Abstract
Kisspeptin-neurokinin B-dynorphin (KNDy) cells of the hypothalamus are a key component in the neuroendocrine regulation of GnRH secretion. Evidence in sheep and other species suggests that dynorphin released by KNDy cells inhibits pulsatile GnRH secretion by acting upon κ-opioid receptors (KOR). However, the precise anatomical location and neurochemical phenotype of KOR-expressing cells in sheep remain unknown. To this end, we determined the distribution of KOR mRNA and protein in the brains of luteal phase ewes, using an ovine specific KOR mRNA probe for in situ hybridization and an antibody whose specificity we confirmed by Western blot analyses and blocking peptide controls. KOR cells were observed in a number of regions, including the preoptic area (POA); anterior hypothalamic area; supraoptic and paraventricular nuclei; ventromedial, dorsomedial, and lateral hypothalamus; and arcuate nucleus. Next, we determined whether KOR is colocalized in KNDy and/or GnRH cells. Dual-label immunofluorescence and confocal analysis of the KNDy population showed a high degree of colocalization, with greater than 90% of these neurons containing KOR. Surprisingly, GnRH cells also showed high levels of colocalization in sheep, ranging from 74.4% to 95.4% for GnRH cells in the POA and medial basal hypothalamus, respectively. Similarly, 97.4% of GnRH neurons in the POA of ovariectomized, steroid-primed female rats also contained immunoreactive KOR protein. These findings suggest that the inhibitory effects of dynorphin on pulsatile GnRH secretion may occur either indirectly by actions upon KOR within the KNDy population and/or directly via the activation of KOR on GnRH cells.
Secretion of GnRH occurs in two major modes, pulsatile and surge secretion, which are primarily under the control of the negative and positive feedback influence of gonadal steroids, respectively (1). A population of neurons postulated to serve as a key component of the GnRH pulse generator, and to participate in steroid feedback control of GnRH secretion, are cells in the arcuate nucleus that coexpress three neuropeptides, kisspeptin, neurokinin B, and dynorphin, termed KNDy cells (2). KNDy cells are highly enriched in receptors for gonadal steroid hormones (3, 4), form a reciprocally interconnected network (5, 6), and have direct projections to GnRH cell bodies and terminals (7), features consistent with their proposed role in conveying the feedback influence of gonadal steroids to GnRH neurons. Current research into KNDy neurons has primarily focused on the stimulatory actions of kisspeptin and neurokinin B (NKB), whereas dynorphin (Dyn), and its role as a signal mediating the inhibition of GnRH pulses, remains the least studied of the three KNDy peptides.
Several lines of evidence have implicated Dyn, a member of the endogenous opioid (EOP) family, as a critical mediator of GnRH inhibition by progesterone. Early work demonstrated that that blockade of EOP action with naloxone, or similar EOP antagonists, increased LH pulse frequency during the luteal phase in several species (8–10), including humans (11). More recent studies in sheep have shown that norbinaltorphimine (nor-BNI), an antagonist to the κ-opioid receptor (KOR), the high-affinity receptor for Dyn, increased LH pulse frequency in luteal phase ewes when implanted in the medial basal hypothalamus (MBH), whereas antagonists to the other two EOP receptors (μ, δ) did not (12). Importantly, Dyn neurons synapse on 40% of GnRH neurons in the preoptic area (POA) and 90% of MBH GnRH cells (12) and a very high percentage (>90%) of Dyn neurons of the arcuate nucleus colocalize progesterone receptors (3), implicating them as possible mediators of progesterone negative feedback via KOR activation. However, it remains unclear precisely where KOR are localized in this circuitry, and consequently where Dyn may act to inhibit GnRH pulses.
KOR is an inhibitory G-coupled receptor with a highly conserved amino acid sequence specifically at the transmembrane domains (13). Although the distribution of KOR has been described in the rat (14, 15), guinea pig (16), and humans (17) using ligand-binding, immunocytochemistry or in situ hybridization techniques, detailed information on the localization of KOR in the sheep is unavailable. Therefore, our first objective was to examine the distribution of KOR-expressing cells in the ovine POA and hypothalamus using in situ hybridization (ISH) and to determine whether KOR mRNA is present in areas of the sheep brain in which GnRH neurons are found. To increase the specificity of this approach, we used a probe that targeted a sequence of the ovine KOR mRNA encoding portions of greatest sequence diversity among opioid receptors (13, 18). Second, using a specific antibody against KOR, we performed single-label immunohistochemistry to determine whether KOR protein was present in the same areas in which we detected KOR mRNA. In addition, we used dual-label immunocytochemistry to determine whether KNDy cells and/or GnRH cells within the POA and MBH colocalize KOR. Surprisingly, we found KOR colocalized not only in KNDy neurons but also in a majority of ovine GnRH neurons, contrary to previous reports in the rodent (19, 20). Therefore, to determine whether there are species differences in the colocalization of KOR, we also examined GnRH cells in the POA of female rats. The results suggest that Dyn may exert its inhibitory effects on GnRH secretion by acting not only directly upon KNDy cells of the arcuate nucleus but also upon GnRH neurons, in both sheep and rats.
Materials and Methods
Animals
Adult, black-faced ewes of mixed breeding were maintained in an open barn with free access to water and fed once daily with a maintenance regimen of silage. Ewes demonstrating regular estrous cycles determined by monitoring estrous behavior with a vasectomized ram were moved to an indoor facility 3–7 days before tissue collection and housed two per pen under artificial lighting that mimicked the natural day-length environmental photoperiod for the breeding season. Tissue was collected during the breeding season (October through January) from two groups of ewes sacrificed during the luteal phase (d 9 of the estrous cycle) and used to determine the distribution of KOR mRNA (n = 3) and KOR protein immunoreactivity and colocalization (n = 4). We used tissue from luteal phase ewes because previous work demonstrated that signaling via KOR inhibits GnRH pulse frequency at this time (12, 21). All procedures were approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for the use of animals in research.
Adult female Sprague Dawley rats (Charles River) were housed in pairs in standard Plexiglass cages. Food and water were provided ad libitum, and animals were maintained in temperature- and humidity-controlled rooms on a 12-hour dark, 12-hour light cycle with lights off at 9:00 am. Females were bilaterally ovariectomized and implanted with sc capsules (Dow Corning tubing; 1.98 mm internal diameter) containing 5% 17β-estradiol-benzoate (in cholesterol; Sigma-Aldrich; 1 cm filled area) and received 500 μg progesterone in 0.1 mL of sesame oil (Sigma-Aldrich; sc) 5 hours prior to tissue collection similar to previous studies (19). This was done to mimic the physiological steroid levels during the proestrous phase of the rodent estrous cycle when GnRH secretion is inhibited by Dyn (16, 22). All procedures were approved by the University of Mississippi Medical Center Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
In situ hybridization
Ewes were euthanized with iv sodium pentobarbital (2–3 g; Sigma-Aldrich) and decapitated. A tissue block containing the septal region, POA, and hypothalamus was rapidly dissected, placed to freeze on dry ice, and stored at −80ºC. Coronal sections (12 μm) were cut using a cryostat in 12 parallel series, separated by 144 μm.
To detect KOR mRNA expression in the sheep POA and hypothalamus by ISH, a cDNA fragment of ovine KOR was isolated using RT-PCR specific for the second and third extracellular loop, the fifth, sixth, and seventh transmembrane domains, and the third intracellular loop and a portion of the carboxy-terminus of the receptor, bases 1–432 (GenBank; DQ065757). A fresh frozen sheep hypothalamic block was collected and total RNA was extracted. After the extraction, 2 μg of RNA was reverse transcribed using avian myeloblastosis virus-reverse transcriptase (30 U; Promega). This was followed by PCR using Taq polymerase (Life Technologies) for 35 cycles (Amplitron II thermocycler) at 51ºC for 2 minutes. A PCR fragment was then ligated into the PGEM-T Easy Vector (Promega), and subcloned into JM109 competent cells (Promega). The KOR sequence was determined using T7 and SP6 primers and screened for sequence identity with the basic local alignment search tool search tool (National Center for Biotechnology Information).
