitis | 15 (31) |
| Hereditary | 8 (17) |
| Drug toxicity | 5 (11) |
| Other | 7 (15) |
| Comorbidity | |
| Mean no. of comorbid conditions | 2.9±1.4 |
| Patients with ≥2 comorbid conditions, n (%) | 38 (81) |
| Median (quartiles 1, 3) | 3 (2, 4) |
| CKD stage, n (%) | |
| 4 | 22 (47) |
| 5 | 25 (53) |
RMC, renal management clinic.
n=45 (information missing on two patients).
Outcomes
Acceptability.
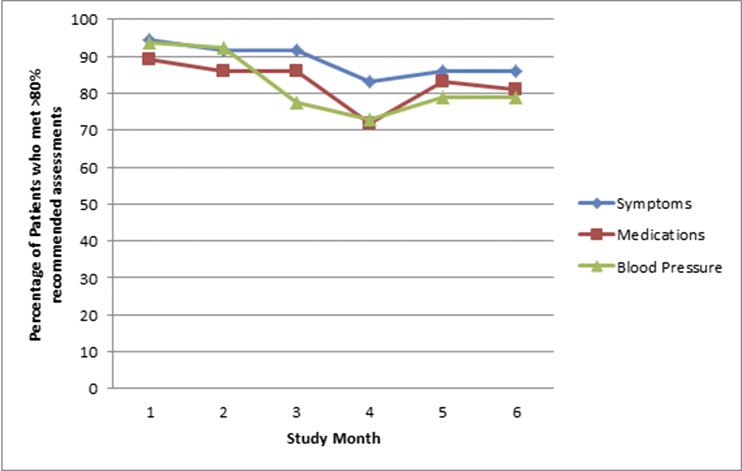
Adoption of the smartphone application is shown in Figure 2. The average number of clinical BP readings (second of two taken at each sitting) was 4.5±0.8 per week. The total numbers of medication and symptom reviews submitted during the 6-month study were 250 and 489, respectively; 96% of study participants completed the required clinic blood work during the study period (baseline, interim, and study end). Monthly adherence rates were mostly >80%. The test for trend showed no drop off in interest over time (P>0.05). The transient decline at 3 and 4 months coincided with Christmas/New Year (Figure 2).
Figure 2.
Adherence rates per month (symptoms, medications, and BP assessments using the mobile application).
Of 47 patients, 38 patients participated in an exit interview; four declined, three had severe medical complications or were hospitalized at the time of interviews, and two had died. All but two indicated that the application made them feel more connected with their health care providers and that they wished to continue using it after the study. They also reported that they would be willing to buy the mobile application kit (including the Bluetooth–enabled BP device) if it were commercially available. Two patients stated that they would not continue using the application after the study: one patient said that it caused anxiety, and the other patient, an individual who was already monitoring BP routinely, found the additional features unhelpful. The clinician interviews (nine participating nephrologists, three nurses, and three pharmacists) were uniformly positive. The nephrologists felt that the patients were better informed, and this greatly improved the quality of the clinic visits. The nurses found that the system helped them prioritize the patients who needed more attention. The pharmacists felt that the medication review alerts lead to interventions that mitigated adverse drug events. None reported disruption in their workflow, and all endorsed future use of the smartphone system.
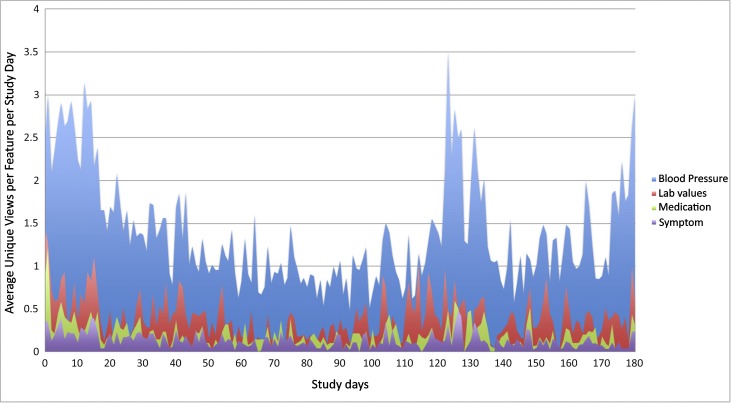
Unique mobile application views were captured and summarized in Figure 3 using Google Analytics (12). Throughout the study, patient users viewed and used the BP feature most frequently followed by laboratory results, medications, and symptoms. Care team information and links to health resources were used the least. Increased use of the smartphone application, especially BP and laboratory results, seemed to coincide with days surrounding patients’ clinic visits.
