Abstract
Maintenance of a normal serum phosphate level depends on absorption in the gut, reabsorption and excretion by the kidney, and the flux between the extracellular and skeletal pools. Phosphate homeostasis is a coordinated, complex system of crosstalk between the bone, intestine, kidney, and parathyroid gland. Dysfunction of this system has serious clinical consequences in healthy individuals and those with conditions, such as CKD, in which hyperphosphatemia is associated with increased risks of cardiovascular morbidity and mortality. The last half-century of renal research has helped define the contribution of the parathyroid hormone, calcitriol, fibroblast growth factor 23, and Klotho in the regulation of phosphate. However, despite new discoveries and insights gained during this time, what remains unchanged is the recognition that phosphate retention is the initiating factor for the development of many of the complications observed in CKD, namely secondary hyperparathyroidism and bone and cardiovascular diseases. Controlling phosphate load remains the primary goal in the treatment of CKD. This review discusses the clinical effects of dysregulated phosphate metabolism, particularly in CKD, and its association with cardiovascular disease. The importance of early control of phosphate load in the treatment of CKD is emphasized, and the latest research in the treatment of phosphate retention is discussed.
Keywords: phosphate, klotho, parathyroid hormone, chronic kidney disease, calcitriol, cardiovascular diseases, humans, hyperparathyroidism, secondary, hyperphosphatemia, fibroblast growth factor 23
Introduction
Phosphorous, in the form of inorganic phosphate, is a macronutrient essential to a variety of cellular functions, including structure, energy production, metabolic pathways, and signaling. A complex system involving diet, multiorgan crosstalk, hormones, and other factors coordinates to regulate phosphate and keep serum levels within a normal range of 2.48–4.65 mg/dl for adults and 4.65–8.22 mg/dl for infants. Maintenance of normal serum phosphate levels is dependent on the absorption of dietary phosphate in the gut, reabsorption and excretion of phosphate in the kidney, and the flux of phosphate between the extracellular and skeletal pools. A regular Western diet provides approximately 20 mg/kg per day of phosphate, of which approximately 13 mg/kg per day are absorbed in the proximal intestine (mainly the jejunum) and approximately 7 mg/kg per day are eliminated in the feces (1). The absorbed phosphate enters the extracellular fluid and moves in and out of the skeletal pool as needed (approximately 3 mg/kg per day). Phosphate is freely filtered through the glomerulus and reabsorbed via the renal sodium/phosphate type 2 cotransporters NaPi-2a and NaPi-2c, which are expressed on the luminal side of the proximal tubular epithelial cells. Phosphate reabsorption in the kidney is greatly affected by parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and dietary phosphate. In the gut, dietary phosphate is absorbed through passive paracellular diffusion (driven by high luminal phosphate concentration) and by active cell–mediated transport of phosphate via the NaPi-2b cotransporter on the luminal side of the enterocyte (regulated by dietary phosphate, calcitriol, and FGF23).
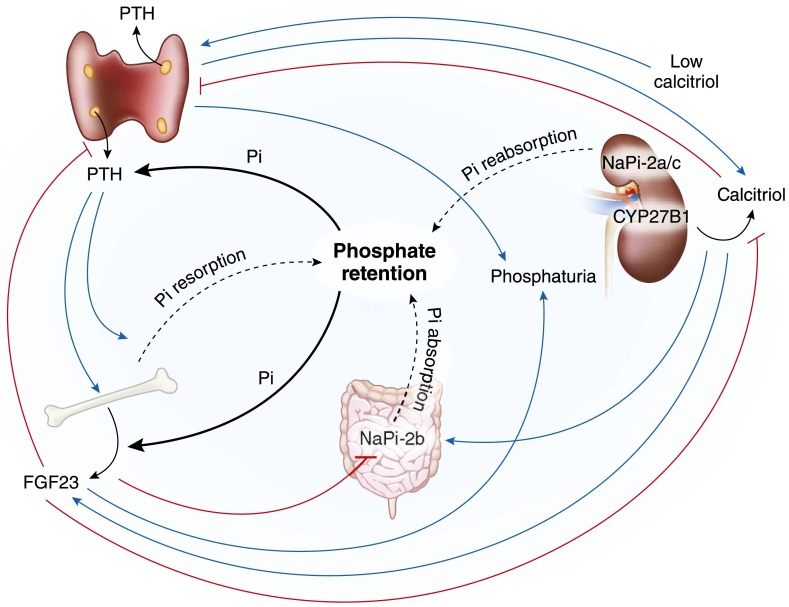
Phosphate homeostasis is regulated by a system of positive and negative feedback loops involving the bone, intestine, kidney, and parathyroid gland (PTG) (Figure 1). The main players in the regulation of phosphate involve a trio of hormones: PTH, calcitriol, and FGF23, a potent phosphaturic glycoprotein secreted by osteoblasts and osteocytes, which binds to the fibroblastic growth receptor 1 (FGFR1) in the presence of its coreceptor Klotho (2). Elevated levels of serum phosphate increase secretion of PTH and FGF23, both of which inhibit phosphate reabsorption in the kidney by decreasing the expression of NaPi-2a and NaPi-2c and, thus, promote phosphaturia (3). PTH is reported to act promptly to induce phosphaturia, whereas the FGF23 response is more delayed and may be more important in long-term regulation (4). High circulating levels of calcitriol or PTH (directly or indirectly via calcitriol) increase synthesis and secretion of FGF23 (5). The increase in FGF23 decreases phosphate absorption in the gut by inhibiting NaPi-2b expression and suppressing circulating calcitriol, which will, in turn, inhibit intestinal absorption of phosphate (6). FGF23 decreases circulating calcitriol by decreasing the renal 1α-hydroxylase, the enzyme that converts 25-hydroxyvitamin D3 to its active form calcitriol, and increasing renal 24-hydroxylase, the enzyme responsible for the catabolism of calcitriol. Therefore, increased renal excretion of phosphate and decreased intestinal phosphate absorption will lower the concentration of phosphate in the circulation. An increase in FGF23 also suppresses levels of PTH (7,8).
Figure 1.
Phosphate homeostasis: A complex crosstalk between the kidney, parathyroid gland (PTG), bone, and intestine. Phosphate reabsorption in the kidney via NaPi-2a/c cotransporters, absorption in the gut via NaPi-2b cotransporter, and resorption from the bone contribute to the retention of phosphate (black dashed lines). Phosphate retention increases levels of the parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) hormones (black solid lines), both of which inhibit phosphate reabsorption in the kidney by decreasing expression of NaPi-2a/c, resulting in phosphaturia. The increase in FGF23 decreases phosphate absorption in the gut by inhibiting NaPi-2b expression and suppressing circulating calcitriol, which in turn, will inhibit intestinal absorption of phosphate. A negative feedback loop exists between PTH and FGF23; PTH increases FGF23 (both directly and indirectly via calcitriol), whereas FGF23 inhibits PTH. High calcitriol levels inhibit PTH and stimulate FGF23, whereas low calcitriol levels stimulate PTH. Dysregulation of this homeostasis in CKD results in excess phosphate retention, high levels of FGF23 and PTH, and low levels of circulating calcitriol. Solid blue lines indicate stimulation, and red lines indicate inhibition. Pi, phosphate; NaPi-2a/c, sodium/phosphate type 2 cotransporters.
Dysfunction of phosphate regulation has serious clinical consequences. Indeed, studies in humans show that even small increases in serum phosphate levels (within the normal or near-normal range) correlate with increased morbidity and mortality (9). This indicates that detection and treatment of elevated serum phosphate may be important in healthy individuals as well as those with conditions, such as CKD, in which hyperphosphatemia is associated with increased risks of cardiovascular events and death (10,11).