Detection of KOR mRNA in the POA and hypothalamus was performed by an ISH procedure using probes synthesized from a linearized ovine KOR cDNA described above; unless noted otherwise all reagents were purchased from Sigma-Aldrich. Sense and antisense radiolabeled cRNA probes were generated by in vitro transcription in a reaction containing transcription buffer (Promega), 0.5 mM ribonucleotide triphosphates (rATP, rGTP, rCTP), 10 μM rat uridine 5-triphosphate, 1 μg DNA template, 10 μM dithiothreitol, 5 μM ribonuclease inhibitor (Rnasin; Promega), 20 U of T7 or SP6 RNA polymerases (Promega), and 50 μCi 35S-uridine 5-triphosphate (MP Biomedicals). After incubating in the reaction tube for 2 hours at 37ºC, deoxyribonuclease I was added to the reaction to degrade the DNA template followed by an additional incubation at 37ºC for 15 minutes. Reaction was stopped with 0.5 M EDTA, unincorporated nucleotides were removed using microquick spin columns (Roche) and 10 μg tRNA was added to the probe solution.
Slides containing tissue sections were removed from the freezer, placed in racks, and air dried for 30–40 seconds. Sections were then fixed for 15 minutes in 4% paraformaldehyde in phosphate buffer (PB; pH 7.4) while shaking at room temperature and washed twice in PB. Slides were dipped in water twice and in triethanolamine (0.1M, pH 8) once and then incubated for 10 minutes in triethanolamine containing acetic anhydride (2.5 μL/mL). Slides were washed in 2× saline sodium citrate (SSC; 0.15 M NaCl, 0.015 M sodium citrate) solution and sections dehydrated through 70%, 95%, and 100% EtOH, 3 minutes each, and delipidated in chloroform for 5 minutes. Sections were then washed in 100% and 95% EtOH, 3 minutes each, and air dried. Sections were hybridized overnight with sense or antisense radiolabeled cRNA probes for KOR at 55ºC in a humidified chamber. Before hybridization, probes were diluted (1 × 106 cpm per 150 μL) in hybridization buffer (50% deionized formamide, 0.3 M NaCl, 20 mM Tris-HCl, 5 mM EDTA, 10 mM NaPO4, Denhanrdt's solution, 10% dextran sulfate, 0.5 mg/mL yeast tRNA, and 100 mM dithiothreitol) and denatured at 70ºC for 10 minutes. Hybridization solution (150 μL) was applied to tissue sections and a parafilm coverslip placed on each slide. On the following day, parafilm coverslips were removed and slides were washed in 5× SSC containing 10 mM β-mercaptoethanol (βME) for 30 minutes at 55ºC followed by TEN buffer (10 mM Tris-HCl, pH 8; 5 mM EDTA, pH 8; 0.5 M NaCl) for 10 minutes at 37ºC three times. Sections were treated with ribonuclease (Sigma; 10 μg/mL in TEN buffer) for 30 minutes at 37ºC to remove nonspecific bound probe followed by a series of washes in TEN buffer for 30 minutes at 37ºC, 2× SSC containing 10 mM βME for 30 minutes at 55ºC twice, 0.1× SSC containing 10 mM βME for 15 minutes at 55ºC, and 0.1× SSC for 15 minutes at room temperature. Finally, sections were dehydrated through ethanol (70% EtOH + 0.3 M ammonium acetate for 5 minutes, twice; 95% EtOH + 0.3 ammonium acetate for 3 minutes; 100% EtOH for 2 minutes, twice) and air dried for 1–3 hours at 37ºC. Slides were then dipped in Kodak nitroblue tetrazolium salt emulsion (Kodak), dried, and exposed in the dark for 6 weeks at 4ºC. Slides were developed in D-19 developer (Kodak) and sections counterstained with cresyl violet, dehydrated, and coverslipped with DPX (EM Sciences).
Every 12th section (144 μm apart) through the POA and hypothalamus was analyzed for each ewe. Cells expressing KOR mRNA were identified using a dark- and bright-field microscope (Leica Corp model) on the basis of silver grain density (at least 5 times above background levels [23]). Drawings of representative sections were made using a camera lucida attached to a Nikon Optiphot microscope (Nikon Corp). The number of KOR-expressing cells was determined in two representative sections of each ewe for the following regions: POA; anterior hypothalamic area (AHA); supraoptic nucleus (SON); paraventricular nucleus (PVN); lateral hypothalamus; ventromedial hypothalamus (VMH); dorsomedial hypothalamus (DMH); the rostral, medial, and caudal aspects of the arcuate nucleus (ARC); and the ventral aspect of the premammillary region. Data are shown in a semiquantitative manner (Table 1). Sections hybridized with sense control probe did not show any labeling, and no silver grains clustered over neurons were detected in any areas of the brain.
Table 1.
Distribution of KOR Cells Throughout the POA and MBH
| Hypothalamic Area | KOR-mRNA Cells, n | KOR-ir Cells, n |
|---|---|---|
| POA | ++++ | +++ |
| SON | +++ | ++++ |
| PVN | +++ | ++++ |
| AHA | +++ | ++ |
| VMH | +++ | ++ |
| DMH | +++ | ++ |
| ARC | ++++ | ++++ |
The total number of cells in each area was calculated for each animal and expressed as a group mean. Numbers of cells are as follows: +, 1–50 cells; ++, 50–100 cells; +++, 100–150 cells; ++++, more than 150 cells.
Immunohistochemistry
Ewes were euthanized with iv sodium pentobarbital and rapidly decapitated. The heads were perfused via both internal carotids with 6 L of 4% paraformaldehyde in 0.1 M PB (pH 7.3) mixed with 0.1% sodium nitrite and administered with 10 U/mL heparin. After perfusion, the brain was removed, and a tissue block containing the septal region, POA, and hypothalamus was dissected out. Blocks were incubated in 4% paraformaldehyde at 4ºC overnight for postfixation and then transferred into 30% sucrose in 0.1 M PB for cryoprotection until infiltration took place. A sliding freezing microtome (SM 200R; Leica Biosystems) was used to cut coronal sections (45 μm) into six parallel series. Sections were stored in cryoprotectant solution (30% ethylene glycol, 1% polyvinylpyrrolidone, 30% sucrose in sodium phosphate buffer) at −20ºC until needed.
Female rats were deeply anesthetized using sodium pentobarbital (270 mg/kg; ip) and perfused intracardially with 10 mL of 0.9% saline, followed by 500 mL of 4% paraformaldehyde in 0.1 M PB. Brains were removed and postfixed for 1 hour at room temperature in the same fixative and then immersed in 20% sucrose and 0.01% sodium azide in 0.1 M PB and stored at 4ºC. Coronal sections (35 μm) were cut with a freezing microtome (H400R; Micron), collected in four parallel series in cryoprotectant solution, and stored at −20ºC until needed (see Supplemental Table 1 for information on all the primary antibodies used herein).
