Abstract
A strategic selection of tree species will shift the type and quality of litter input, and subsequently magnitude and composition of the soil organic carbon (SOC) through soil microbial community. We conducted a manipulative experiment in randomized block design with leaf litter inputs of four native subtropical tree species in a Pinus massoniana plantation in southern China and found that the chemical composition of SOC did not differ significantly among treatments until after 28 months of the experiment. Contrasting leaf litter inputs had significant impacts on the amounts of total microbial, Gram-positive bacterial, and actinomycic PLFAs, but not on the amounts of total bacterial, Gram-negative bacterial, and fungal PLFAs. There were significant differences in alkyl/O-alkyl C in soils among the leaf litter input treatments, but no apparent differences in the proportions of chemical compositions (alkyl, O-alkyl, aromatic, and carbonyl C) in SOC. Soil alkyl/O-alkyl C was significantly related to the amounts of total microbial, and Gram-positive bacterial PLFAs, but not to the chemical compositions of leaf litter. Our findings suggest that changes in forest leaf litter inputs could result in changes in chemical stability of SOC through the altered microbial community composition.
The composition of plant litter and its rate of input to the forest floor will change due to climate, land-use change, and ecosystem disturbance1. A strategic selection of tree species will shift the type and quality of litter inputs, and subsequently the chemical composition of soil organic carbon (SOC) through the altered soil microbial community composition. The changes in soil microorganisms may lead to changes in enzyme activities that can give rise to selective decomposition of some compounds2, the metabolic capabilities of decomposers3, and/or the chemical composition of soil microbial necromass4. The potential for SOC sequestration could accordingly vary with litter type and soil microbial community; however, large uncertainties remain concerning the effects of litter input on soil microbial community composition and the retention of litter-derived C in soils5.
Soil contains a mixture of heterogeneous C pools. Through chemical and/or physical methods, bulk soils can be separated into different fractions that differ in chemical composition and/or location in the soil matrix6. Soil fractionations based on carbon (C)-rich organic molecules allow bulk soil to be partitioned into functional pools of recalcitrant and labile chemical compositions and can help to assess the degrees of stability against decomposition in the environment7,8. For example, alkyl C chains in lipids and aromatic structures in aromatics and phenolics are more recalcitrant than carbohydrates, such as cellulose containing abundant O-alkyl groups9. Aliphatic compounds often accumulate in soil, thus contributing to increasingly stable SOC pools10,11. The alkyl/O-alkyl C of soil, which has been recommended as an index of the extent of decomposition12 and of the quality of SOC13,14, was also calculated in this study as an indicator of the chemical stability of SOC.
Chemically complex plant-derived compounds are known to be selectively preserved in the SOC fractions in terrestrial ecosystems15,16. Crow et al. (2009) showed that needle-derived aliphatic compounds and root-derived lignin were preferentially preserved in soils of coniferous forests, whereas root-derived aliphatic compounds were a source of SOC with greater stability than leaf-derived C in soils of deciduous forests, indicating that the dominant sources of SOC can differ substantially between forest types8. Mueller et al. (2013) reported that nearly 70% of the variation in individual soil lipid contents was explained by lipid contents in tree leaves and roots, whereas biological compositions, including bacteria and fungi, of soils had little impact on soil lipid contents15.
Although most SOC is initially derived from plant materials4, microbial-mediated decomposition and re-synthesis of plant input as the key processes shape stable soil C stocks5,17,18,19, giving rise to a theory that the plant litter has a rather fleeting influence on the quantity and composition of SOC20,21. This concept implies that plant litter chemistry may not be the main regulator controlling the amount and form of litter-derived C stabilized in soil22. Soil microbial biomass represents a significant source of SOC and contributes to the maintenance of soil organic matter (SOM) as a biochemical precursor23. The cell wall compounds, metabolites, and C use efficiencies all varied with microbial community compositions24. About 50% of bacterial biomass-derived C was found to remain in the soils25. The dynamics of root-associated fungi is also an important regulator of ecosystem C accumulation in boreal forests26. However, the input of easily degradable plant metabolites would destabilize SOC stocks by facilitating microbial co-metabolism of recalcitrant compounds27, or by enhancing microbial access to previously mineral-protected C compounds28, leading to a net loss of C from soil. In addition, You et al. (2016) pointed to differential controls on soil C density and mineralization among contrasting forest types and complex interplays among various biotic and environmental factors in regulating soil C dynamics29. The above complex processes highlight the existing disconnection between litter quality, variations in soil microbial community composition, and formation pathways of SOC.
Quantifying the effects of tree species on SOC is inevitably limited by soil background conditions in exiting forests, and the randomized block experiments with diverse tree species combinations may not be easily managed on the same site if long-term experimental maintenance and associated costs are taken into consideration. Thus, we conducted a manipulative leaf litter inputs field experiment under the same edaphic condition and site environment while excluding roots influence to examine the effects of leaf litter inputs from different tree species on SOC accumulation, and the associated underlying mechanisms. The first experiments on Detritus Input and Removal Treatments (DIRT) were established at Harvard Forest in temperate region in 1990 to address the gap between the above-ground versus below-ground litter input and their roles in determining soil C content and nutrient cycling in forest ecosystems30. There have been a few studies on the effects of plant litter inputs of aboveground and/or belowground on soil C cycling in tropical and subtropical forests31,32,33, in particular, under abundant water and heat conditions different from temperate climate33. However, data on the effects of different tree species’ litter inputs on chemical composition of SOC is lacking.