Phosphate and CKD
In 1960, Bricker et al. (12) proposed the “intact nephron hypothesis” that set the path for kidney research for the next 50 years. The hypothesis states that, although the diseased kidney consists of a diminished number of nephrons, the remaining nephrons are functionally normal. To maintain homeostasis of any given solute, renal function of the diseased kidney must undergo adaptive changes, wherein the excretion rate of each functioning nephron must increase progressively to compensate for damaged nephrons. With regard to phosphate, an adaptive decrease in tubular reabsorption will prevent a rise in the serum levels of phosphate until advanced deterioration of renal function occurs. However, a biologic price is paid for these adaptive changes. As Bricker (13) proposed in his “trade-off hypothesis,” increasing nephron function to maintain solute homeostasis can result in abnormalities of the uremic state that will adversely contribute to the uremic syndrome. The most common example of this tradeoff is the development of secondary hyperparathyroidism (2° HPT). The traditional hypothesis that development of 2° HPT was caused by phosphate retention and low levels of calcium and calcitriol has recently been updated to include the contribution of FGF23 (14). The updated hypothesis maintains that decreased phosphate clearance, caused by loss of functioning renal mass, leads to an increase in FGF23 secretion, which will, in turn, act on the kidney to decrease phosphate reabsorption, suppress calcitriol synthesis, and stimulate its degradation. Decreased calcitriol levels stimulate an increase in PTH levels early in renal disease, whereas the elevated phosphate levels that occur later in renal disease cause a further increase in PTH secretion.
The importance of phosphate retention in CKD was appreciated early on in renal research. Daily maintenance of phosphate requires that the amount of phosphate that enters the extracellular fluid equals the amount that is excreted into the urine (13). In 1968, our laboratory found that the tubular reabsorption of phosphate decreases in proportion to the severity of CKD (15). With a normal GFR of 120 ml/min, approximately 10% of filtered phosphate is excreted, whereas at a very low GFR of <20 ml/min, the excretion of phosphate per nephron increases to approximately 80%–90%. We were able to prevent development of 2° HPT by severely restricting dietary phosphate at the beginning of nephron damage (16). In subsequent studies, we found that when GFR was reduced in a stepwise manner, while at the same time proportionally restricting phosphate intake, there was no need for nephron adaptation and ultimately, no development of 2° HPT (17). In essence, preventing the adaptation of phosphate excretion prevented the development of 2° HPT. These studies, however, provided only indirect evidence of the effect of phosphate on development of 2° HPT. Portale et al. (18) subsequently found that a low-phosphate diet was able to normalize calcitriol and PTH levels in patients with CKD. Conversely, with a high-phosphate diet, calcitriol levels decreased even further than the already low levels found in the patients, and PTH levels increased even more. Therefore, manipulation of dietary phosphate induced changes in the circulating levels of calcitriol, and this was responsible, at least in part, for the observed changes in PTH.
Although it is generally accepted that a transient and recurrent or sustained hypocalcemia is an important factor in the development of 2° HPT, early studies in our laboratory showed that hypocalcemia, per se, was not essential for the development of 2° HPT in CKD (19). When ionized calcium was maintained at a normal (or even increased) level in nephrectomized dogs, the development of 2° HPT still occurred, indicating that other mechanisms, perhaps involving phosphorus or calcitriol, were at play in the abnormal secretion of PTH.
PTH is directly regulated by calcium via the calcium-sensing receptor (CaSR) and by calcitriol via the vitamin D receptor. Although the search for a phosphate receptor has so far been unsuccessful, studies in our laboratory and by others have shown that phosphate does directly regulate PTH, independent of calcitriol and calcium. Both work in our laboratory (20) and the work by Almaden et al. (21) showed that high phosphate directly stimulated secretion of PTH in organoid cultures of fresh rat glands. Maintenance of the three-dimensional architecture of the parathyroid tissue is mandatory to obtain the stimulatory effect of phosphate, because a direct effect of in vitro phosphate was obtained when using bovine parathyroid tissue sections but not dispersed cells (22). Phosphate, therefore, has both direct and indirect effects on PTH secretion and the development of 2° HPT.
Dietary phosphate may control 2° HPT by affecting the ability of the PTGs to sense calcium. It is well accepted that the PTGs of patients with CKD have reduced expression of the CaSR (23–25). Brown and coworkers (26,27) from our group found that a high-phosphate diet that promoted 2° HPT led to downregulation of the CaSR and reduced the sensitivity of the PTGs to suppression of PTH by calcium. A diet low in phosphate maintained and restored expression of the CaSR to normal levels and restored the sensitivity of the PTG to calcium (26,28). The study was also the first to show that the effect of dietary phosphate on PTH was rapid (28); PTH levels were normalized within 1 day of changing the diet of uremic rats from high to low phosphate.
It should be pointed out, however, that despite recognition of the importance of phosphate retention in CKD, it is not a settled issue, at least in terms of traditional phosphate balance studies. In a recent study by Hill et al. (29), patients with stages 3 and 4 CKD remained in overall neutral phosphate balance. Such findings highlight the fact that many factors (including FGF23 and Klotho, as discussed below) are at play in the complex nature of phosphate homeostasis.
FGF23 and Klotho in CKD
FGF23 is proposed to be an early biomarker of abnormal mineral metabolism in CKD. In rats with induced nephritis, a significant increase in FGF23 and PTH levels was evident before a rise in serum phosphate (30). In patients who were predialysis, Isakova et al. (31) found that FGF23 is elevated before PTH and phosphate in CKD (the Chronic Renal Insufficiency Cohort Study). A study by Chudek et al. (32), however, disputes that FGF23 is a biomarker of abnormal mineral metabolism in early CKD, at least not in the elderly. As well, Evenepoel et al. (33) support a “phosphate-centric” paradigm for the development of 2° HPT; a rise in serum phosphate levels was not prevented by FGF23 in patients with early CKD (stages 1–3). In addition, serum phosphate levels were inversely associated with eGFR in early CKD, and there was no independent effect of FGF23 on the fractional excretion of phosphate.
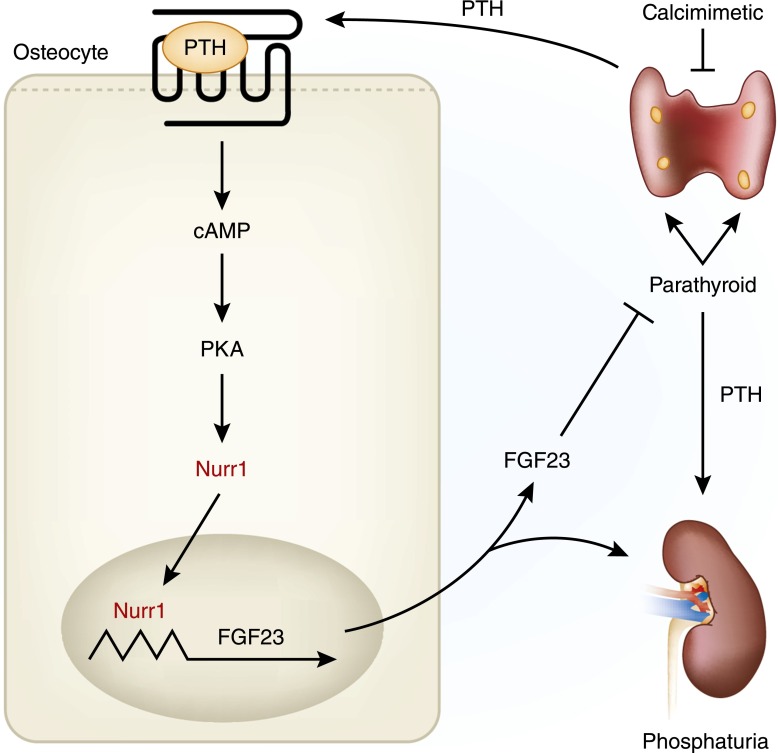
The effect of PTH on FGF23 is also important in the regulation of phosphate in CKD. Lavi-Moshayoff et al. (34) showed that PTH increases FGF23 expression and found that parathyroidectomy prevented and corrected the increased serum FGF23 that develops during short–term kidney failure in rats. Likewise, López et al. (35) showed both direct and indirect effects of PTH on FGF23 secretion in rats, with the indirect effects being mediated by changes in calcitriol concentration. Recently, Meir et al. (36) showed that PTH increases FGF23 via nuclear receptor–associated protein 1, a nuclear orphan receptor, in UMR106 cells (Figure 2). In addition, the calcimimetic cinacalcet decreased PTH, nuclear receptor–associated protein 1, and FGF23 expression in uremic rats. These studies support other findings in patients on dialysis that cinacalcet decreased serum PTH and led to decreased serum FGF23 levels (37). Moreover, studies in our laboratory showed that cinacalcet decreases PTH and FGF23 and induces hyperphosphatemia in rats with renal failure (38).