Single-label immunohistochemistry for KOR
The distribution and quantification of KOR cells in ovine tissue was determined in a series of every sixth section (270 μm apart) using immunoperoxidase. Free-floating sections were washed thoroughly in 0.1 M PBS for several hours to remove excess cryoprotectant prior to incubation with antigen unmasking solution (1:100 in distilled H2O; Vector Laboratories; catalog number H-3300) in an 80ºC water bath for 30 minutes. This antigen retrieval protocol has been previously shown to increase the visualization of many antigens not otherwise detectable (3). Sections were allowed to cool for 20 minutes and then incubated for 10 minutes in 1% H2O2 diluted in PBS to block endogenous peroxidase activity. Next, sections were incubated in a blocking solution (PBS+) containing 4% normal goat serum (Jackson ImmunoResearch Laboratories, Inc) in PBS containing 0.4% Triton X-100 (TX-100; Fisher Scientific) for 1 hour and then 17 hours with monoclonal mouse anti-KOR (1:250; Santa Cruz Biotechnology, Inc; catalog number SC-374479) in PBS+ at room temperature. After incubation with the primary antiserum, sections were incubated with biotinylated goat antimouse secondary antibody (1:500; Vector Laboratories) diluted in PBS+ for 30 minutes followed by ABC-elite (1:500 in PBS; Vector Laboratories) for 1 hour. KOR labeling was visualized using 3,3′-diaminobenzidine with 0.02% nickel sulfate and 0.003% hydrogen peroxide as substrate for 10 minutes. Finally, the sections were washed in PB, mounted onto Superfrost slides (Fisher Scientific), dehydrated, and coverslipped using Depex Mountant (EM Sciences).
Peptide-blocking controls
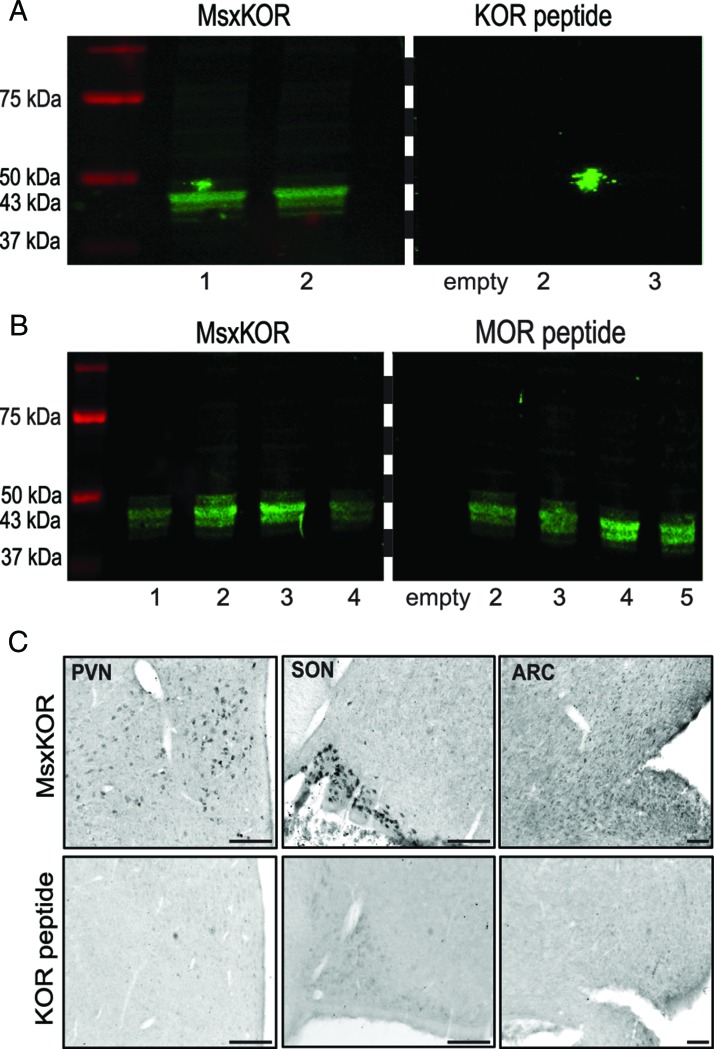
Specificity of KOR antibody was tested using recommended controls for immunostaining (24) including the following: peptide-blocking controls, primary antibody omissions controls, Western blot analysis, and comparison with the pattern of mRNA expression (see below). Single-label immunoperoxidase detection revealed KOR-positive cells in a number of hypothalamic regions, including the POA, the PVN, and the ARC, areas that also contain KOR mRNA. Preabsorption (overnight at 4ºC) of the KOR antibody (mouse xKOR) with 25 μg/mL of the peptide against which it was made (exact sequence; amino acids 351–380 [C terminal] of the human KOR, custom made by Phoenix Pharmaceuticals Inc) abolished all staining in the ovine POA, PVN, SON, and ARC (Figure 1C, lower panel) but preabsorption with a peptide for μ-receptor (MOR) antibody (Santa Cruz Biotechnology; AG-375) did not (Figure 1C, upper panel). In addition, we performed primary antibody omission controls for both single-label and dual-label procedures involving detection of KOR (see below), and in all cases, removal of the primary antibody incubation from the protocols eliminated all KOR staining.
Figure 1.
KOR antibody validation. Western blots illustrating immunoreactive single bands after exposure to mouse anti-KOR (A, left panel, samples 1 and 2, and B, left panel, samples 1–4) at the appropriate mass: 43 kDa. This immunoreactivity was blocked by preabsorption of mouse anti-KOR preabsorption with KOR peptide (A, right panel, samples 1–3) but not MOR peptide (B, right panel, samples 2–5). Empty lanes in right panels of A and B were not loaded with peptide and served as negative controls. All samples in A and B were loaded and processed on the same gels and membranes. Membrane was cut at dashed line for exposure to either mouse anti-KOR (MsxKOR) or preabsorption with KOR (A) or MOR peptide (B). Images for immunohistochemical controls are shown in panel C for sections of SON, PVN, or ARC incubated with either mouse-anti-KOR or preabsorption control with KOR peptide. Scale bars, 500 μm.
Western blot
Protein was isolated from adult ewe hypothalamus. Tissue blocks containing the hypothalamus were frozen on dry ice and stored at −80ºC until processing. The hypothalamus was microdissected and homogenized in radioimmunoprecipitation assay buffer (50 nM Tris-HCl; 150 mM NaCl; 1% Nonidet P-40; 0.1% sodium dodecyl sulfate [SDS]; 0.5% sodium deoxycholate). The homogenate was centrifuged at 12 000 rpm for 20 minutes at 4ºC, supernatant was collected and protein concentrations determined using a bicinchoninic assay (ThermoFisher Scientific) and NanoDrop ND-1000 spectrophotometer (ThermoFisher Scientific). Protein samples were boiled at 96ºC for 4 minutes in an Accublock digital dry bath (Labnet International), and 10 μg was loaded for each sample on a 10% polyacrylamide gel and separated under reducing conditions using a Mini Trans-Blot cell system (Bio-Rad Laboratories Ltd) and Tris-glycine-SDS running buffer (25 mM Tris; 192 mM glycine; 0.1% SDS [pH 8.3]). Precision Plus protein All Blue standards (Bio-Rad Laboratories Ltd) were used to mark molecular weights. Next, proteins were transferred to Millipore Immobilon-FL polyvinylidene difluoride membranes (Millipore) using Trans-Blot cell wet blotting system (Bio-Rad Laboratories Ltd) in transfer buffer (20% methanol and 0.037% SDS in Tris-glycine (25 mM Tris; 192 mM glycine [pH 8.3]) (Bio-Rad Laboratories) at 82 V for 1 hour at room temperature. Next, membranes were incubated in an Odyssey blocking buffer (2:3 solution; LI-COR Biosciences) and then Tris-buffered saline (TBS; 50 mM Tris and 150 mM NaCl [pH 8.0]) for 1 hour at room temperature. This was followed with mouse anti-KOR (Santa Cruz Biotechnology; 1:250), which was either not preabsorbed or preabsorbed with 10 μg/mL KOR or MOR antigen, in a 2:3 mix of Odyssey blocking buffer with TBS + 0.05% Tween-20 (pH8.0) for 16 hours at 4ºC under gentle agitation. Next, membranes were incubated for 1 hour in IR Dye 800 CW-conjugated goat antimouse (1:10 000; in Odyssey blocking buffer with TBS + 0.05% Tween-20 [pH8.0]). Images of fluorescent bands were captured using Odyssey 2.1 scanner (LI-COR Biosciences).