The objectives of this experiment were to examine the effects of leaf litter inputs from different tree species characterized with different chemical properties on soil microbial community composition and the chemical compositions of SOC in a Pinus massoniana plantation. Our hypotheses are as follows: (i) soil microbial community composition differ among the leaf litter inputs of four tree species; (ii) the soils that received different leaf litter inputs will exhibit the corresponding changes in chemical composition of SOC; (iii) the chemical composition of SOC is related to either leaf litter C chemical composition or soil microbial community composition, or their combinations and interactions.
Results
Leaf litter C chemical compositions
Significant differences were detected in the proportions of C chemical compositions (alkyl, O-alkyl, aromatic, and carbonyl C) and alkyl/O-alkyl C among the leaf litter samples of four tree species (p < 0.01). The proportions of alkyl C in Castanopsis hystrix and Erythrophleum fordii were significantly higher than in P. massoniana and Cunninghamia lanceolate (Table 1). The proportions of O-alkyl C in P. massoniana and C. lanceolata were higher than in C. hystrix and E. fordii (Table 1). Alkyl/O-alkyl C in C. hystrix and E. fordii were significantly higher than in P. massoniana and C. lanceolate (Table 1).
Table 1. Leaf litter C chemical compositions of the four tree species in subtropical China (means with SE in brackets).
| Leaf litter C chemical composition | P. massoniana | C. lanceolata | C.hystrix | E. fordii |
|---|---|---|---|---|
| Proportion of alkyl C (%) | 15.2 (0.5) a | 15.4 (0.4) a | 16.7 (0.2) b | 19.3 (0.1) c |
| Proportion of O-alkyl C (%) | 60.1 (0.6) c | 58.9 (0.7) c | 56.2 (0.1) b | 53.0 (0.1) a |
| Proportion of aromatic C (%) | 18.2 (0.5) a | 18.9 (0.1) a | 19.8 (0.2) b | 18.3 (0.1) a |
| Proportion of carbonyl C (%) | 6.5 (0.5) a | 6.8 (0.2) a | 7.4 (0.1) a | 9.5 (0.1) b |
| Alkyl/O-alkyl C | 0.25 (0.01) a | 0.26 (0.01) a | 0.30 (0.01) b | 0.36 (0.01) c |
Different lowercase letters indicate significant differences among different tree species at p < 0.05 (n = 3).
Soil temperature and moisture, and microbial community composition
Neither soil temperature nor soil moisture significantly differed monthly from January 2012 to December 2013 among the four leaf litter input treatments (Fig. 1). In January 2014, the amounts of total microbial PLFAs, Gram-positive bacterial PLFAs, and actinomycic PLFAs were significantly different among the four treatments (p < 0.05). The amounts of total microbial PLFAs and Gram-positive bacterial PLFAs in the needle litter input treatments were significantly higher than in the C. hystrix leaf litter input treatments, but did not significantly differ with the E. fordii leaf litter input treatments (Fig. 2a,b). The amount of actinomycic PLFAs in the broadleaf litter input treatment was significantly lower than in the P. massoniana needle litter input treatment, but was not significantly different from that in the C. lanceolata needle litter input treatment (Fig. 2c). No significant variation occurred in the amounts of total bacterial PLFAs, Gram-negative bacterial PLFAs, fungal PLFAs, or in the fungi/bacteria (F/B) ratio among the four leaf litter input treatments (p > 0.05).
Figure 1. Soil temperature (a) and soil moisture (b) on a monthly basis from January 2012 to December 2013 in the different treatments of leaf litter inputs in a Pinus massoniana plantation in subtropical China.
Bars show standard errors of the means (n = 6).
Figure 2. Soil phospholipid fatty acid (PLFA) amounts in the different treatments of leaf litter inputs sampled after 28 months of experiment, in a Pinus massoniana plantation in subtropical China.