Figure 2.
Parathyroid hormone (PTH) -fibroblast growth factor 23 (FGF23) feedback loop. PTH binds to the parathyroid hormone receptor (PTH1R) on osteocytes and osteoblasts and activates protein kinase A (PKA), which increases nuclear receptor–associated protein 1 (Nurr1) expression. Nurr1 then binds to defined nerve growth factor-induced clone B response elements in the FGF23 promoter and induces FGF23 transcription. FGF23, in turn, inhibits PTH production. In CKD, however, hyperparathyroidism increases Nurr1 and FGF23 expression, but downregulation of the FGF23 receptor complex (Klotho-fibroblastic growth receptor 1 [FGFR1]) makes the parathyroid gland (PTG) and kidney resistant to the actions of FGF23. Calcimimetics decrease PTH, Nurr1, and FGF23. Modified from reference 36, with permission.
Phosphate itself is an important regulator of FGF23 both indirectly and directly. In normal mice, dietary phosphate regulates serum FGF23 levels, which correspond to changes in the bone FGF23 gene expression (39). In healthy human subjects, oral phosphate loading increased serum FGF23 levels (40). In addition, Takasugi et al. (41) showed in rats that oral phosphate administration increases serum FGF23 levels, at least partially, by stimulation of PTH secretion. In vitro studies have shown a direct effect of phosphate on FGF23. IDG-SW3 cells differentiate from osteoblast to late osteocyte–like cells and express relatively abundant levels of FGF23 after 3–5 weeks in culture (42); Ito et al. (43) reported that high-phosphate concentrations upregulated FGF23 mRNA in these cells. An earlier study by Ito et al. (44) shows that the FGF23 promoter is regulated by both phosphate and calcitriol in the K-562 erythroleukemia cell line and that the two factors have a synergistic effect on FGF23 expression. In an established rat calvaria osteoblast developmental model, Yamamoto et al. (45) showed that phosphate alone, and even more so when combined with calcitriol, increased FGF23 production. In the rat osteoblastic UMR106 cell line, Hori et al. (46) showed that phosphate and calcitriol directly enhance FGF23 transcription by different mechanisms. Additional studies are needed to clarify both the direct and indirect mechanisms of action by which phosphate regulates FGF23. Although there has been much debate in the literature as to whether PTH or FGF23 constitutes the primary impetus in the development of abnormal mineral metabolism in CKD, the fact remains that phosphate retention is the driving force for elevation of both hormones.
It should be noted that in addition to the well known stimulators of FGF23 (i.e., calcitriol, PTH, and phosphate), other factors, such as inflammation, calcium, metabolic acidosis, leptin, and iron deficiency, as well as certain intravenous iron preparations are also known inducers of FGF23 (47–51). An increase in FGF23 can be the result of increased transcription or a decrease in cleavage of FGF23, which is known to be impaired in CKD (52).
α-Klotho (Klotho) is a single–pass transmembrane 130-kD protein that originally was identified as an antiaging factor, but now is recognized as a key player in calcium and phosphate homeostasis. A soluble form of Klotho, which has endocrine and physiologic effects and acts independently of FGF23, is found in the blood, urine, and cerebrospinal fluid (53). Soluble Klotho arises by alternative splicing of its transcript or from proteolytic cleavage of the extracellular domain of the transmembrane form (ectodomain shedding) (54). The kidney is thought to be the main source for the production of soluble Klotho (55).
Klotho acts directly on calcium channels and phosphate transporters (via soluble Klotho) and indirectly as a membrane cofactor that converts FGFR1 into a specific receptor for FGF23. The expression of membranous Klotho, therefore, determines the tissue specificity of the function of FGF23. Klotho is highly expressed in the kidney and by varying degrees in other tissue, including the PTG, pituitary, pancreas, ovary, testis, placenta, choroid plexus of the brain, and aorta (56,57). There has been a great deal of controversy concerning the presence of Klotho in the vasculature. Studies show the presence (57–60) or absence (61–63) of endogenous Klotho in the vasculature. This discrepancy could very well be caused by experimental conditions, sample preparation, or more likely, the nature of antibodies used for analysis (64). However, a recent study may put an end to the debate of Klotho expression in the vasculature. The group of Lim et al. (65) is the first to use next generation–targeted proteomic analysis using parallel reaction monitoring (PRM) together with antibody-based methods to examine the expression and spatial distribution of Klotho in human tissue. PRM is a state of the art technique that uses high–resolution mass spectrometry to identify a peptide signature for a protein of interest. Importantly, PRM is able to distinguish between membrane-bound Klotho and soluble Klotho. The group confirmed the presence of membrane-bound Klotho in the human aorta and the renal and epigastric arteries.
Klotho may be one of the earliest biomarkers of AKI. Hu et al. (66) showed that a reduction of renal Klotho protein precedes the increase in creatinine in an AKI rat model. In humans, soluble Klotho levels were decreased in patients with AKI (67). In another study, the expression of renal Klotho decreased in patients with AKI according to the severity of the disease, regardless of the etiology, and low Klotho expression associated with a poor short–term outcome (68). Therefore, decreased Klotho may be a key pathologic feature in AKI development and progression to CKD.
CKD is generally considered to be a state of severe Klotho deficiency, and the reduction in renal Klotho expression is one of the earliest changes observed in CKD (69,70). In advanced CKD, FGF23 resistance is attributed to the reduced expression of the parathyroid Klotho-FGFR1 receptor complex in the kidney, PTG, and aorta (59,66,71). Reduced concentrations of renal and soluble Klotho are found in mice with experimentally induced CKD (72), and our laboratory recently reported that renal Klotho expression is drastically reduced in uremic rats (57). As well, reduced renal and soluble Klotho levels are found in patients with CKD (73,74). Lower levels of serum Klotho significantly correlate with lower eGFR levels in patients with CKD (75). Importantly, Kim et al. (76) found that low levels of serum Klotho are linked to the progression of CKD independently of FGF23, proteinuria, or PTH, suggesting that α-Klotho may serve as a useful clinical biomarker for progression of CKD. In addition, Barker et al. (77), using a novel synthetic antibody, recently found that soluble Klotho was decreased early in CKD, preceding hyperphosphatemia and increases in FGF23 and PTH. This further emphasizes the role of Klotho as a biomarker of kidney injury.