Figure 3.
Average unique views per application feature by day over the course of the study.
Clinical Measures.
BP.
The mean BP readings at the beginning and the end of the study and the changes in the subset of patients with both sets of readings (n=36) are presented in Table 2. BP measured both in the clinic and at home fell from entry to exit. Although changes in office BP were not statistically significant, the mean changes in home readings were significant (systolic BP, −3.4 mmHg; 95% confidence interval, −5.0 to −1.8 and diastolic BP, −2.1 mmHg; 95% confidence interval, −2.9 to −1.2). For those with uncontrolled hypertension at baseline, the mean falls in systolic and diastolic BP were greater than those of patients who were normotensive; 27% with normal clinic BP readings had newly identified masked hypertension (normal clinic and elevated home BP readings). Finally, 34 interventions resulted from the critical BP alerts (32 high and two low alerts). Of these, seven patients required medication changes, but none required an extra clinic visit.
Table 2.
Mean BP at the beginning and end of the study by site of assessment
| Site, Patients, and Visit | No. | SBP Mean, mmHg | SD | DBP Mean, mmHg | SD |
|---|---|---|---|---|---|
| Clinic | |||||
| All | |||||
| Baseline | 47 | 130.6 | 17.4 | 78.9 | 11.1 |
| Exit | 36 | 122.8 | 15.5 | 77.3 | 13.4 |
| ∂ | 36 | −6.8 | −0.9 | ||
| 95% CI | 36 | −48.2 to 34.7 | −19.4 to 17.7 | ||
| Uncontrolled hypertension | |||||
| Baseline | 25 | 141.9 | 14.9 | 84.4 | 10.2 |
| Exit | 20 | 126.3 | 13.6 | 80.0 | 13.7 |
| ∂ | 20 | −13.5 | −2.6 | ||
| 95% CI | 20 | −58.5 to 31.5 | −22.1 to 17.0 | ||
| Normotensives | |||||
| Baseline | 22 | 117.9 | 9.5 | 72.7 | 8.5 |
| Exit | 16 | 118.6 | 17.1 | 73.9 | 12.6 |
| ∂ | 16 | 1.6 | 1.2 | ||
| 95% CI | 16 | −32.0 to 35.3 | −17.3 to 19.8 | ||
| Home | |||||
| All | |||||
| Baseline | 47 | 134.5 | 13.8 | 79.4 | 11.2 |
| Exit | 36 | 131.3 | 13.5 | 77.3 | 12.4 |
| ∂ | 36 | −3.4 | −2.1 | ||
| 95% CI | 36 | −5.0 to −1.8 | −2.9 to −1.2 | ||
| Uncontrolled hypertension | |||||
| Baseline | 25 | 139.6 | 15.9 | 80.4 | 11.9 |
| Exit | 20 | 133.9 | 13.2 | 77.1 | 12.3 |
| ∂ | 20 | −4.3 | −1.5 | ||
| 95% CI | 20 | −6.6 to −2.1 | −2.6 to −0.4 | ||
| Normotensives | |||||
| Baseline | 22 | 128.7 | 8.1 | 78.2 | 10.6 |
| Exit | 16 | 128.0 | 13.5 | 77.6 | 12.8 |
| ∂ | 16 | −2.2 | −2.8 | ||
| 95% CI | 16 | −4.4 to −0.1 | −4.2 to −1.5 |
SBP, systolic BP; DBP, diastolic BP; ∂, change in BP readings from baseline to exit; 95% CI, 95% confidence interval.
Medication.
The percentage of patients with one or more clinically relevant discrepancies was 80% at baseline and 72% at exit. Of the 250 medication assessments performed by patients, 153 (61%) reported no change. For the remainder (n=97), there were 127 medication discrepancies (Table 3), with 59% requiring intervention(s) to prevent harm.
Table 3.