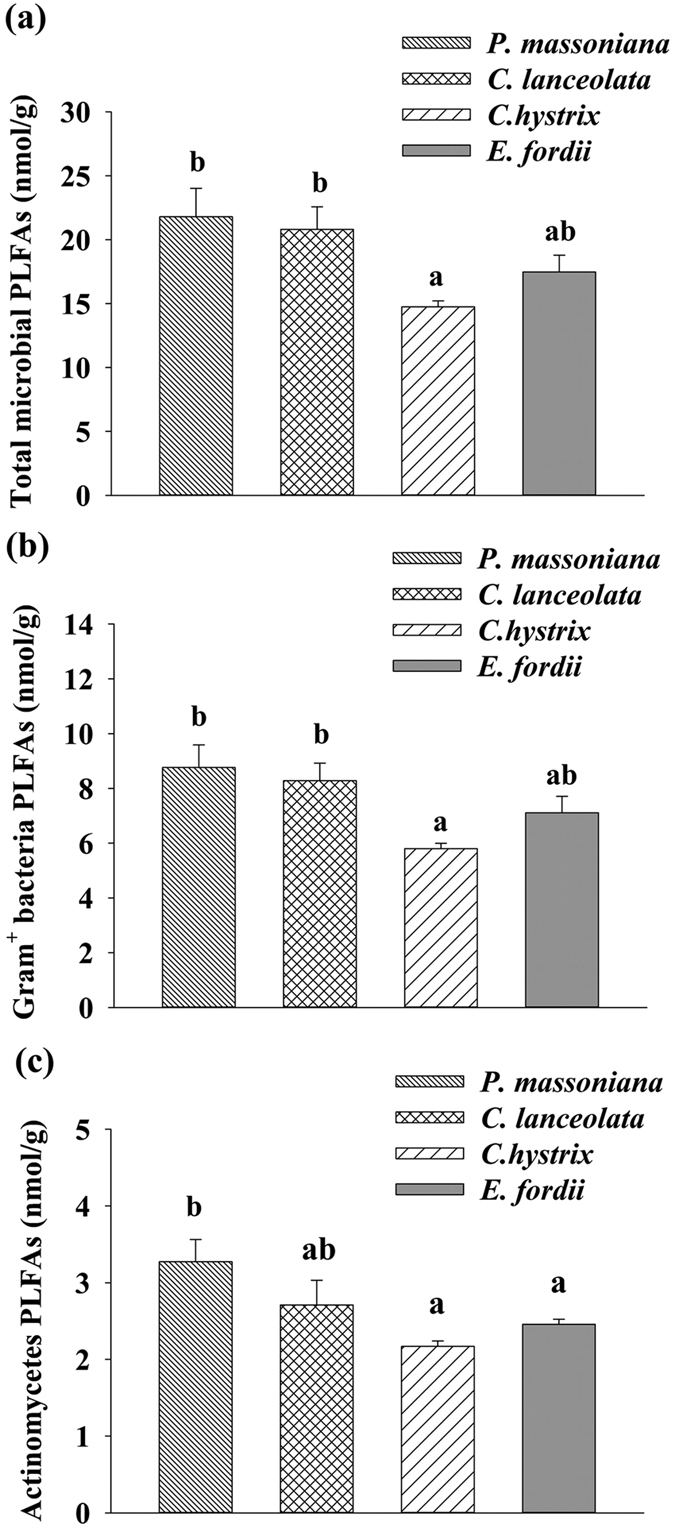
Bars show standard errors of the means (n = 3).
SOC chemical compositions
After 14 months of the experiment, the proportions of alkyl, O-alkyl, aromatic, and carbonyl C in SOC, and soil alkyl/O-alkyl C did not differ significantly among the four leaf litter input treatments (Table 2; p > 0.05). However, after 28 months, soil alkyl/O-alkyl C significantly differed among the four leaf litter input treatments (p < 0.05); soil alkyl/O-alkyl C was significantly lower in the broadleaf litter input treatments than in the C. lanceolata needle litter input treatment, but was not significantly different with that in the P. massoniana needle litter input treatment (Fig. 3). However, the proportions of alkyl, O-alkyl, aromatic, and carbonyl C in SOC were not significantly different among the four leaf litter input treatments (p > 0.05).
Table 2. Soil organic C chemical compositions of different leaf litter input treatments after 14 months in a Pinus massoniana plantation in subtropical China (means with SE in brackets).
| Soil organic C chemical composition | P. massoniana | C. lanceolata | C.hystrix | E. fordii |
|---|---|---|---|---|
| Proportion of alkyl C (%) | 25.0 (0.2) a | 24.4 (0.3) a | 23.1 (1.6) a | 26.2 (1.0) a |
| Proportion of O-alkyl C (%) | 41.7 (1.2) a | 40.6 (1.4) a | 40.3 (1.7) a | 42.1 (2.7) a |
| Proportion of aromatic C (%) | 23.9 (0.2) a | 24.4 (0.9) a | 26.0 (2.0) a | 23.1 (1.2) a |
| Proportion of carbonyl C (%) | 9.4 (1.3) a | 10.5 (0.9) a | 10.5 (1.5) a | 8.5 (1.1) a |
| Alkyl/O-alkyl C | 0.60 (0.01) a | 0.60 (0.03) a | 0.57 (0.02) a | 0.63 (0.07) a |
Different lowercase letters indicate significant differences among different tree species at p < 0.05 (n = 3).
Figure 3. Soil alkyl/O-alkyl C in the different treatments of leaf litter inputs after 28 months of experiment in a Pinus massoniana plantation in subtropical China.
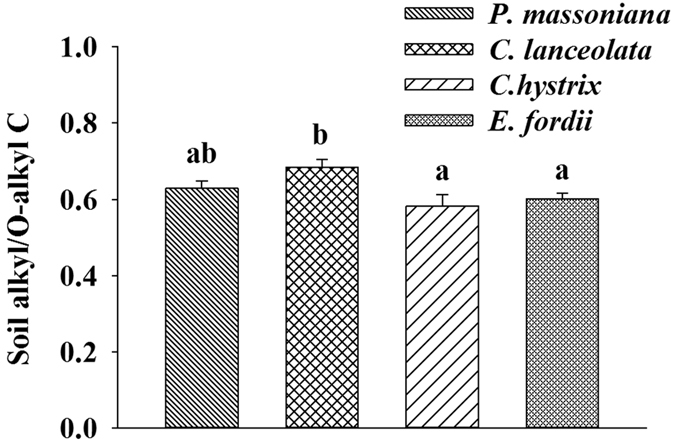
Bars show standard errors of the means (n = 3).