Phosphate and Cardiovascular Disease
Cardiovascular disease is the leading cause of death in patients with CKD in a manner independent of risk factors, such as a history of cardiovascular disease or the presence of documented proteinuria (78,79). The increased morbidity and mortality resulting from cardiovascular disease are associated with vascular calcification in these patients. Hyperphosphatemia is a serious complication in late-stage CKD, and a well known association exists between hyperphosphatemia and cardiovascular disease in patients undergoing dialysis.
Vascular calcification is an active, cell–regulated process in which ectopic deposition of calcium-phosphate salts occurs in blood vessels, mainly in the arteries or cardiac valves. Intimal calcification (calcification of the innermost layer of the vasculature) is associated with atherosclerotic plaque, and medial calcification (i.e., Mönckeberg sclerosis) is associated with stiffening of the blood vessels. The vascular smooth muscle cell (VSMC), which makes up the majority of cells in the media, is central to phosphate-induced calcification. High-phosphate levels in culture induce transformation of VSMCs into osteoblast-like cells by mediating the activity of Pit-1, a type 3 sodium–dependent phosphate cotransporter (80). Shroff et al. (81) showed that high levels of phosphate in cultures of arterial rings from patients with CKD promoted calcification of the vessels, with resulting apoptosis and death of the VSMCs. Culture of vessel rings from normal subjects exhibited no calcification, suggesting that normal VSMCs have inherent pathways to prevent calcification, whereas CKD primes the VSMCs and makes them susceptible to calcification.
CKD is known to activate endothelial cells and generate membrane-derived microparticles (MPs), which are vesicles shed from plasma membranes of cells, such as platelets, endothelial cells, and leukocytes (82). Endothelial MPs are markers for vascular dysfunction in CKD and possible causes of thrombosis and cardiovascular diseases (83,84). High levels of extracellular phosphate induce MP production in cultured endothelial cells (85). In human vascular endothelial cells, Abbasian et al. (85) found that high levels of extracellular phosphate resulted in an increase in intracellular phosphate concentration mediated via Pit-1/slc20a1 transporters. The increase in intracellular phosphate leads to changes in protein phosphorylation, which in turn, cause the release of strongly procoagulant MPs. The MPs, therefore, may be part of a pathologic signaling pathway that links hyperphosphatemia in patients with CKD and cardiovascular events.
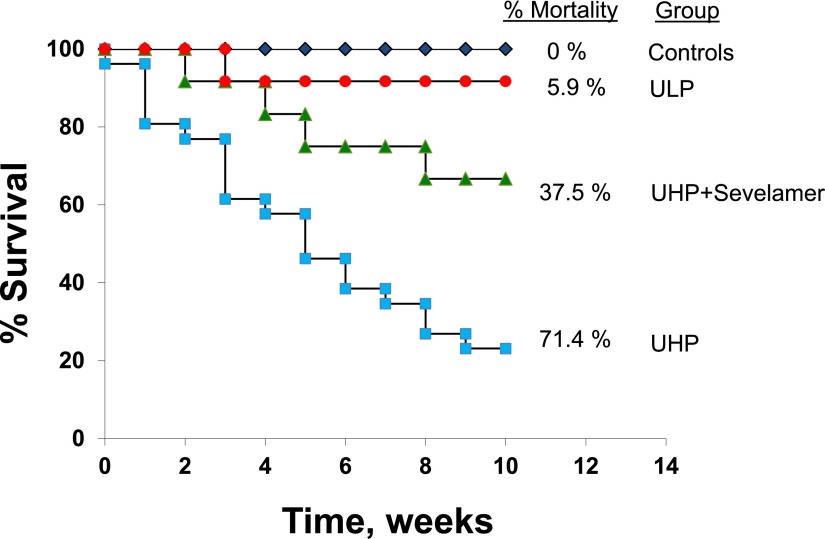
In humans, even mild elevations in serum phosphate may increase the risk of cardiovascular calcification. Higher phosphate levels within the normal range are associated with vascular and valvular calcification in patients with moderate CKD, independent of PTH and calcitriol levels (86). In fact, higher serum phosphate concentrations falling within the normal range are associated with cardiovascular events and mortality in patients with normal and abnormal kidney function (9,87). A recent study by Shang et al. (88) showed that hyperphosphatemia is an independent risk factor in the progression of coronary artery calcification. Phosphate levels were positively associated with protein intake and peritoneal dialysis adequacy, suggesting that restriction of phosphate intake may be useful in slowing the progression of vascular calcification. Our laboratory recently reported a significant reduction in aortic calcification and decreased mortality as well as preserved renal function using phosphate binders and dietary phosphate restriction in uremic rats; the Kaplan–Meier analysis of the mortality and survival rates in this study is shown in Figure 3 (89).
Figure 3.
Phosphate restriction reduces mortality in uremic rats with established vascular calcification. Uremic rats were fed a high-phosphate diet (1.4%) for 3 months. They were then divided into three groups that were fed the high-phosphate diet (UHP), a low-phosphate diet (ULP; 0.1%), or the high-phosphate diet to which 4% of the phosphate binder sevelamer carbonate was added (UHP + Sevelamer). Tracking of mortality was begun at this point and continued for an additional 3 months. Normal rats fed UHP served as controls. Kaplan–Meier analysis shows the mortality and survival rates during the final 3-month period. There was a 71.4% mortality rate for uremic rats fed the UHP; of 35 rats initially placed in this group, only 10 survived. Conversely, rats that were switched to the ULP experienced a mortality rate of only 5.9% (16 of 17 rats that started in this group survived; P<0.001 versus UHP). Rats fed UHP + Sevelamer had a mortality rate of 37.5% (five of the original eight rats survived; P=0.07 versus UHP and P<0.05 versus ULP). Modified from reference 89, with permission.
The procalcification effects of phosphate are independent of FGF23 (62). The calcification observed in soft tissues and vasculature in FGF23 null mice is attributed to the accompanying hyperphosphatemia, because dietary phosphate restriction attenuated the calcification (90). Likewise, in humans, arterial and capillary calcification resulting from a novel mutation in FGF23 is attributed to concomitant hyperphosphatemia (91).
FGF23 and Klotho in Cardiovascular Disease
High levels of FGF23 are associated with all-cause mortality and cardiovascular events in CKD (92,93). Unlike phosphate, however, the effect of FGF23 on arterial calcification is associated with the degree of kidney function. FGF23 is not associated with coronary artery calcification in individuals with normal renal function (94), and there are conflicting reports of the association in patients with CKD predialysis (62,95). However, FGF23 does correlate with peripheral and aortic calcification in patients on hemodialysis (96,97).
The effect of FGF23 on left ventricular hypertrophy (LVH) is well established. FGF23 is independently associated with greater left ventricular mass and higher prevalence of LVH and plays a role in the molecular pathogenesis of LVH, independently of Klotho (98,99). Faul et al. (99) propose that high concentrations of serum FGF23 may be accentuated in CKD because of the downregulation of membranous Klotho in the kidney and PTG; this may promote “promiscuous” binding of FGF23 to FGFR in other tissues, such as the heart (99). Alternatively, Faul et al. (99) state that high-affinity binding of FGF23 to specific cardiac FGFRs (i.e., FGFR4) may induce LVH. Therefore, phosphate and FGF23 seem to have distinct effects on the cardiovascular system, with elevated FGF23 directly promoting cardiac remodeling and hyperphosphatemia directly promoting arterial injury (62,100).