Mouse anti-KOR revealed a single band at the appropriate mass (43 kDa). Preabsorption of the KOR antibody with KOR peptide, but not with peptide for MOR antibody (Santa Cruz Biotechnology; AG-375) abolished all signal (Figure 1, A and B). All samples were processed simultaneously on the same gels and membranes and scanned in parallel.
Dual-label immunofluorescent detection of KOR in the ewe
To determine whether KORs are colocalized within the hypothalamic KNDy and/or GnRH cell populations, a series of sections containing the POA or ARC were processed for dual-label immunofluorescence and confocal microscopic analysis using a biotinylated tyramine amplification procedure. The procedure was similar to that described above and in our previous papers (7, 25), including antigen unmasking steps. After antigen unmasking, sections were incubated with mouse anti-KOR (1:250; 17 h; Santa Cruz Biotechnology), biotinylated goat antimouse secondary antibody (1:500; 1 h; Vector Laboratories), ABC-elite (1:500; 1 h; Vector Laboratories), biotinylated tyramine (tyramide signal amplification; 1:250 in PBS containing 3% H2O2 per 1 mL, 10 min; PerkinElmer), and Alexa 555-streptavidin (1:100; 30 min; Molecular Probes; KOR/kisspeptin; KOR/GnRH) or Alexa 488-streptavidin (1:100; 30 min; Invitrogen, Grand Island, NY; KOR/NKB). Next, sections were incubated in either rabbit antikisspeptin (1:10 000, gifted by A. Caraty, French National Institute for Agricultural Research, Paris), rabbit anti-NKB (1:500; Phoenix Pharmaceuticals, Inc), or rabbit anti-GnRH (1:400; Immunostar) in PBS+ for 17 hours. Subsequently, sections were incubated in either Dylight 488 goat antirabbit (1:100; 30 min; Thermo Scientific; KOR/kisspeptin, KOR/GnRH) or Alexa 555 goat antirabbit (1:100; 30 min; KOR/NKB). Sections stained for KOR/kisspeptin were then incubated in Neurotrace 640/660 Deep Red fluorescent Nissl stain (1:100; Molecular Probes) for 20 minutes to visualize nuclear boundaries. All sections were mounted onto Superfrost slides (Fisher Scientific), dried, and coverslipped with an aqueous mounting medium (Gelvatol) containing an antifading agent (1,4-diazabicyclo[2,2]octane (DABCO); 50 mg/mL). Antibody controls again showed that preabsorption (overnight at 4 C) of the KOR antibody (mouse anti-KOR) with 25 μg/mL of the peptide against which it was made (amino acids 351–380 [C terminal] of the human KOR, custom made by Phoenix Pharmaceuticals Inc), abolished all immunofluorescent staining for KOR, whereas staining for kisspeptin or GnRH remained intact.
Dual-label immunofluorescent detection of KOR in the female rat
To ascertain whether any findings were specific to ewes or conserved between animal species, dual-fluorescence immunocytochemistry (ICC) was repeated in ovariectomized (OVX) estradiol (E)+progesterone (P) female rats (n = 3). Procedures were similar as described above. Antibody incubation solution consisted of PBS containing 0.1% bovine serum albumin (Fisher Scientific) and 0.4% TX-100 (Fisher Scientific). Sections were incubated with mouse anti-KOR (1:100; 17 h; Santa Cruz Biotechnology), biotinylated goat antimouse secondary antibody (1:500; Vector Laboratories), ABC-elite (1:500 in PBS; Vector Laboratories), biotinylated tyramine (1:250; PerkinElmer), and Alexa 555-streptavidin (1:100; Molecular Probes). Next, sections were incubated with rabbit anti-GnRH (1:400; Immunostar) and Dylight 488 goat antirabbit (1:100; Thermo Scientific).
Data analysis
Immunocytochemistry
For single-label KOR, the distribution of immunoreactive cells was examined in sections through the POA and hypothalamus of each ewe. Three representative sections of the rostral, middle, and caudal divisions of the POA, SON, PVN, AHA, retrochiasmatic area of the hypothalamus, VMH, DMH, and ARC were quantitatively analyzed per animal.
Colocalization of KOR in KNDy neurons in the ARC and kisspeptin neurons in the POA was analyzed in two sections per brain area per ewe at ×20 magnification using Neurolucida software (MicroBrightfield Bioscience) and a digital camera (Microfire A/R; Optronics) attached to a microscope (DM500B; Leica Microsystems). The total number of single- and double-labeled cells were analyzed for each section, averaged per animal, and reported as the percentages of the following: kisspeptin- or NKB-immunoreactive (ir) cells containing KOR, single labeled kisspeptin- or NKB-ir cells, KOR-ir cells containing kisspeptin or NKB and single-labeled KOR cells. All data are expressed as the mean ± SEM.
Neurons immunopositive for GnRH or both KOR and GnRH were counted in ovine sections containing the POA, AHA, and MBH at ×20 magnification (Leica Microsystems). The total number of single- and double-labeled GnRH-ir cells were calculated and reported as percentages ± SEM.
Colocalization analysis for GnRH/KOR in female rats was analyzed in sections containing the POA following the methods stated above.
Confocal imaging
Sections processed for dual immunofluorescence were imaged using a Nikon D-Eclipse C1 laser-scanning confocal system (Nikon Corp) attached to a Nikon Eclipse E800 microscope (Nikon Corp). Fluorophores are detected by three lasers at wave lengths of 488, 543, and 633 nm, filtered by two dichroic mirrors filtering at 530 and 625 nm, respectively. Confocal Z-stacks of optical sections (1 μm optical sections, at ×60 magnification) were captured through KOR, kisspeptin, NKB, and GnRH-ir neurons using EZ-C1 Gold version 3.80 software (Nikon Corp). Z-stacks from the middle and caudal ARC of each animal were used for analysis of KOR/kisspeptin and KOR/NKB colocalization. For the examination of the colocalization in the POA, the Z-stacks were analyzed for KOR/kisspeptin and KOR/GnRH-immunostained sections.