Relationships of soil alkyl/O-alkyl C with leaf litter C chemical compositions and soil microbial community composition
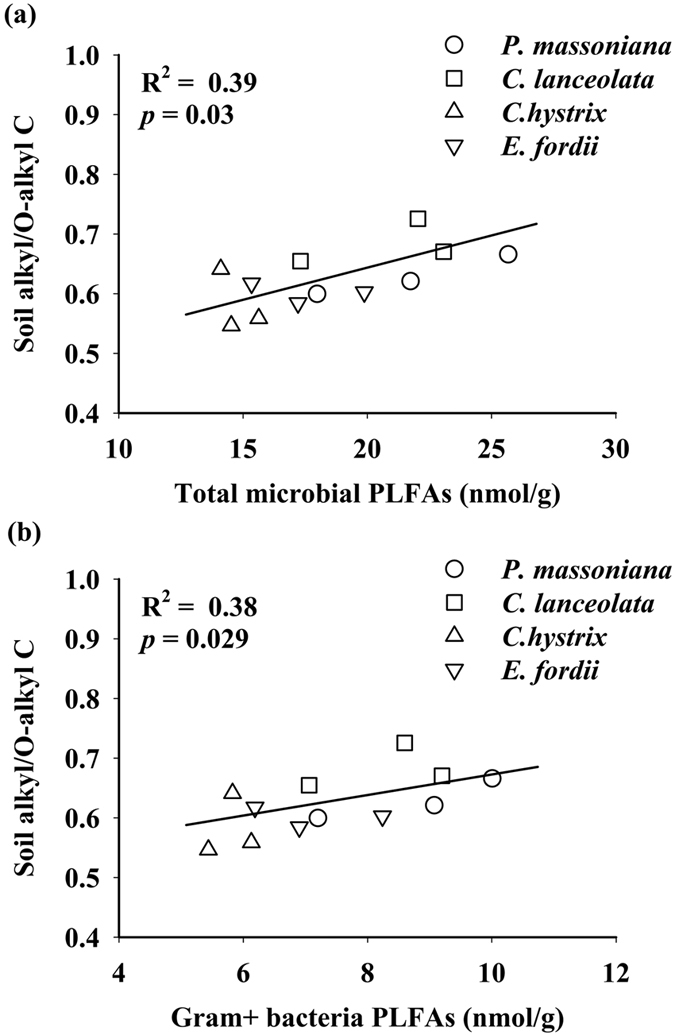
Soil alkyl/O-alkyl C sampled in January 2014 was not correlated to the proportions of alkyl, O-alkyl, aromatic, and carbonyl C in leaf litter, nor was it to the leaf litter alkyl/O-alkyl C across the four leaf litter input treatments. However, the soil alkyl/O-alkyl C was significantly correlated to the amounts of total soil microbial PLFAs and Gram-positive bacterial PLFAs (Fig. 4a,b).
Figure 4. Relationships between soil alkyl/O-alkyl C and the amounts of total soil microbial and Gram-positive phospholipid fatty acids (PLFAs) across the leaf litter input treatments of the four tree species in a Pinus massoniana plantation in subtropical China.
Discussion
Changes in soil microbial community composition induced by leaf litter inputs of different tree species
There were significant differences in the chemical compositions of leaf litter C among the four leaf litter types (Table 1). This helps understanding the role of chemical complexity of leaf litter C inputs in regulating the chemical composition of SOC. The chemical differences in leaf litter inputs can influence the chemical properties of both aggregated and non-aggregated SOM34. Changes in vegetation types and environmental conditions may affect the composition and function of decomposer community. In this study, we found that the amounts of total microbial, Gram-positive bacterial, and actinomycic PLFAs differed among the four leaf litter input treatments after 28 months of the experiment (Fig. 2), indicating shifts of soil microbial community composition with changes in litter type. Although very few studies have directly examined the effects of different tree species’ litter inputs on soil microbial community composition and consequently SOC dynamics, many studies, including the DIRT experiments and forest vegetation conversion studies31,35, showed the altered soil microbial community composition in response to the changes in plant C input. For example, Wang et al. (2013) reported that litter input and removal manipulation affected the soil microbial community composition in a central subtropical coniferous plantation36. We also found that the amounts of total PLFAs, fungal PLFAs, bacterial PLFAs, and Gram-positive and negative bacterial PLFAs significantly differed among four southern subtropical monospecific plantations37.
Linkages of SOC chemical composition to leaf litter C chemical composition and soil microbial community composition
When the leaf litter input treatments persisted for 28 months, the chemical compositions of SOC in the 0–10 cm soil layer differed among the four leaf litter input treatments, as indicated by the significant differences in soil alkyl/O-alkyl C (Fig. 3). Furthermore, soil alkyl/O-alkyl C under the C. lanceolata needle litter input was significantly higher than in the broadleaf litter inputs (Fig. 3). The results suggest that the greater amount of relative stable C components accumulated in soils with treatments of the coniferous C. lanceolata leaf litter compared to the soils with treatments of the broadleaf litter of C. hystrix and E. fordii. Our results are supported by findings in a previous study that the chemical compositions of SOC differed among the four subtropical plantations, with a greater proportion of alkyl C and a lower proportion of O-alkyl C in SOC in P. massoniana plantation relative to the other three broadleaf plantations38. Also, the similar result was reported in a temperate forest that needle derived aliphatic compounds were preferentially preserved in soils of coniferous forests39.
The effects of vegetation type on SOC chemical composition could result from the diversity in chemical compositions of litter C and soil microbial necromass40,41. Plant litter contains various organic compounds including polysaccharides, aromatics, and aliphatics8. The quantity of litter and its chemical properties are the key factors influencing the formation of SOM in terrestrial ecosystems42. However, we did not find a clear relationship of the chemical compositions between SOC and leaf litter C in this study, suggesting that chemical composition of SOC could not be related to the chemical composition of leaf litter C; instead, some other alternative processes could be involved in the formation of soil C fractions. This result is in contrary to a previous study in temperate plantations that the concentrations of individual lipid in soil were very strongly correlated with their concentrations in leaves and roots15. The different results could be attributed to the different climate because fast leaf litter decomposition and fine roots turnover in tropical and subtropical forests. Our previous research has also showed no significant linkage between the chemical compositions of SOC and plant litter C in four subtropical plantations37.