The severe deficiency of Klotho observed in CKD is not only reflective of the state of kidney injury, but it is also associated with extrarenal complications of CKD. In the initial report of a defect in the Klotho gene by Kuro-o et al. (56), affected mice exhibited striking changes in the vasculature, including medial calcification of the aorta, medial calcification and intimal thickening in middle–sized muscular arteries, and calcification of small renal arteries. In humans, a decreased serum Klotho and a decreased vascular Klotho expression are associated with an increased risk of coronary artery disease (60), and a variant of the Klotho gene is associated with early–onset ischemic stroke (101). Navarro-González et al. (60) found reduced levels of Klotho in the serum and the vasculature in humans and that the relationship between Klotho expression and coronary artery disease was independent of other cardiovascular risk factors. In patients on maintenance hemodialysis, lower serum Klotho levels were independently associated with severe abdominal aortic calcification (102). In addition, Hu et al. (103) recently found that high FGF23 levels correlated with the severity of cardiomyopathy but only in the presence of low plasma Klotho levels.
Lim et al. (59), who were the first to describe endogenous Klotho expression in human artery, found that lower levels of vascular Klotho result in a vascular resistance to FGF23 and that vitamin D receptor activators (VDRAs) restored Klotho expression and re-established FGF23-responsive signaling in human aortic smooth muscle cells. Our laboratory found a significant decrease in Klotho expression in the medial layer of the aorta of rats with moderate CKD and mild to moderate hyperphosphatemia in a stage of uremia in which there was no detection of calcification (57). Surprisingly, we found an increase in Klotho expression in the adventitial (outermost) layer of the aorta of these uremic rats. The expression of Klotho in the adventitia is most likely caused by its expression in mature and undifferentiated fibroblasts. Although the function of the increase in adventitial Klotho is unknown, it is of particular interest that areas of calcification progress concentrically from the adventitia to the intima in patients who are uremic with increasing degrees of vascular calcification (104). As well, in mice, activated myofibroblasts in the adventitia promoted vascular calcification of the media by inducing paracrine Wnt signals (105). Additional studies are needed to better define the function of differential expression and the regulation of Klotho in the media and adventitia to clarify what role the Klotho plays in vascular calcification.
Treatment of Hyperphosphatemia
Despite all that has been learned in the last half-century of renal research, the importance of controlling phosphate load remains the primary goal in treatment of CKD. Although there is an abundance of basic science work and observational studies that support the role of phosphate toxicity in CKD, large clinical trials showing a reduction in rates of adverse clinical outcomes with interventions that reduce phosphate burden are lacking. Nevertheless, the list of current therapeutic approaches used to lower serum phosphate (acting directly or indirectly) continues to grow. Because each treatment has its advantages and disadvantages and because no one optimal treatment exists, a growing consensus is that a multipronged approach may be the best way to control hyperphosphatemia. In addition, there is also a question as to when to start intervention. Although treatment of phosphate retention is generally initiated after the development of hyperphosphatemia, it has been suggested that it may be important to treat phosphate retention at earlier stages of CKD to maintain near–normal phosphorus levels as long as possible as CKD advances (106), and early studies in animals support this approach (107). At this time, however, there is no hard evidence indicating when or if early intervention should begin. Clinical studies are clearly needed to address this issue. Current therapeutic approaches used for lowering serum phosphate are discussed below.
Dietary Phosphate Control
Restricting dietary phosphate lowers serum phosphate and is recommended in both the Kidney Disease Improving Global Outcomes and Kidney Disease Outcomes Quality Initiative guidelines. When prescribing a low-phosphate diet, the phosphate content, the source of the phosphate, and the phosphate-to-protein ratio of the diet are all important considerations (108). It is highly recommended that the diet consist of foods high in protein but low in phosphate (109). The phosphate-to-protein ratio should be <10 mg/g; as an example, egg whites are <2 mg/g. A diet rich in plant-based phosphate is also recommended, because it is less absorbable in the gut. In uremic rats and patients with CKD stage 3 or 4, a grain-based diet results in a lower serum phosphate level compared with a meat-based diet (110). In addition, patients should be made aware of how to avoid the hidden inorganic phosphate additives in food as an additional means to reduce dietary phosphate. A reduced protein intake alleviates uremic symptoms and slows the progression of kidney failure (111). However, there can be drawbacks to restricting dietary phosphate. Although a reduced protein diet can be renoprotective, protein-energy wasting, which is an independent determinant of morbidity and mortality in patients on dialysis, can develop if the diet is not properly implemented or followed (112–114). This highlights the importance of monitoring the nutritional status of the prescribed diet. It should be pointed out that studies have indicated that there is only a modest or no correlation between dietary phosphate intake and serum phosphate levels (110,115). In a recent study, Selamet et al. (116) found that dietary phosphate intake may not be the major determinant of serum phosphate concentrations in patients with CKD and that there was no significant correlation between greater phosphate intake and risk of ESRD, cardiovascular disease mortality, noncardiovascular disease mortality, or all-cause mortality.
Binders
Phosphate binders are commonly used in patients who are hyperphosphatemic. The compounds (such as sevelamer carbonate, lanthanum, and iron salts) directly bind luminal phosphate, making it unavailable for absorption (117). The binders effectively reduce dietary phosphate absorption in patients on dialysis (118) and reduce urinary phosphate excretion by 20%–50% in patients with CKD stage 3 or 4 who are normophosphatemic (119). A well known drawback to phosphate binders is poor patient compliance for certain binders, mainly because of side effects, frequent administration, and high pill burden (120). However, phosphate-depleted diets or phosphate binders can actually upregulate NaPi-2b expression in the gut, leading to an increase in dietary phosphate absorption when the dietary phosphate load is re-established, thus counteracting the binder’s potency (121). A study by Block et al. (122) in patients with moderate to advanced CKD and normal to near–normal serum phosphate levels found that, although phosphate binders significantly lowered serum and urinary phosphate and attenuated progression of 2° HPT, treatment resulted in the development of vascular calcification. The adverse effect on vascular calcification was most pronounced in patients treated with the calcium acetate binder, but it also occurred with use of lanthanum and sevelamer binders. It was postulated that the calcium acetate binder could have induced a positive calcium balance that resulted in dystrophic calcification; the noncalcium phosphate binders could have enhanced the availability of free intestinal calcium, which likewise, resulted in a positive calcium balance. A National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases pilot study (the CKD Optimal Management with Binders and Nicotinamide study) that will evaluate the effects of lanthanum carbonate and nicotinamide on serum phosphate and FGF23 in patients with CKD stages 3 and 4 is currently underway; the estimated study completion date is June of 2018 (123).
Phosphate Transporters
Phosphate transporters are therapeutic targets in the control of hyperphosphatemia in patients with CKD. Nicotinamide (niacinamide and vitamin B3) reduces dietary phosphate absorption and decreases serum phosphate levels in patients by inhibiting expression of NaPi-2b (124,125). Nicotinamide also has a phosphaturic effect independent of PTH, which may be the result of nicotinamide reducing levels of renal NaPi-2a and NaPi-2c in the kidney (126). Most recently, Bobeck et al. (127) generated oral antibodies that bind to NaPi-2b on the enterocyte and prevent active uptake of phosphate.
Indirect Approaches
Other treatments, such as those targeting FGF23, Klotho, and PTH, have an indirect effect on serum phosphate in patients with CKD. Shaloub et al. (128) developed therapeutic antibodies to neutralize FGF23 in uremic rats. Unfortunately, although the antibodies lowered circulating levels of FGF23, which had a positive effect on 2° HPT (i.e., reduced PTH, increased calcitriol, increased serum calcium, and normalization of numerous bone markers), there were accompanying negative effects of a dose-dependent hyperphosphatemia and aortic calcification associated with an increased risk of death. The investigators suggested that the hyperphosphatemia was caused by inhibition of FGF23’s phosphaturic effect on the residual kidney and called for additional studies that would control phosphate levels while assessing the effect of FGF23 neutralization in the different stages of CKD. In patients on dialysis, studies have shown that cinacalcet reduces serum FGF23 levels (37,129). In the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events trial, although cinacalcet did not significantly reduce the risk of composite cardiovascular end points, in a post hoc analysis, there was an association between a lower serum FGF23 and cardiovascular events and mortality in patients on hemodialysis treated with cinacalcet (130).