Types and frequency of medication discrepancies reported (n=127)
| Type | No. of Discrepancies (%) | Clinical Examples of Medication Errors Detected through the Smartphone Application |
|---|---|---|
| Dose of medication incorrect | 53 (42) | Patient started on pregabalin. Dose adjustment identified by pharmacist and intervention made to adjust dose according to renal function. Follow-up monitoring with serum creatinine made and instructions to the patient to monitor for increased drowsiness to determine if readjustment of dosing is needed in the future. |
| Taking drug not on the medication list | 39 (31) | Patient was prescribed indomethacin from an emergency visit for an acute attack of gout. Patient reported the new medication using the mobile application. The pharmacist advised the patient to stop taking it and communicated with the nephrologist to prescribe colchicine instead. Serum creatinine on repeat testing was elevated and did not return to baseline on additional testing. |
| Not on or no longer taking drug on the medication list | 19 (15) | Patient reported not taking sodium bicarbonate that was on the medication list. Clinic requested patient to get repeat blood work to reassess therapy. |
| Dosing frequency incorrect | 5 (4) | Patient reported taking hydralazine once daily and not twice daily as prescribed. The pharmacist contacted the patient, determined that the patient was confused over frequency of doses, and advised how to make necessary dosing adjustments. Patient was hypertensive before dose adjustment, and BP was regulated with the new dose. |
| Adverse drug reaction | 4 (3) | Patient was prescribed gemfibrozil by the endocrinologist for high triglycerides. Patient reported the new medication through the mobile application. Serum creatinine increase was noted during a follow-up renal visit and did not return to baseline after stopping gemfibrozil. |
| Taking two or more drugs from the same drug class or indication with no clinical justification | 3 (2) | Patient was prescribed isosorbide dinitrate by a cardiologist who was unaware that the patient was already on the nitroglycerin patch. Patient reported the new medication through the mobile application, and the pharmacist advised the patient to stop using the patch. Patient experienced decreased BP and dizziness while on both nitrates for a few days. |
| Taking medication on their own without medical advice or altered medication without medical consultation | 4 (3) | Patient started taking hydrochlorothiazide because of edema without medical advice. This medication discrepancy was identified through the smartphone application on a monthly medication assessment. |
CKD-Related Symptoms.
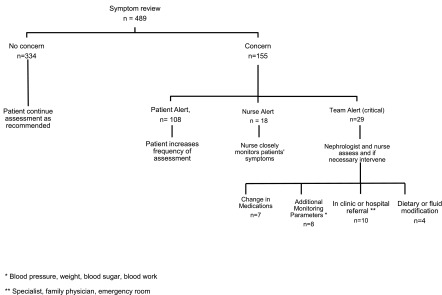
Symptom assessments resulted in 108 alerts being sent to patients advising an increase in the frequency of monitoring. There were 47 alerts for 13 patients that were also sent to the clinical team. These alerts resulted in telephone contact with the kidney care team that further led to interventions in 29 instances (Figure 4).
Figure 4.
Flow diagram illustrating the sequence of events that occurred after patients completed their symptom assessments. *BP, weight, blood sugar, or blood work; **specialist, family physician, or emergency room.
CKD–Specific Laboratory Tests.
There were no appreciable differences in the proportions of patients in the target ranges for potassium, phosphate, or hemoglobin between baseline and exit (Table 4).
Table 4.
Comparison of CKD–specific laboratory results between baseline and 6 months
| Laboratory Values (Range) | Baseline, n=47 | End, n=36 | P Value |
|---|---|---|---|
| Serum potassium (3.2–5.0), mEq/L | 4.7±0.5 | 4.6±0.6 | 0.18 |
| Patients within range, % | 78.7 | 80.6 | 0.22 |
| Serum phosphate (<4.6), mg/dl | 4.21±0.41 | 4.21±0.87 | 0.28 |
| Patients within range, % | 74.5 | 75 | >0.99 |
| Hemoglobin (10.0–12.0), g/dl | 11.8±1.7 | 11.8±1.5 | 0.52 |
| Patients within range, % | 29.8 | 25 | >0.99 |
| Patients receiving ESA, % | 40.4 | 52.8 | 0.51 |
ESA, erythropoietin-stimulating agent.