The significant relationships between soil alkyl/O-alkyl C and the amounts of total microbial PLFAs, and Gram-positive bacterial PLFAs (Fig. 4) indicate that the chemical composition of SOC could be likely related to soil microbial community composition. It is consistent with the findings that litter loses mostly non-structural compounds, which are incorporated into microbial biomass at high rates, resulting in efficient SOM formation43. Soil microbes are considered as transformers of plant residue, and they use plant material as their C source, transforming it to CO2, intermediate metabolites, and soil microbial biomass25. The high abundance of submicrometer structures including fragments of hyphae, cells, cell wall fragments, and extracellular polysaccharides are related to microbes found in the soil44. In this study, only soil alkyl/O-alkyl C significantly differed among the four leaf litter input treatments. This is supported by the results of incubation experiment that microbial materials synthesized from glucose by soil microorganisms are mostly O-alkyl, alkyl, and carbonyl C, while phenolic and aromatic structures are only found in small amounts45. Furthermore, the positive relationship between the total soil microbial PLFAs and soil alkyl/O-alkyl C (Fig. 4a) suggests that the high abundance of soil bacteria and fungi could be linked to the high proportion of relatively stable aliphatic C in SOC. Webster et al. (2000) also reported that the soil alkyl/O-alkyl C increased in concomitant with the increased microbial activity during a 28-day incubation46. In addition, the positive relationship between Gram-positive bacterial PLFAs and soil alkyl/O-alkyl C (Fig. 4b) indicates that Gram-positive bacteria could be more related to the high proportion of stable aliphatic C in SOC than other soil microbial community compositions in this study. This is because that bacteria contains more alkyl C and less O-alkyl C than fungi47, and unique to Gram-positive bacteria is the presence of teichoic acids (containing lipid components) in the cell wall.
Conclusion
Leaf litter inputs of the four subtropical tree species had the significant impact on the composition of soil microbial community, and consequently affected SOC chemical composition in the P. massoniana plantation. Organic C input from leaf litter materials did not directly contribute to the formation of SOC chemical fractions, whereas soil microbial community could be a main factor influencing the chemical composition of SOC. Our findings suggest that leaf litter input could induce changes in SOC chemical stability if a close-to-nature management is adopted in the subtropical region by substituting coniferous monospecific plantations with the native broadleaved tree species.
Materials and Methods
Site description
The experimental site is located at the Experimental Center for Tropical Forestry, the Chinese Academy of Forestry (22°05′N, 106°86′E), Pingxiang city, Guangxi Zhuang Autonomous Region, P. R. China. The climate is subtropical monsoon climate, with mean annual temperature of 22.3 °C and annual rainfall of 1400 mm falling mainly from April through September. The soils are classified as red soils based on Chinese soil classification, which is equivalent to Oxisol in USDA Soil Taxonomy48. Since 1950s, the majority of subtropical evergreen broadleaf forests have been harvested and subsequently replaced by Chinese fir (C. lanceolata) and masson pine (P. massoniana). In this study, a coniferous monospecific plantation dominated by P. massoniana at an elevation of 550 m was selected as experimental stand, which was established in 1983 after a clear-cut of a C. lanceolata plantation on the site. The mean diameter of trees measured at breast height (DBH), total tree height, and stem density of the P. massoniana plantation were 24.6 cm, 17.2 m, and 404 trees/ha, respectively38.
Experimental design
In September 2011, a randomized complete block design with three blocks was established in the P. massoniana plantation. Each block was randomly designed to have four leaf litter input treatments. The area of each treatment is 2 m × 2 m. A total of 12 plots were included in the experiment. The four leaf litter input treatments of native tree species contained two coniferous tree species (P. massoniana and C. lanceolata) and two broadleaved tree species (C. hystrix and E. fordii). A 7–10 m buffer zone was in-between the plots to eliminate the interfering effect among the treatments. The averaged content of SOC across the 12 plots before the experiment was 37.6 ± 0.53 g/kg, with no significant difference in SOC among the four treatments (p > 0.05). Each of the plots was trenched to a depth of 1 m to minimize root growth entering the plots and the outside edges of the trenches were lined with thick plastic sheets and backfilled with soil. There were no trees and shrubs inside the plots, and litter detritus and understory were removed from the plots before the experiment. Natural litterfall from the adjacent trees was blocked by nylon mesh spread at a height of 1.6 m above each plot. The plots were monitored and kept free from plants throughout the experiment. The P. massoniana needle litter was collected in the same plantation site, and the C. lanceolata, C. hystrix, and E. fordii leaf litter were collected in the three adjacent monospecific plantation sites, respectively, with the similar topography, soil texture, stand age, and site history. In each of the four plantation sites, 20 litter traps (each 1 m × 1 m) with a mesh size of 1 mm were randomly installed at a height of 1 m above the ground. This work was conducted based on the Forestry Standards “Observation Methodology for Long-term Forest Ecosystem Research” of the People’s Republic of China (LY/T 1952–2011). Leaf litter of each monospecific plantation was monthly collected and mixed together. One fifth of the collected leaf litterfall was oven dried at 65 °C for a constant weight and then weighed for calculating moisture content. Another one fifth of the leaf litterfall was reserved for chemical analysis. The remaining leaf litter inputs was monthly added on the soil surface in each of experimental plots at the same mass of dry weight, which was defined by the minimum value of leaf litter mass of dry weight among the four monospecific plantations. From September 2011 to December 2013, 241.5 g m−2 yr−1 of litter mass of dry weight was added to the 12 plots.