Results
Distribution of KOR mRNA in the POA and hypothalamus of ewes
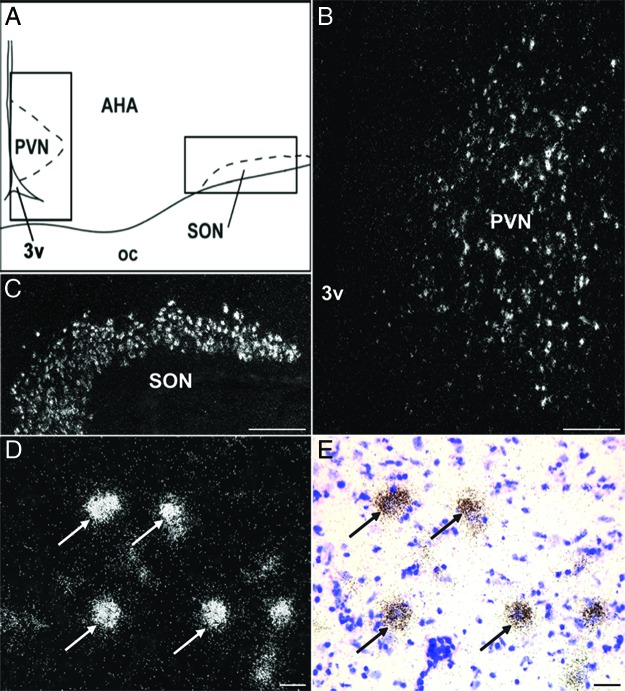
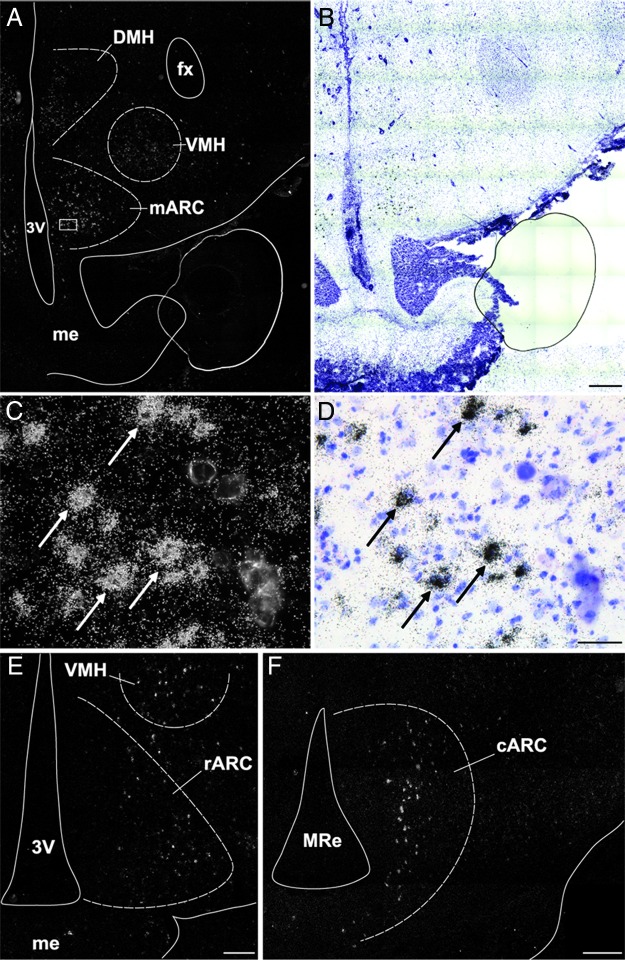
Cells expressing KOR were distributed widely in the ovine diencephalon (Table 1). KOR mRNA-containing neurons were observed in the POA, specifically in its medial portion, and extended rostrally into the diagonal band of Broca. In the SON, cells containing KOR mRNA were distributed densely and provided a clear delineation of the nucleus (Figure 2C). Cells containing KOR mRNA were also observed in the PVN; rostrally these appeared as a small group of cells in the ventral anterior hypothalamus (Figure 2B), which extended caudally and dorsally along the third ventricle in the periventricular zone in a wing-like shape that corresponded to the Nissl-defined boundaries of the nucleus. In addition, isolated KOR-expressing cells were observed scattered throughout the anterior hypothalamic area. In the middle levels of the hypothalamus, cells containing KOR mRNA were observed in the ventromedial and dorsomedial nuclei and in the lateral hypothalamus. In the ARC (Figure 3), KOR mRNA-expressing cells were observed throughout the rostral-caudal extent of the nucleus, with the largest number of cells seen in middle and caudal divisions (Figures 3, A and F). The location of the KOR mRNA-positive cells also varied according to the rostral-caudal position in the ARC, KOR cells being primarily laterally in the rostral ARC but located in a more central and medial location in the middle and caudal divisions of this nucleus. Cells expressing KOR mRNA were also observed in the premammillary region of the hypothalamus.
Figure 2.
KOR mRNA in PVN and SON. Camera lucida drawing (A) displaying areas (solid box) depicted in dark-field photomicrographs (B and C) of a representative hypothalamic section hybridized with antisense probe to ovine KOR mRNA. Cells expressing KOR were observed in the PVN (B) and SON (C). High-power dark- (D) and bright-field (E) photomicrograph of PVN cells expressing KOR (arrows). Scale bar (B and C) 500 μm; (D and E) 100 μm. oc, optic chiasm; 3v, third ventricle.
Figure 3.
KOR mRNA in ARC. Representative dark- (A) and bright-field (B) photomicrographs of a hypothalamic section at the level of the middle ARC hybridized with antisense probe to ovine KOR mRNA. High-power dark- (C) and bright-field (D) photomicrograph of ARC cells expressing KOR (arrows) from outlined area (white box) in panel A. E and F, Representative dark-field photomicrographs showing the distribution of KOR mRNA-containing cells in the rostral (E) and caudal (F) ARC. Scale bar (B, E, and F), 500 μm; (D), 100 μm. cARC, caudal ARC; fx, fornix; mARC, middle ARC; me, median eminence; MRe, mammillary recess of the third ventricle; rARC, rostral ARC; 3v, third ventricle.
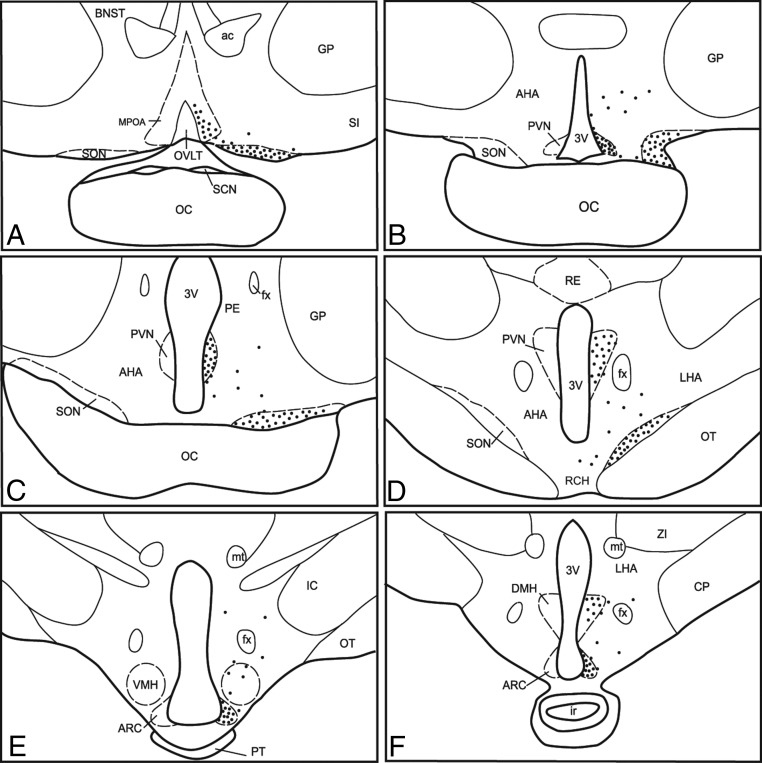
Anatomical distribution of KOR-ir in the ovine hypothalamus
The distribution pattern of cells containing immunoreactive KOR protein in the ovine hypothalamus was very similar to that described above for KOR mRNA (Table 1). Intense immunolabeling for KOR was observed in the SON and PVN, in which numerous KOR-positive cells were densely packed. In addition, KOR-ir cells were observed in the POA, AHA (Figure 4, A and B), ARC (Figure 4E), ventromedial (Figure 4E) and dorsomedial nuclei of the hypothalamus (Figure 4F), and premammillary nucleus. In general, these cells were less densely labeled compared with those in the SON and PVN and were smaller in soma size. In addition, KOR-ir axon terminals were observed throughout the external zone of the median eminence in which they were located near GnRH axons.
Figure 4.
Camera lucida drawings illustrating representative distribution of KOR-ir cells in the POA (A), SON (B–D), PVN (B–D), AHA; anterior hypothalamic area (B–D), retrochiasmatic area (RCH; D), VMH (E) and DMH (F) nuclei of the hypothalamus; and ARC (E and F). Each black dot represents approximately 10 KOR-ir cells. The distribution of KOR-ir was identical on both sides of the POA and hypothalamus and is shown unilaterally to allow visualization of labels. ac, anterior commissure; BNST, bed nucleus of the stria terminalis; CP, cerebral peduncle; fx, fornix; GP, globus pallidus; ir, infundibular recess; mt, mammillary tract; OC, optic chiasm; OT, optic tract; OVLT, organum vasculosum of lamina terminalis; PE, periventricular nucleus; PT, pars tuberalis of the adenohypophysis; RE, reticular nucleus; SCN, suprachiasmatic nucleus; SI, substantia innominata; ZI, zona incerta; 3v, third ventricle.