Discussion
Our findings provide valuable information on the use of a smartphone–based self–management system to supplement the care of patients with advanced CKD. The smartphone application streamlined important tasks and supported patient decision making in real time through built-in algorithms for each behavioral component. It also connected the patient to their health care team without being intrusive on either side and had a dynamic and customizable alerting system. Moreover, it allowed for bidirectional communication of pertinent health information through seamless integration with peripheral monitoring devices (Bluetooth–enabled BP cuff) and interfaced with the pharmacy and laboratory data repositories, so that patients and clinicians had useful and accurate information immediately available to them. This led to early identification of health problems that arose between scheduled clinic visits. To the best of our knowledge, this is the first mobile health system for CKD care trialed, which embodies important elements of the Chronic Care Model, because it promotes self-care, integrates clinical information systems, provides decision support, and enhances the delivery of health care (17–19).
Our study showed that patients maintained a high level of use of the system throughout the 6-month study, suggesting sustainability. It was developed using an iterative, user–centered design method to ensure an exceptional user experience, even for those with a disability or unfamiliar with mobile technology. The automatic transfer of BP readings circumvented the pitfalls of manual entry (20), and user exit interviews suggested that health care providers had more confidence in the values transmitted. Of equal importance, the significant reduction in home BP levels, consistent with our previous work, implies that the smartphone system may be clinically effective (3,6).
Of the many application features, patients used test result features most often. For BP, this may, in part, be related to the preprescribed BP monitoring schedule, which required patients to measure their BP relatively frequently. However, next to BP, patients viewed their laboratory results most frequently, although there were few new results over the course of the study period. The reasons for the patients’ focus on test results are unclear. One might conjecture that they viewed the information, which generally is not readily available to patients, to guide decision making or objectively gauge their clinical status. In contrast, the symptom feature was used infrequently. Despite the relatively few symptom views, there was a large number of symptom alerts, of which almost one third were judged by clinicians to be critical. This discordance warrants additional exploration.
The smartphone system identified a large number of medication discrepancies by patients reconciling their medications with the smartphone application. More than one half required an intervention by the pharmacist to prevent harm to the patient from the error, suggesting that the technology may contribute to patient safety and quality initiatives. Unlike other mobile applications, we believe that this is the only mobile application for outpatient use that supports patient–driven medication reconciliation with a feedback loop to correct errors (10,21,22). Communication of medication-related information is particularly crucial for this patient population, because patients frequently experience medication errors as a result of fragmented communication between multiple prescribers (23–26).
Our study is limited by the lack of a comparison group to control for secular trends and the possibility of selection bias. Thus, our results should be viewed as hypothesis generating. Changes in BP could not be linked to a particular component of the intervention because of the pilot nature of the study. There was also no formal cost–effectiveness analysis. Nevertheless, this system takes advantage of consumer-ready products and rapidly growing smartphone ownership by American adults (64% in 2015 compared with 35% in 2011) (27). Furthermore, we used automated messaging and alerts to mitigate increases in health care personnel costs.
The smartphone–based self–management system appeared to be clinically useful in improving CKD care, had a high level of acceptance by patients, and provided timely and reliable information to the clinical care team without disrupting their workflow pattern. These data provide a strong rationale for undertaking a longer RCT to test whether it can cost-effectively improve health outcomes when used as an adjunct to usual care.
Disclosures
None.
Acknowledgments
We thank the patients who agreed to participate in the study, the clinical staff in the Renal Management Clinic at the University Health Network for their help and support, the pharmacy students from the University of Toronto for their contribution in data collection, Kelly Min for her dedication in ensuring the success of the project, Akib Uddin for shepherding the technological aspects of the study, and the software developers and engineers at the Centre for Global eHealth Innovation (Stephanie So, Mark Iantorno, David Ngo, Anthony Mei, John Li, and Melanie Yeung).
The project sponsors and funding sources were the Ontario Ministry of Health Alternative Funding Program, the Ontario Renal Network, and the University Health Network Fast Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Smartphone Apps: A Patient’s New Best Friend?,” on pages 935–937.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10681015/-/DCSupplemental.