Samplings and measurements
The reserved samples of leaf litterfall from each of the four monospecific plantations which were collected monthly from January 2012 to December 2013, were pooled together, respectively, for analysis of major C chemical compositions (i.e., alkyl, O-alkyl, aromatic, and carbonyl C) by using solid-state 13C cross polarization with magic angle spinning nuclear magnetic resonance (CPMAS NMR)49,50. The 13C CPMAS NMR spectra were obtained at a frequency of 100.64 MHz on a Bruker AVANCE III 400 spectrometer (Bruker, Karlsruhe, Germany). Samples were packed in a ZrO2 rotor (OD = 7 mm) and spun at 5 kHz at the magic angle. Single contact time of 1 ms was applied with an acquisition time of 42 ms, and a recycle delay of 1 s. Transients (20,000) were collected for all samples and a Lorentzian line broadening function of 50 Hz was applied to all spectra. Chemical shift values were referenced externally to glycine at 176.03 ppm, which is equivalent to tetramethylsilane at 0 ppm. The 13C CPMAS NMR spectra were divided into four chemical regions that were assigned to specific organic C functional groups4,51: 0–45 ppm, alkyl C (lipids, cutin, and suberin): 0–45 ppm, alkyl C (lipids, cutin, and suberin); 45–110 ppm, O-alkyl C (carbohydrates, cellulose, hemicelluloses, and methoxyl C); 110–160 ppm, aromatic C (lignin, tannin, olefins, and aromatic compounds); and 160–220 ppm, carbonyl C (carboxylic acid, amide, and ketone groups). The corresponding areas under the curve of the above four regions were quantified by integration.
Mineral soil samples were collected in November 2012 and January 2014, respectively, i.e. 14 and 28 months after the start of the experiment, to determine the effects of different characterized leaf litter inputs on the chemical composition of SOC. Four 5-cm-diameter soil cores were randomly taken to a depth of 10 cm in each plot. The four cores of each plot were combined and sieved through a 2-mm sieve to carefully remove plant material, roots, and gravel to minimize the influence of the plant residues on both chemical and microbial analyses. Soil samples were ground for analyzing for major organic C chemical compositions. The soil samples were pretreated with 10% (v/v) hydrofluoric acid solution prior to solid-state 13C CPMAS NMR spectra analysis, to remove a substantial amount of Fe3+ and Mn2+ from soils and to concentrate SOC for improving the signal/noise ratio of NMR49.
The fresh soil samples in January 2014 were analyzed for phospholipid fatty acids (PLFAs) following Bossio and Scow52. The abundance of individual fatty acids was determined as nmol per g of dry soil using standard nomenclature53. In this study, the content of each PLFA was calculated based on the contents of the 19:0 internal standards. Bacteria were identified by the following PLFAs: i14:0, i15:0, a15:0, 15:0, i16:0, a17:0, i17:0, 15:0 3OH, 16:1 2OH, cy17:0, 17:0, 16:1ω7c, and 18:1ω7c54,55. We calculated the sum of i14:0, i15:0, a15:0, 15:0, i16:0, a17:0, and i17:0 as Gram-positive bacteria56,57 and the sum of 15:0 3OH, 16:1 2OH, cy17:0, 17:0, 16:1ω7c, and 18:1ω7c as Gram-negative bacteria55. The content of PLFAs methyl branched fatty acids (Me) was calculated as a group of actinomycetes58. Fungi were identified by the PLFAs 18:2ω6, 9c, and 18:1ω9c58,59. All of the above PLFAs were used to represent the total PLFAs of the soil microbial community.
Soil temperature and moisture at 5 cm below the soil surface were monitored in each plot. Soil temperature was measured using a digital thermometer (Harvesting Science and Technology Co., Ltd, BJ, China). Volumetric soil moisture (% v/v) was measured simultaneously using Mpkit-B (NTZT Inc., Nantong, JS, China), which consisted of four amplitude domain reflectometry (ADR) moisture probes (MP406). The measurements of soil temperature and soil moisture were made twice a month from January 2012 to December 2013. The 2-monthly measurements of soil temperature and soil moisture in each plot were averaged for each month.
Statistical analysis
One-way analysis of variance was used to examine leaf litter input effects of the four tree species on the proportions of organic C chemical compositions (alkyl, O-alkyl, aromatic, and carbonyl C) in SOC, soil alkyl/O-alkyl C, and the amounts of soil microbial PLFAs. The differences in the proportions of C chemical compositions (alkyl, O-alkyl, aromatic, and carbonyl C) in leaf litter C, and leaf litter alkyl/O-alkyl C among the four tree species were also analyzed using one-way analysis of variance. The statistically significant level was set at p < 0.05. Multiple comparisons of mean among the four treatments were subjected to Duncan’s test. Soil alkyl/O-alkyl C was related to leaf litter C chemical compositions and the amounts of soil microbial PLFAs across the four treatments using bivariate linear regressions. All analyses were performed using SPSS 19.0 for Windows.