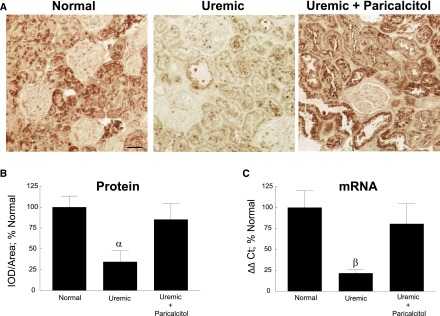
Because decreased membrane–bound Klotho in CKD contributes to target tissue FGF23 resistance, it has been proposed that, instead of neutralizing/reducing FGF23, the Klotho coreceptor should be upregulated to redirect FGF23 to its original function (131). This would prevent FGF23 from having off-target effects, such as cardiotoxicity (99). In our study in uremic rats, a drastic decrease in Klotho in the kidney was prevented by treatment with the VDRA paricalcitol, as shown in Figure 4 (57). Lim et al. (59) found that lower levels of vascular Klotho result in a vascular resistance to FGF23 and that VDRAs restored Klotho expression and re-established FGF23-responsive signaling in human aortic smooth muscle cells. Methods using soluble/exogenous Klotho as a therapeutic agent have also been used. Treatment with recombinant Klotho attenuated renal failure in a rat AKI model (66), and when mice with AKI were administered an adenovirus–mediated Klotho gene, an increase in serum creatinine level was suppressed, and renal histologic damage was prevented (132). With regard to cardiovascular dysfunction, Xie et al. (133) showed that soluble Klotho protects the heart against stress–induced cardiac hypertrophy by inhibiting abnormal calcium signaling. Additional studies showed that intravenous delivery of a transgene coding for soluble Klotho protects against cardiomyopathy in Klotho–deficient CKD mice in a manner independent of FGF23 or phosphate (134). Intraperitoneal administration of Klotho protein to Klotho-deficient mice extended lifespan and attenuated systemic calcification and renal fibrosis; the protective effect on renal function was via downregulation of renal TGF-β mRNA expression, a main regulator of renal fibrosis (135). In addition, Takenaka et al. (136) recently showed that exogenous or xeno-Klotho directly interacts with the PTH receptor to inhibit PTH signaling. Moreover, xeno-Klotho inhibited the PTH-induced expression of renal 1α-hydroxylase both in vitro and in vivo, indicating that Klotho may act as a second messenger for FGF23 in its inhibition of vitamin D. Although these results in animals are exciting, more research is needed to determine if soluble Klotho replacement and/or upregulation of membrane-bound Klotho will provide beneficial therapy for renal and cardiovascular dysfunction in patients with CKD.
Figure 4.
Vitamin D receptor activator paricalcitol prevents downregulation of renal Klotho in uremic rats. (A) Representative images of Klotho immunostaining of normal, uremic, and uremic paricalcitol–treated rat kidney tissue. Klotho was detected in the proximal tubules, distal tubules, and collecting ducts of kidney tissue. Original magnification, ×200. Scale bar, 50 μm. (B) Quantitation of Klotho immunostaining shows that Klotho protein was significantly decreased 65.5% in the uremic rats; this decrease was blocked by treatment with paricalcitol. IOD, integrated optical density. αP≤0.01 versus normal (n=6 each; average±SEM). (C) Quantitation of renal Klotho mRNA showed a significant decrease of 78.3% in Klotho expression in uremic rats, which was blocked by paricalcitol treatment. βP≤0.05 versus uremic (n=6 each; average±SEM). Ct, PCR cycle number at threshold value. Modified from reference 57, with permission.
The excess PTH seen in 2° HPT increases bone resorption and leads to the skeleton contributing to the hyperphosphatemia observed in CKD. VDRAs have been used for many years to treat patients with 2° HPT. Initially, calcitriol and alfacalcidol were used, and although these compounds do, indeed, lower PTH levels, they can lead to an increase in serum calcium and phosphorus levels because of increased intestinal absorption. Numerous vitamin D receptor analogs were subsequently developed (i.e., 22-oxacalcitriol, doxercalciferol, paricalcitol, and falecalcitriol) that were reported to have similar or superior dose–equivalent suppression of PTH but less calcemic and phosphatemic activity. In patients on dialysis, calcimimetic drugs reduce PTH, calcium, phosphorus, and the calcium-phosphate product (137,138). In patients with CKD not receiving dialysis, cinacalcet decreases PTH but results in frequent instances of hypocalcemia and hyperphosphatemia (139). Although VDRAs and calcimimetics have advantages and disadvantages in different stages of CKD, their importance in reducing bone resorption and decreasing serum phosphate is well accepted. However, the true benefit of VDRAs on hard patient–level outcomes and their value in treatment of CKD are still in question because of a lack of randomized, controlled trials (130,140,141).
Conclusion
Our understanding of the dysregulated mineral metabolism found in CKD has expanded greatly over the past 50 years of renal research. In particular, the recent discovery of FGF23 and Klotho has helped clarify aspects of endocrine regulation of mineral metabolism in both health and disease and necessitated the updating of well established hypotheses. What has remained unchanged all of these years, however, is the recognition that phosphate retention is the initiating factor for the development of many of the complications observed in CKD, namely 2° HPT and bone and cardiovascular diseases. The association of phosphate retention with patient morbidity and mortality makes the regulation of serum phosphate a priority in the patient with CKD. However, the key to any effective treatment of hyperphosphatemia is adherence, and current treatments are not free of side effects or inconveniences. Therefore, combining therapies that target dietary phosphate, phosphate binders, manipulators of the phosphate transporters, suppressors of PTH, and methods to upregulate Klotho and/or decrease FGF23 levels may be optimal in the treatment of phosphate retention in CKD.
Disclosures
Washington University and E.S. may receive income on the basis of a license of related technology by the University of Wisconsin (Madison, WI). C.S.R. has no disclosures to report.