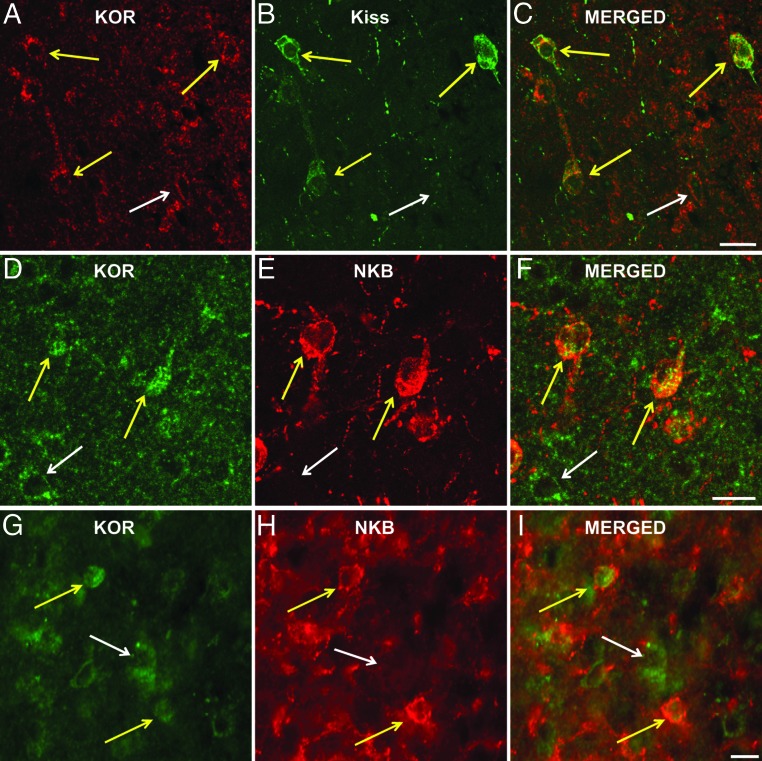
Colocalization of KOR with kisspeptin and NKB in the ewe
Analysis of dual-labeled sections stained for KOR and kisspeptin revealed that KOR was colocalized in the vast majority of ARC KNDy neurons (Figure 5, A–C); specifically, 97.8% ± 1.5% of ARC kisspeptin neurons were seen to contain KOR (Table 2). In contrast, only 11.1% ± 3.7% of kisspeptin cells in the POA colocalized KOR (Table 2). Similar to the high degree of colocalization seen for ARC kisspeptin and KOR, an analysis of the dual-labeled sections stained for KOR and NKB showed that 93.5% ± 1.6% of the arcuate NKB cells colocalize KOR (Table 3). In addition to dual-labeled kisspeptin/KOR and NKB/KOR cells, numerous single-labeled KOR cells were also observed throughout the ARC (Figure 5, D–I); specifically, 57.7% ± 2.5% of all KOR neurons did not colocalize kisspeptin (Table 2) and 53.9% ± 3.8% did not colocalize NKB (Table 3).
Figure 5.
Representative images showing KOR colocalization in KNDy cells. Upper panels (A–C) are confocal images of 1-μm-thick optical sections showing KOR-ir (red) and kisspeptin-ir (green) cells in the ARC. Middle and lower panels are confocal (D–F) and light microscopic fluorescent (G–I) images of KOR-ir (green) and NKB-ir (red) in the ARC. Cells colocalizing KOR and either kisspeptin or NKB are denoted by yellow arrows. Single-labeled KOR cells are denoted by white arrows. Scale bars, 50 μm. Kiss, kisspeptin.
Table 2.
Percent Colocalization of KOR in Kisspeptin Neurons
| Area | Kiss/KOR, % | Kiss Only, % | Kiss Cells, n | KOR/Kiss, % | KOR Only, % | KOR Cells, n |
|---|---|---|---|---|---|---|
| ARC | 97.8 ± 1.5 | 2.2 ± 1.5 | 69.0 ± 1.6 | 42.3 ± 2.5 | 57.67 ± 2.5 | 162.8 ± 3.7 |
| POA | 11.1 ± 3.7 | 88.9 ± 2.4 | 10.8 ± 4.1 |
Abbreviation: Kiss, kisspeptin. Values are expressed as group mean ± SEM. Kiss/KOR, % indicates percentage of kisspeptin-ir cells containing KOR; Kiss only, % indicates percentage of kisspeptin-ir cells not containing KOR; Kiss cells, n indicates number of kisspeptin-ir cells per animal; KOR/Kiss, % indicates percentage of KOR-ir cells containing kisspeptin; KOR only, % indicates percentage of KOR-ir cells not containing kisspeptin; KOR cells, n indicates number of KOR-ir cells per animal.
Table 3.
Percent Colocalization of KOR in NKB Neurons
| Area | NKB/ KOR, % | NKB Only, % | NKB Cells, n | KOR/NKB, % | KOR Only, % | KOR Cells, n |
|---|---|---|---|---|---|---|
| ARC | 93.5 ± 1.6 | 6.5 ± 1.6 | 63.8 ± 8.6 | 46.1 ± 3.8 | 53.9 ± 3.8 | 127.9 ± 13.2 |
Values are expressed as group mean ± SEM. NKB/KOR, % indicates percentage of NKB-ir cells containing KOR; NKB only, % indicates percentage NKB-ir cells not containing KOR; NKB cells, n indicates mean number of NKB-ir cells per animal; KOR/NKB, % indicates percentage of KOR-ir cells containing NKB; KOR only, % indicates percentage of KOR-ir cells not containing NKB; KOR cells, n indicates mean number of KOR-ir cells per animal.
Colocalization of KOR in GnRH cells in the ewe
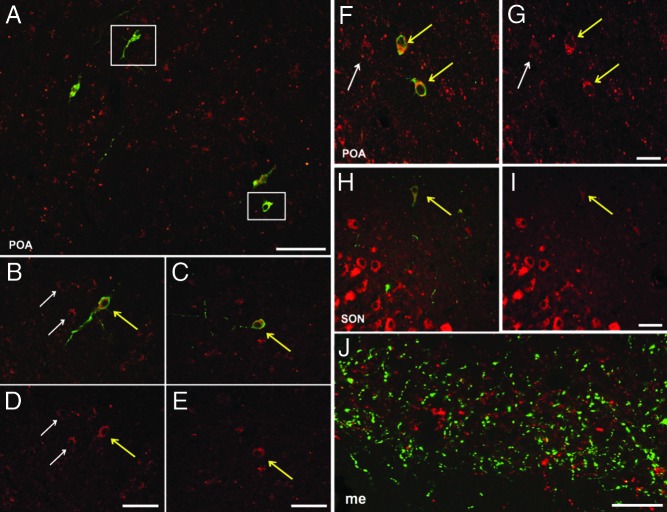
A quantitative analysis of sections dual labeled for GnRH and KOR revealed high levels of colocalization (Figure 6) with 74.4% ± 2.6% of GnRH cells in the POA, 87.6% ± 2.9% of GnRH cells in the AHA, and 95.4% ± 1.8% of GnRH cells in the MBH, containing KOR (Table 4). However, despite close examination of GnRH fibers within the median eminence, we found no examples of KOR colocalization in GnRH fibers or terminals in this region in any animal (Figure 6J).
Figure 6.