References
- 1.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bodenheimer T, Lorig K, Holman H, Grumbach K: Patient self-management of chronic disease in primary care. JAMA 288: 2469–2475, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Logan AG, McIsaac WJ, Tisler A, Irvine MJ, Saunders A, Dunai A, Rizo CA, Feig DS, Hamill M, Trudel M, Cafazzo JA: Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens 20: 942–948, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR: Design of an mHealth app for the self-management of adolescent type 1 diabetes: A pilot study. J Med Internet Res 14: e70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ: Mobile phone-based telemonitoring for heart failure management: A randomized controlled trial. J Med Internet Res 14: e31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, Feig DS, Cafazzo JA: Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension 60: 51–57, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Institue of Medicine Committee on Health Care in America : Crossing the Quality Chasm: A New Health System for the 21st Century, Washington, DC, National Academy Press, Institute of Medicine, 2001 [Google Scholar]
- 8.Ong SW, Jassal SV, Porter E, Logan AG, Miller JA: Using an electronic self-management tool to support patients with chronic kidney disease (CKD): A CKD clinic self-care model. Semin Dial 26: 195–202, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Becker S, Kribben A, Meister S, Diamantidis CJ, Unger N, Mitchell A: User profiles of a smartphone application to support drug adherence--experiences from the iNephro project. PLoS One 8: e78547, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamantidis CJ, Ginsberg JS, Yoffe M, Lucas L, Prakash D, Aggarwal S, Fink W, Becker S, Fink JC: Remote Usability testing and satisfaction with a mobile health medication inquiry system in CKD. Clin J Am Soc Nephrol 10: 1364–1370, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HIMSS Mobile Security Working Group: Security of Mobile Computing Devices in the Healthcare Environment, 2011. Available at: www.himss.org/files/HIMSSorg/content/files/PrivacySecurity/HIMSS_Mobility_Security_in_Healthcare_Final.pdf. Accessed August 28, 2015
- 12.Google: Analytics for Mobile Apps, 2015. Available at: www.google.com/analytics/mobile/. Accessed August 28, 2015
- 13.Myers MG, Kaczorowski J, Paterson JM, Dolovich L, Tu K: Thresholds for diagnosing hypertension based on automated office blood pressure measurements and cardiovascular risk. Hypertension 66: 489–495, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F Task Force Members : 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31: 1281–1357, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA: Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 61: 1689–1695, 2004 [DOI] [PubMed] [Google Scholar]
- 16.R Core Team: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2014. Available at: http://www.R-project.org/ Accessed August 28, 2015
- 17.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A: Improving chronic illness care: Translating evidence into action. Health Aff (Millwood) 20: 64–78, 2001 [DOI] [PubMed] [Google Scholar]
- 18.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R: Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev 12: CD007459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A: The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med 10: e1001362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H: Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens 11: 1413–1417, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Mira JJ, Navarro I, Botella F, Borrás F, Nuño-Solinís R, Orozco D, Iglesias-Alonso F, Pérez-Pérez P, Lorenzo S, Toro N: A Spanish pillbox app for elderly patients taking multiple medications: Randomized controlled trial. J Med Internet Res 16: e99, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC: Smartphone medication adherence apps: Potential benefits to patients and providers. J Am Pharm Assoc (2003) 53: 172–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong SW, Fernandes OA, Cesta A, Bajcar JM: Drug-related problems on hospital admission: Relationship to medication information transfer. Ann Pharmacother 40: 408–413, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Cardone KE, Bacchus S, Assimon MM, Pai AB, Manley HJ: Medication-related problems in CKD. Adv Chronic Kidney Dis 17: 404–412, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Manley HJ, Drayer DK, McClaran M, Bender W, Muther RS: Drug record discrepancies in an outpatient electronic medical record: Frequency, type, and potential impact on patient care at a hemodialysis center. Pharmacotherapy 23: 231–239, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Pai AB, Cardone KE, Manley HJ, St Peter WL, Shaffer R, Somers M, Mehrotra R Dialysis Advisory Group of American Society of Nephrology : Medication reconciliation and therapy management in dialysis-dependent patients: Need for a systematic approach. Clin J Am Soc Nephrol 8: 1988–1999, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A: Pew Research Survey on U.S. Smartphone Use in 2015. Available at: www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/#smartphones. Accessed December 11, 2015