Acknowledgments
This work was supported by a Washington University Research in Renal Diseases grant (3068-31030A), a Washington University Center for Kidney Disease Research O'Brian Center grant (P30DK079333), and an AbbVie IIS Program grant.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tenenhouse HS: Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr 25: 197–214, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Ix JH, Ketteler M, Martin KJ, Thadhani RI, Tonelli M, Wolf M, Jüppner H, Hruska K, Wheeler DC: Phosphate homeostasis in CKD: Report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 62: 457–473, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC: FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284: 977–981, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Felsenfeld AJ, Levine BS, Rodriguez M: Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial 28: 564–577, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Quarles LD: Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiavi SC, Kumar R: The phosphatonin pathway: New insights in phosphate homeostasis. Kidney Int 65: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE: Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, Hegarty J, New J, O’Donoghue DJ, Middleton RJ, Kalra PA: Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bricker NS, Morrin PA, Kime SW Jr.: The pathologic physiology of chronic Bright’s disease. An exposition of the “intact nephron hypothesis”. Am J Med 28: 77–98, 1960 [DOI] [PubMed] [Google Scholar]
- 13.Bricker NS: On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med 286: 1093–1099, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez OM: Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clin J Am Soc Nephrol 5: 1710–1716, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Slatopolsky E, Robson AM, Elkan I, Bricker NS: Control of phosphate excretion in uremic man. J Clin Invest 47: 1865–1874, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slatopolsky E, Caglar S, Pennell JP, Taggart DD, Canterbury JM, Reiss E, Bricker NS: On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest 50: 492–499, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slatopolsky E, Caglar S, Gradowska L, Canterbury J, Reiss E, Bricker NS: On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using “proportional reduction” of dietary phosphorus intake. Kidney Int 2: 147–151, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Portale AA, Booth BE, Halloran BP, Morris RC Jr.: Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest 73: 1580–1589, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Hilker S, Galceran T, Chan YL, Rapp N, Martin KJ, Slatopolsky E: Hypocalcemia may not be essential for the development of secondary hyperparathyroidism in chronic renal failure. J Clin Invest 78: 1097–1102, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ: Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M: Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11: 970–976, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Nielsen PK, Feldt-Rasmussen U, Olgaard K: A direct effect in vitro of phosphate on PTH release from bovine parathyroid tissue slices but not from dispersed parathyroid cells. Nephrol Dial Transplant 11: 1762–1768, 1996 [PubMed] [Google Scholar]
- 23.Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, Drüeke TB: Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 51: 328–336, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Kifor O, Moore FD Jr., Wang P, Goldstein M, Vassilev P, Kifor I, Hebert SC, Brown EM: Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab 81: 1598–1606, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, Maeda S, Kitazawa R: Association of decreased calcium-sensing receptor expression with proliferation of parathyroid cells in secondary hyperparathyroidism. Kidney Int 58: 1980–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Ritter CS, Martin DR, Lu Y, Slatopolsky E, Brown AJ: Reversal of secondary hyperparathyroidism by phosphate restriction restores parathyroid calcium-sensing receptor expression and function. J Bone Miner Res 17: 2206–2213, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Brown AJ, Ritter CS, Finch JL, Slatopolsky EA: Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: Role of dietary phosphate. Kidney Int 55: 1284–1292, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Ritter CS, Finch JL, Slatopolsky EA, Brown AJ: Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int 60: 1737–1744, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M: Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 83: 959–966, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chudek J, Kocełak P, Owczarek A, Bożentowicz-Wikarek M, Mossakowska M, Olszanecka-Glinianowicz M, Wiecek A: Fibroblast growth factor 23 (FGF23) and early chronic kidney disease in the elderly. Nephrol Dial Transplant 29: 1757–1763, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, Vanderschueren D, Vanrenterghem Y: Fibroblast growth factor-23 in early chronic kidney disease: Additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol 5: 1268–1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T: PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 35.López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E: Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 80: 475–482, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Meir T, Durlacher K, Pan Z, Amir G, Richards WG, Silver J, Naveh-Many T: Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int 86: 1106–1115, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M: Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant 27: 784–790, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Finch JL, Tokumoto M, Nakamura H, Yao W, Shahnazari M, Lane N, Slatopolsky E: Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298: F1315–F1322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Vervloet MG, van Ittersum FJ, Büttler RM, Heijboer AC, Blankenstein MA, ter Wee PM: Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol 6: 383–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takasugi S, Akutsu M, Nagata M: Oral phosphorus supplementation secondarily increases circulating fibroblast growth factor 23 levels at least partially via stimulation of parathyroid hormone secretion. J Nutr Sci Vitaminol (Tokyo) 60: 140–144, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF: Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res 26: 2634–2646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito N, Findlay DM, Anderson PH, Bonewald LF, Atkins GJ: Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J Steroid Biochem Mol Biol 136: 183–186, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K: Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab 288: E1101–E1109, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto R, Minamizaki T, Yoshiko Y, Yoshioka H, Tanne K, Aubin JE, Maeda N: 1alpha,25-dihydroxyvitamin D3 acts predominately in mature osteoblasts under conditions of high extracellular phosphate to increase fibroblast growth factor 23 production in vitro. J Endocrinol 206: 279–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hori M, Kinoshita Y, Taguchi M, Fukumoto S: Phosphate enhances Fgf23 expression through reactive oxygen species in UMR-106 cells [published online ahead of print March 21, 2015]. J Bone Miner Metab [DOI] [PubMed] [Google Scholar]
- 47.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M: Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production [published online ahead of print November 4, 2015]. Kidney Int doi: 10.1038/ki.2015.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda Y, Komaba H, Goto S, Fujii H, Umezu M, Hasegawa H, Fujimori A, Nishioka M, Nishi S, Fukagawa M: Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol 33: 421–426, 2011 [DOI] [PubMed] [Google Scholar]
- 49.David V, Dai B, Martin A, Huang J, Han X, Quarles LD: Calcium regulates FGF-23 expression in bone. Endocrinology 154: 4469–4482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quarles LD: A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin Nephrol 33: 130–142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krieger NS, Culbertson CD, Kyker-Snowman K, Bushinsky DA: Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am J Physiol Renal Physiol 303: F431–F436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H: Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab 95: 578–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akimoto T, Kimura T, Watanabe Y, Ishikawa N, Iwazu Y, Saito O, Muto S, Yagisawa T, Kusano E: The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc 45: 134–136, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Ritter CS, Zhang S, Delmez J, Finch JL, Slatopolsky E: Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int 87: 1141–1152, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA: Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int 85: 142–150, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL: Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125: 2243–2255, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R, Mora-Fernández C: Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 100: 34–40, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82: 1261–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP, Hamano T, Master SR, Nessel L, Chai B, Xie D, Kallem RR, Chen J, Lash JP, Kusek JW, Budoff MJ, Giachelli CM, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators: Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83: 1159–1168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mencke R, Harms G, Mirković K, Struik J, Van Ark J, Van Loon E, Verkaik M, De Borst MH, Zeebregts CJ, Hoenderop JG, Vervloet MG, Hillebrands JL; NIGRAM Consortium: Membrane-bound Klotho is not expressed endogenously in healthy or uraemic human vascular tissue. Cardiovasc Res 108: 220–231, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Lewin E, Olgaard K: The vascular secret of Klotho. Kidney Int 87: 1089–1091, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D: α-Klotho expression in human tissues. J Clin Endocrinol Metab 100: E1308–E1318, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu YJ, Sun HD, Chen J, Chen MY, Ouyang B, Guan XD: Klotho: A novel and early biomarker of acute kidney injury after cardiac valve replacement surgery in adults. Int J Clin Exp Med 8: 7351–7358, 2015 [PMC free article] [PubMed] [Google Scholar]