Representative confocal images of KOR colocalization in GnRH cells. A, Composite image of a confocal microscopy z-stack showing immunofluorescent detection of KOR-ir (red) and GnRH-ir (green) cells within the POA. B and C, Single 1-μm optical sections from outlined areas (white box) in the same Z-stack (panel A). F, Confocal microscopy images of 1-μm optical sections illustrating additional KOR-ir (red) and GnRH-ir (green) cells in the POA. H, Representative GnRH neuron shown is located near the SON. Colocalization is denoted by yellow arrows; white arrows denote single-labeled KOR cells. J, Representative image of GnRH fibers (green) and KOR (red) depicting no colocalization within the median eminence (me). D, E, G, and I, Single-channel images displaying KOR-ir labeling. Scale bar (A), 500 μm; (D, E, G, I, and J), 100 μm.
Table 4.
Percentage Colocalization of KOR in GnRH Neurons
| Area | GnRH/ KOR, % | GnRH Only, % | GnRH Cells, n |
|---|---|---|---|
| MBH | 95.4 ± 1.8 | 4.6 ± 1.8 | 45.5 ± 0.7 |
| AHA | 87.6 ± 2.9 | 12.4 ± 2.9 | 19.3 ± 8.7 |
| POA | 74.4 ± 2.6 | 25.6 ± 2.6 | 73.5 ± 6.2 |
Values are expressed as group mean ± SEM. GnRH/KOR, % indicates percentage of GnRH-ir cells containing KOR; GnRH only, % indicates percentage of GnRH-ir cells not containing KOR; GnRH cells, n indicates mean number of GnRH-ir cells per animal.
Colocalization of KOR in GnRH cells in the rat
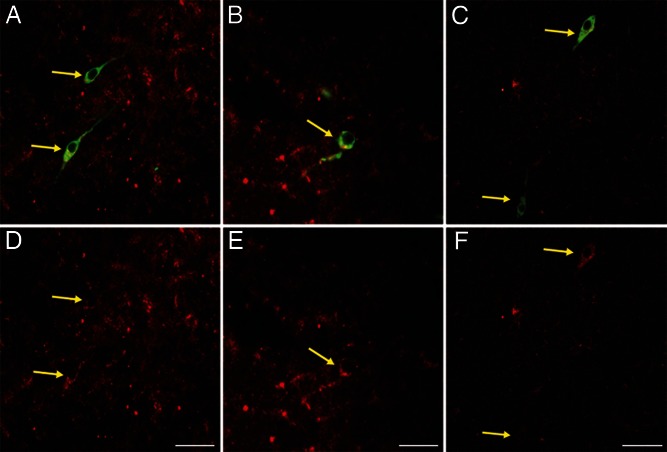
To determine whether colocalization of KOR in GnRH cells was seen in other species in addition to the sheep, the same dual-label immunocytochemical procedure and analysis was performed in sections of the POA and hypothalamus from OVX, steroid-primed female rats. The distribution of KOR-ir cells was nearly identical to that observed in ewe, with dense labeling in the SON and PVN, and KOR-ir cells in the POA, ARC, AHA, VMH, and DMH (data not shown). More importantly, dual labeling showed that 97.4% ± 0.5% of GnRH cells in the POA colocalized KOR (Figure 7).
Figure 7.
Representative images of KOR colocalization in GnRH cells in the rodent POA. Light microscopy images (A and B) and confocal microscopy images (C) of tissue processed for immunofluorescent detection of KOR-ir (red) and GnRH-ir (green) cells. D, E, and F, Single-channel images displaying KOR-ir labeling. Yellow arrows denote colocalization. Scale bars, 100 μm.
Discussion
In this study, we first described the general distribution of KOR mRNA and protein throughout the POA and MBH of luteal-phase ewes and reported their presence in areas, including the POA and ARC, shown to be important for reproductive neuroendocrine function. Second, we observed that KOR is colocalized in greater than 94% of ovine KNDy neurons within the ARC, results that are consistent with its proposed role in the inhibition of GnRH pulse frequency in this species (26). Finally, we reported novel evidence for the colocalization of KOR in GnRH neurons, suggesting the possibility of direct Dyn actions on these neurons to control GnRH release.
The results of the in situ hybridization and single-label immunohistochemical analyses reported above are the first in the sheep model to show that KOR mRNA and protein are distributed widely within the POA and hypothalamus. Earlier reports had demonstrated the presence of KOR in the POA and hypothalamus of the sheep using ligand-binding procedures (16, 27). However, those studies used hypothalamic tissue extracts, a methodology that provides limited resolution of regional or cellular location of the receptor. In the current study, techniques that allow resolution at cellular level were used. Our observations are in general agreement with findings of the distribution of KOR mRNA in the mouse (15, 28, 29), guinea pig (30), and human (17). KOR expression was dense in the SON and PVN. This is consistent with other studies (15, 28) and supports the role for Dyn and KOR in the neuroendocrine regulation of fluid homeostasis controlling vasopressin and oxytocin release (31, 32). Vasopressin/Dyn neurons of the SON and PVN have been shown to contain KOR in the guinea pig (33). We do not know whether KOR cells in the ewe SON and PVN coexpress vasopressin and oxytocin, although this seems likely, given their distribution and large soma size. Finally, we would note that the labeling for both KOR mRNA and protein was markedly more intense in the SON and PVN than in the other preoptic and hypothalamic areas in which it was observed, including the ARC. This may reflect the difference in cell size between magnocellular SON/PVN neurons and the smaller cells of the ARC and other areas as well as differences in the turnover/accumulation of mRNA and protein. For example, vasopressin immunoreactivity is also markedly more intense in SON and PVN magnocellular cells than in the much smaller neurons of the suprachiasmatic nucleus (34).
The distribution of KOR in the ovine POA and hypothalamus we observed is largely consistent with previous studies in the ewe that described the anatomical distribution of Dyn cells and their fibers. Thus, Dyn-ir fibers and terminals have been identified in the SON, the AHA, and the ARC (23), and retrograde-tracing studies have revealed that 22% of ARC Dyn neurons project to the POA in luteal phase ewes (35). Dyn cells have also been identified in the PVN at the level of the anterior hypothalamus; however, no fibers were reported in this area (23).
In addition to describing the general distribution of KOR, we also observed that nearly all ARC KNDy neurons contain KOR. The presence of KOR within the ovine ARC KNDy population is consistent with its proposed role in GnRH pulse regulation (26). This model predicts that each GnRH pulse is triggered by NKB binding to KNDy cells stimulating the release of kisspeptin onto GnRH neurons, resulting in a large spike of GnRH secretion. The same neurons stimulated by NKB then release Dyn inhibiting activity of the KNDy network and thus terminating GnRH release. Two studies using nor-BNI have supported the proposed role of Dyn in the termination of GnRH release. They reported that this KOR antagonist increased bursts of multiunit activity in OVX goats when given intracerebroventricularly (36) and significantly increased LH pulse frequency when given into the ARC of OVX ewes (26). However, colocalization of KOR in KNDy cells had not previously been shown in either species.