Additional Information
How to cite this article: Wang, H. et al. Differential effects of conifer and broadleaf litter inputs on soil organic carbon chemical composition through altered soil microbial community composition. Sci. Rep. 6, 27097; doi: 10.1038/srep27097 (2016).
Acknowledgments
We would like to thank the Guangxi Youyiguan Forest Ecosystem Research Station for experimental stand maintenance and logistical support; RM He, H Chen, Y Wang, D Luo, X Tang, X Zhang and CY Li for assistance in field work and laboratory analysis; YT Fang and XH Wei for reviewing earlier drafts of this manuscript. Grants from the China National Natural Science Foundation (31100380, 31290223), the Ministry of Science and Technology (2015DFA31440, 2012BAD22B01), and the Lecture and Study Program for Outstanding Scholars from Home and Abroad (CAFYBB2011007) supported this work. This paper was also supported by CFERN & GENE Award Funds on Ecological Paper.
Footnotes
Author Contributions H.W. and S.L. conceived the experiment and planned the study. J.W. guided data analysis. Z.S. directed plots design. H.W., J.X., P.H., A.M., H.Y. and L.C. carried out the data collections. L.L. and D.C. supported the experiment management. H.W. wrote the first manuscript with contributions from all co-authors. All authors discussed the results and commented on the manuscript.
References
- Jobbágy E. G. & Jackson R. B. Patterns and mechanisms of soil acidification in the conversion of grasslands to forests. Biogeochemistry 64, 205–229 (2003). [Google Scholar]
- Grandy A. S. & Neff J. C. Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Science of the Total Environment 404, 297–307 (2008). [DOI] [PubMed] [Google Scholar]
- Balser T. C. & Firestone M. K. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73, 395–415 (2005). [Google Scholar]
- Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry 34, 139–162 (2002). [Google Scholar]
- Prescott C. E. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101, 133–149 (2010). [Google Scholar]
- Wang F., Zhu W. & Chen H. Changes of soil C stocks and stability after 70-year afforestation in the Northeast USA. Plant and Soil 401, 319–329 (2016). [Google Scholar]
- Solomon D. et al. Long-term impacts of anthropogenic perturbations on dynamics and speciation of organic carbon in tropical forest and subtropical grassland ecosystems. Global Change Biology 13, 511–530 (2007). [Google Scholar]
- Crow S. E. et al. Sources of plant-derived carbon and stability of organic matter in soil: implications for global change. Global Change Biology 15, 2003–2019 (2009). [Google Scholar]
- Baldock J. et al. Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy. Biogeochemistry 16, 1–42 (1992). [Google Scholar]
- Lorenz K., Lal R., Preston C. M. & Nierop K. G. Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio (macro) molecules. Geoderma 142, 1–10 (2007). [Google Scholar]
- Mikutta R., Kleber M., Torn M. S. & Jahn R. Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77, 25–56 (2006). [Google Scholar]
- Baldock J. & Preston C. Chemistry of carbon decomposition processes in forests as revealed by solid-state carbon-13 nuclear magnetic resonance. Carbon Forms and Functions in Forest Soils, 89–117 (1995). [Google Scholar]
- Chen C., Xu Z. & Mathers N. Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Science Society of America Journal 68, 282–291 (2004). [Google Scholar]
- Huang Z., Xu Z., Chen C. & Boyd S. Changes in soil carbon during the establishment of a hardwood plantation in subtropical Australia. Forest Ecology and management 254, 46–55 (2008). [Google Scholar]
- Mueller K. E. et al. What controls the concentration of various aliphatic lipids in soil? Soil Biology and Biochemistry 63, 14–17 (2013). [Google Scholar]
- Stark S. et al. Composition of lipophilic compounds and carbohydrates in the accumulated plant litter and soil organic matter in boreal forests. European Journal of Soil Science 63, 65–74 (2012). [Google Scholar]
- Cotrufo M. F., Wallenstein M. D., Boot C. M., Denef K. & Paul E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biology 19, 988–995 (2013). [DOI] [PubMed] [Google Scholar]
- Schmidt M. W. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011). [DOI] [PubMed] [Google Scholar]
- You Y. et al. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecology and evolution 4, 633–647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile R., Vanlauwe B. & Six J. Litter quality impacts short-but not long-term soil carbon dynamics in soil aggregate fractions. Ecological Applications 21, 695–703 (2011). [DOI] [PubMed] [Google Scholar]
- Mambelli S., Bird J. A., Gleixner G., Dawson T. E. & Torn M. S. Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Organic Geochemistry 42, 1099–1108 (2011). [Google Scholar]
- Kögel-Knabner I. & Kleber M. Mineralogical, physicochemical, and microbiological controls on soil organic matter stabilization and turnover. Handbook of Soil Sciences Resource Management and Environmental Impacts, second ed. CRC Press Taylor and Francis Group, Boca Raton/London/New York. Part I section (2012). [Google Scholar]
- Simpson A. J., Simpson M. J., Smith E. & Kelleher B. P. Microbially derived inputs to soil organic matter: are current estimates too low? Environmental Science & Technology 41, 8070–8076 (2007). [DOI] [PubMed] [Google Scholar]
- Six J., Frey S. & Thiet R. & Batten, K. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Science Society of America Journal 70, 555–569 (2006). [Google Scholar]
- Miltner A., Bombach P., Schmidt-Brücken B. & Kästner M. SOM genesis: microbial biomass as a significant source. Biogeochemistry 111, 41–55 (2012). [Google Scholar]