- 68.Seo MY, Yang J, Lee JY, Kim K, Kim SC, Chang H, Won NH, Kim MG, Jo SK, Cho W, Kim HK: Renal Klotho expression in patients with acute kidney injury is associated with the severity of the injury. Korean J Intern Med 30: 489–495, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T: Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77: 211–218, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y: Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 9: e86301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H: A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8: e56695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo TH, Kang SW, Han DS, Han SH: Circulating α-klotho levels in CKD and relationship to progression. Am J Kidney Dis 61: 899–909, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS: The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Kiu Weber CI, Duchateau-Nguyen G, Solier C, Schell-Steven A, Hermosilla R, Nogoceke E, Block G: Cardiovascular risk markers associated with arterial calcification in patients with chronic kidney disease Stages 3 and 4. Clin Kidney J 7: 167–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lau WL, Festing MH, Giachelli CM: Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost 104: 464–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM: Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 21: 103–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burton JO, Hamali HA, Singh R, Abbasian N, Parsons R, Patel AK, Goodall AH, Brunskill NJ: Elevated levels of procoagulant plasma microvesicles in dialysis patients. PLoS One 8: e72663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A: Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 101: 841–843, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, Patyroglu T, Gurgoze M: The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant 24: 2511–2518, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Abbasian N, Burton JO, Herbert KE, Tregunna BE, Brown JR, Ghaderi-Najafabadi M, Brunskill NJ, Goodall AH, Bevington A: Hyperphosphatemia, phosphoprotein phosphatases, and microparticle release in vascular endothelial cells. J Am Soc Nephrol 26: 2152–2162, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cancela AL, Santos RD, Titan SM, Goldenstein PT, Rochitte CE, Lemos PA, dos Reis LM, Graciolli FG, Jorgetti V, Moysés RM: Phosphorus is associated with coronary artery disease in patients with preserved renal function. PLoS One 7: e36883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shang D, Xie Q, Ge X, Yan H, Tian J, Kuang D, Hao CM, Zhu T: Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol 16: 107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finch JL, Lee DH, Liapis H, Ritter C, Zhang S, Suarez E, Ferder L, Slatopolsky E: Phosphate restriction significantly reduces mortality in uremic rats with established vascular calcification. Kidney Int 84: 1145–1153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD: Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18: 2116–2124, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Shah A, Miller CJ, Nast CC, Adams MD, Truitt B, Tayek JA, Tong L, Mehtani P, Monteon F, Sedor JR, Clinkenbeard EL, White K, Mehrotra R, LaPage J, Dickson P, Adler SG, Iyengar SK: Severe vascular calcification and tumoral calcinosis in a family with hyperphosphatemia: A fibroblast growth factor 23 mutation identified by exome sequencing. Nephrol Dial Transplant 29: 2235–2243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M; HOST Investigators: FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roos M, Lutz J, Salmhofer H, Luppa P, Knauss A, Braun S, Martinof S, Schömig A, Heemann U, Kastrati A, Hausleiter J: Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin Endocrinol (Oxf) 68: 660–665, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Nakayama M, Kaizu Y, Nagata M, Ura Y, Ikeda H, Shimamoto S, Kuma K: Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: A cross-sectional study. BMC Nephrol 14: 22, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Peripheral vascular calcification in long-haemodialysis patients: Associated factors and survival consequences. Nephrol Dial Transplant 24: 948–955, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Inaba M, Okuno S, Imanishi Y, Yamada S, Shioi A, Yamakawa T, Ishimura E, Nishizawa Y: Role of fibroblast growth factor-23 in peripheral vascular calcification in non-diabetic and diabetic hemodialysis patients. Osteoporosis Int 17: 1506–1513, 2006 [DOI] [PubMed]
- 98.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf M: Mineral (mal)adaptation to kidney disease--Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol 10: 1875–1885, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majumdar V, Nagaraja D, Christopher R: Association of the functional KL-VS variant of Klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun 403: 412–416, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Cai H, Lu R, Zhang M, Pang H, Zhu M, Zhang W, Ni Z, Qian J, Yan Y: Serum soluble klotho level is associated with abdominal aortic calcification in patients on maintenance hemodialysis. Blood Purif 40: 120–126, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-O M, Hill JA, Moe OW: Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 26: 1290–1302, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ballanti P, Silvestrini G, Pisanò S, De Paolis P, Di Giulio S, Mantella D, Iappelli M, Favarò A, Bonucci E, Coen G: Medial artery calcification of uremic patients: A histological, histochemical and ultrastructural study. Histol Histopathol 26: 191–200, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA: Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115: 1210–1220, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin KJ, González EA: Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 6: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Rutherford WE, Bordier P, Marie P, Hruska K, Harter H, Greenwalt A, Blondin J, Haddad J, Bricker N, Slatopolsky E: Phosphate control and 25-hydroxycholecalciferol administration in preventing experimental renal osteodystrophy in the dog. J Clin Invest 60: 332–341, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noori N, Sims JJ, Kopple JD, Shah A, Colman S, Shinaberger CS, Bross R, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran J Kidney Dis 4: 89–100, 2010 [PubMed] [Google Scholar]
- 109.Streja E, Lau WL, Goldstein L, Sim JJ, Molnar MZ, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K: Hyperphosphatemia is a combined function of high serum PTH and high dietary protein intake in dialysis patients. Kidney Int Suppl (2011) 3: 462–468, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chauveau P, Combe C, Rigalleau V, Vendrely B, Aparicio M: Restricted protein diet is associated with decrease in proteinuria: Consequences on the progression of renal failure. J Ren Nutr 17: 250–257, 2007 [DOI] [PubMed]
- 112.Heng AE, Cano NJM: Nutritional problems in adult patients with stage 5 chronic kidney disease on dialysis (both haemodialysis and peritoneal dialysis). NDT Plus 3: 109–117, 2010 [Google Scholar]
- 113.Cano NJ, Miolane-Debouit M, Léger J, Heng AE: Assessment of body protein: Energy status in chronic kidney disease. Semin Nephrol 29: 59–66, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Isakova T, Gutiérrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M: Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 26: 584–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Selamet U, Tighiouart H, Sarnak MJ, Beck G, Levey AS, Block G, Ix JH: Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3-5: The Modification of Diet in Renal Disease Study [published online ahead of print September 30, 2015]. Kidney Int [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bobeck EA, Meyer KM, Helvig C, Petkovich M, Cook ME: Sevelamer hydrochloride binds phosphate released from phytate in chicks fed 1α-hydroxy cholecalciferol. J Ren Nutr 23: 21–27, 2013 [DOI] [PubMed]
- 118.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 119.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ketteler M, Biggar PH: Use of phosphate binders in chronic kidney disease. Curr Opin Nephrol Hypertens 22: 413–420, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Schiavi SC, Tang W, Bracken C, O’Brien SP, Song W, Boulanger J, Ryan S, Phillips L, Liu S, Arbeeny C, Ledbetter S, Sabbagh Y: Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol 23: 1691–1700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA: Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26: 2328–2339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW: A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC: Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, Ito M, Kido S, Segawa H, Sano M, Fukuwatari T, Shibata K, Miyamoto K: Hepatectomy-related hypophosphatemia: A novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol 25: 761–772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bobeck EA, Hellestad EM, Sand JM, Piccione ML, Bishop JW, Helvig C, Petkovich M, Cook ME: Oral peptide specific egg antibody to intestinal sodium-dependent phosphate co-transporter-2b is effective at altering phosphate transport in vitro and in vivo. Poult Sci 94: 1128–1137, 2015 [DOI] [PubMed] [Google Scholar]
- 128.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG: FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122: 2543–2553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD: Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 5: 110–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 131.Moe OW: Fibroblast growth factor 23: Friend or foe in uremia? J Clin Invest 122: 2354–2356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY: The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698: 67–73, 2013 [DOI] [PubMed] [Google Scholar]
- 136.Takenaka T, Inoue T, Miyazaki T, Hayashi M, Suzuki H: Xeno-Klotho inhibits parathyroid hormone signaling [published online ahead of print August 19, 2015]. J Bone Miner Res doi: 10.1002/jbmr.2691 [DOI] [PubMed] [Google Scholar]
- 137.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 138.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Chonchol M, Locatelli F, Abboud HE, Charytan C, de Francisco AL, Jolly S, Kaplan M, Roger SD, Sarkar S, Albizem MB, Mix TC, Kubo Y, Block GA: A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis 53: 197–207, 2009 [DOI] [PubMed] [Google Scholar]
- 140.Galassi A, Bellasi A, Auricchio S, Papagni S, Cozzolino M: Which vitamin D in CKD-MBD? The time of burning questions. BioMed Res Int 2013: 864012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morrone LF, Cozzolino M: The beneficial impact of vitamin D treatment in CKD patients: What’s next? Clin Kidney J 8: 38–40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]