- Clemmensen K. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013). [DOI] [PubMed] [Google Scholar]
- Fontaine S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 277–280 (2007). [DOI] [PubMed] [Google Scholar]
- Keiluweit M. et al. Mineral protection of soil carbon counteracted by root exudates. Nature Climate Change 5, 588–595 (2015). [Google Scholar]
- You Y.-M. et al. Differential controls on soil carbon density and mineralization among contrasting forest types in a temperate forest ecosystem. Scientific Reports 6, 22411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton P. J., Castanha C., Torn M. S. & Bird J. A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Global change biology 21, 1358–1367 (2015). [DOI] [PubMed] [Google Scholar]
- Feng W., Zou X. & Schaefer D. Above-and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biology and Biochemistry 41, 978–983 (2009). [Google Scholar]
- Sayer E. J., Heard M. S., Grant H. K., Marthews T. R. & Tanner E. V. Soil carbon release enhanced by increased tropical forest litterfall. Nature Climate Change 1, 304–307 (2011). [Google Scholar]
- Leff J. W. et al. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Global change biology 18, 2969–2979 (2012). [DOI] [PubMed] [Google Scholar]
- Stewart C. E., Neff J. C., Amatangelo K. L. & Vitousek P. M. Vegetation effects on soil organic matter chemistry of aggregate fractions in a Hawaiian forest. Ecosystems 14, 382–397 (2011). [Google Scholar]
- Tamura M. & Tharayil N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New phytologist 203, 110–124 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Q., He T., Wang S. & Liu L. Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agricultural and forest meteorology 178, 152–160 (2013). [Google Scholar]
- Wang H. et al. Soil microbial community composition rather than litter quality is linked with soil organic carbon chemical composition in plantations in subtropical China. Journal of Soils and Sediments 15, 1094–1103 (2015). [Google Scholar]
- Wang H. et al. Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecological research 25, 1071–1079 (2010). [Google Scholar]
- Crow S. E. et al. Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. Forest Ecology and Management 258, 2224–2232 (2009). [Google Scholar]
- Hannam K., Quideau S., Oh S.-W., Kishchuk B. & Wasylishen R. Forest floor composition in aspen- and spruce-dominated stands of the boreal mixedwood forest. Soil Science Society of America Journal 68, 1735–1743 (2004). [Google Scholar]
- Quideau S., Chadwick O., Benesi A., Graham R. & Anderson M. A direct link between forest vegetation type and soil organic matter composition. Geoderma 104, 41–60 (2001). [Google Scholar]
- Scholes M. C., Powlson D. & Tian G. Input control of organic matter dynamics. Geoderma 79, 25–47 (1997). [Google Scholar]
- Cotrufo M. F. et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geoscience 8, 776–779 (2015). [Google Scholar]
- Foster R. Microenvironments of soil microorganisms. Biology and fertility of soils 6, 189–203 (1988). [Google Scholar]
- Golchin A., Clarke P. & Oades J. The heterogeneous nature of microbial products as shown by solid-state 13C CP/MAS NMR spectroscopy. Biogeochemistry 34, 71–97 (1996). [Google Scholar]
- Webster E., Chudek J. & Hopkins D. Carbon transformations during decomposition of different components of plant leaves in soil. Soil Biology and Biochemistry 32, 301–314 (2000). [Google Scholar]
- Baldock J., Oades J., Vassallo A. & Wilson M. Solid-state CP/MAS 13C NMR analysis of bacterial and fungal cultures isolated from a soil incubated fith glucose. Soil Research 28, 213–225 (1990). [Google Scholar]
- USDA, S. S. S. o.Keys to Soil Taxonomy. (United States Department of Agriculture, Natural Resources Conservation Service, 1996). [Google Scholar]
- Schmidt M., Knicker H., Hatcher P. G. & Kogel-Knabner I. Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. European Journal of Soil Science 48, 319–328 (1997). [Google Scholar]
- Wang H. et al. Dynamics and speciation of organic carbon during decomposition of leaf litter and fine roots in four subtropical plantations of China. Forest Ecology and Management 300, 43–52 (2013). [Google Scholar]
- Spielvogel S., Prietzel J. & Kögel-Knabner I. Soil organic matter changes in a spruce ecosystem 25 years after disturbance. Soil Science Society of America Journal 70, 2130–2145 (2006). [Google Scholar]
- Bossio D. & Scow K. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecology 35, 265–278 (1998). [DOI] [PubMed] [Google Scholar]
- Tunlid A., Hoitink H., Low C. & White D. Characterization of bacteria that suppress Rhizoctonia damping-off in bark compost media by analysis of fatty acid biomarkers. Applied and Environmental Microbiology 55, 1368–1374 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård Å. & Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils 22, 59–65 (1996). [Google Scholar]
- Zelles L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biology and Fertility of Soils 29, 111–129 (1999). [Google Scholar]
- Kourtev P. S., Ehrenfeld J. G. & Häggblom M. Exotic plant species alter the microbial community structure and function in the soil. Ecology 83, 3152–3166 (2002). [Google Scholar]
- Liu L., Gundersen P., Zhang T. & Mo J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biology and Biochemistry 44, 31–38 (2012). [Google Scholar]
- Cusack D. F., Silver W. L., Torn M. S., Burton S. D. & Firestone M. K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92, 621–632 (2011). [DOI] [PubMed] [Google Scholar]
- Thoms C. & Gleixner G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biology and Biochemistry 66, 239–248 (2013). [Google Scholar]