Our results show that greater than 90% of KNDy neurons in the ewe cells express KOR; thus, Dyn may exert its inhibitory control of GnRH pulse frequency by binding to postsynaptic KOR within KNDy cells. In contrast, previous studies in the mouse have revealed a much lower level of colocalization with approximately 20% of ARC KNDy cells containing KOR in females (37) and approximately 6% in males (38). The lower degree of KOR colocalization in rodent KNDy cells may be due to technical reasons or physiological differences between rodents and ruminants. One possible technical reason is that previous studies used only ISH, which may not have been sensitive enough to detect the relatively low levels KOR mRNA present in these neurons. Supporting this possibility is evidence from single-cell RT-PCR studies that 41% of KNDy neurons in male mice express KOR (39), a higher percentage than that seen in the same sex and species using ISH. Additionally, the lengthy emulsion exposure time required for visualization of the low levels of mRNA within the ovine ARC, and resulting decrease in neuropeptide antigenicity within the same section, makes it impractical to combine ISH for KOR with immunolabeling to determine cell phenotype. In the current study, an antigen-retrieval protocol for ICC combined with tyramide-based signal amplification was required to allow visualization of KOR at the cellular level as well as phenotypic identification. Another possible reason for the difference in percentage colocalization between species is that Dyn may play different functional roles in the control of GnRH pulses. In rodents, in contrast to sheep and goats, administration of nor-BNI has no effect of episodic LH secretion in OVX rats (40). Similarly, nor-BNI has no effect on the spontaneous electrical activity of murine KNDy neurons in slice preparations (39, 41). Thus, the low level of KOR/KNDy colocalization in rodents may reflect the fact that other transmitters, besides or in addition to Dyn, may be responsible for GnRH pulse termination.
We also observed a sizable proportion of KOR-positive cells (>50%) in the ARC, which do not contain NKB or kisspeptin, and hence represent a separate subpopulation from KNDy cells. Thus, Dyn from KNDy or other neurons may also act upon adjacent non-KNDy, KOR-containing neurons in the ARC. In this regard, it is interesting to note that receptors for kisspeptin (Kiss1r) and NKB (NK3R) have also been identified in non-KNDy neurons in the ovine ARC (42, 43). Recent findings in the ewe suggest that kisspeptin, in addition to its role as an output of KNDy cells driving individual pulses (44), also acts within the ARC to modulate pulse frequency (26). Because KNDy cells in the ewe lack the Kiss1r (42), these effects of kisspeptin on pulse frequency are likely mediated by actions on other, adjacent neurons. Single-labeled KOR neurons are potential candidates for this kisspeptin-sensitive population, or for the non-KNDy, NK3R-containing cells, in the ARC; if either, or both, of these receptors colocalize with KOR, then projections from them back onto KNDy cells would allow such cells to participate in both stimulation and inhibition of GnRH pulses (44). Thus, one important future study will be to determine whether single-labeled KOR cells of the ARC colocalize Kiss1R or NK3R and are contacted by KNDy fibers. Preliminary observations support this hypothesis because we noted kisspeptin-ir fibers near single-labeled KOR cells in our material but have not yet carried out quantitative, confocal analysis of those potential contacts.
Several other neural populations within the ARC also represent potential candidates for the non-KNDy KOR containing cells we observed. In addition to their role in reproductive neuroendocrine function, KOR cells of the ARC are likely involved in other aspects of neuroendocrine regulation. For example, Dyn has been shown to regulate energy balance and food intake in both mice (45) and rats (46) via proopiomelanocortin and neuropeptide Y neurons in the ARC. Dyn injections into the hypothalamus have also been shown to induce feeding in sheep (47). In addition, Dyn has been implicated in the suckling-induced prolactin secretory response in rats, primates, and humans. Thus, infusion of Dyn antisera, intracerebroventricularly, abolished the suckling-induced prolactin increase in female rats (48), and a Dyn agonist increased serum prolactin levels in follicular phase female rhesus monkeys (49) and humans (50). Further studies investigating the phenotype of non-KNDy, KOR-containing cells may provide insights into the mechanisms by which Dyn regulates these neuroendocrine functions.
Finally, we observed that greater than 75% of the GnRH neurons in the POA, AHA, and MBH contain KOR in the ewe. Our findings stand in contrast to previous reports in the rodent (see below) and represent the first evidence of KOR colocalization on GnRH neurons in any species. KOR colocalization in GnRH cells in the sheep is also consistent with evidence for a role of dynorphin-KOR signaling in the POA in mediating P-negative feedback in the ewe. Previous studies in sheep have shown the following: 1) Dyn neurons in the POA and AHA as well as the ARC contain nuclear P receptor (23); 2) dynorphin-positive fibers synapse directly onto GnRH neuron cell bodies and dendrites in the POA and MBH (12); and 3) local administration of nor-BNI in both the POA and MBH blocked the inhibitory influence of progesterone on LH pulse frequency during the luteal phase (12). The presence of KOR in ovine GnRH cells are consistent with the ability of nor-BNI microimplants in the POA to block P-negative feedback (12) and suggest a potential direct site of the inhibitory action of dynorphin on GnRH release in addition to its role in pulse termination at the level of KNDy cells and their reciprocal circuitry (26).
To determine whether our finding of KOR colocalization in GnRH neurons was specific to the ewe, we repeated the analysis in the female rat. We observed that 97% of GnRH neurons contained KOR in POA of OVX+E+P rats. This finding contradicts that of previous studies reporting a lack of mRNA for μ-, δ-, or κ-opioid receptors in the GnRH neurons of the rat (19, 20). One possible explanation for this discrepancy may be the technical difficulty of detecting low levels of receptor mRNA in dual-labeled material compared with the ability to more sensitively detect protein using the antigen retrieval and signal amplification procedures used in the current study. The results of studies localizing other endogenous opioid receptors in GnRH neurons also support this possibility. Using single-cell RT-PCR, Ronnekleiv and colleagues (51) reported that 33% of GnRH neurons in the guinea pig contain MOR mRNA and in the same paper note preliminary evidence that 40% of mouse GnRH neurons express MOR mRNA; both results stand in contrast to the complete absence of MOR colocalization reported in the rat by ISH (19). In addition, δ-opioid receptors have been shown to be colocalized by dual-label immunofluorescence within a subset of GnRH fibers in the rat median eminence (52), even though colocalization was not detectable in the GnRH cell bodies in the same study. Collectively these observations are consistent with the possibility that technical limitations render relatively low levels of endogenous opioid receptors (including KOR) present in GnRH cell bodies undetectable either by ISH or by ICC without antigen retrieval and signal amplification.
In addition, we would also note other studies support a potential role for Dyn in mediating the feedback control of GnRH release in rats, which could occur directly via actions on GnRH cells. First, Dyn may mediate E- and/or P-negative feedback because intracerebroventricular administration of nor-BNI increased LH in OVX+E rats (53), and local administration of this antagonist in the POA and MBH increased LH release in pregnant rats (54). Dyn has also been implicated in the positive feedback actions of ovarian steroids: 1) the Dyn message is reduced in the medial POA during the LH surge on proestrus (22) and 2) local blockade of KOR advances the surge in proestrous rats (55).
In summary, this study provides a description of the cellular distribution of KOR mRNA and protein in the ovine POA and hypothalamus and demonstrates that the vast majority of both KNDy and GnRH neurons in the ewe contain this receptor. Together with earlier work (12), our results provide additional neuroanatomical support for the proposed role of Dyn in the regulation of pulsatile GnRH secretion and reveal two potential pathways by which Dyn may modulate GnRH activity: 1) indirectly via the KNDy cell network and/or 2) directly at the level of GnRH cell bodies. Future studies are required to determine the neuropeptide/transmitter phenotype of non-KNDy, KOR-containing cells within the ARC of the ewe and their potential role/s in reproductive, or other neuroendocrine, functions. Moreover, the discrepancy between our finding of KOR colocalization in GnRH cells and previous attempts to identify EOP receptors in GnRH neurons points to the need for additional work to reexamine this issue and determine whether other EOP receptors, other than to KOR, may also be colocalized in GnRH neurons.
Acknowledgments
We recognize, with gratitude, the contributions of our friend and coauthor, Dr Marcel Amstalden, who died on Wednesday, September 3, 2014. Dr Amstalden was responsible for the KOR in situ hybridization results reported in this manuscript. We thank Mr Shadon Rollins and Ms Tina Smith for their excellent technical support.
This work was supported by National Institutes of Health Grant R01 HD039916 (to M.N.L. and R.L.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHA
- anterior hypothalamic area
- ARC
- arcuate nucleus
- DMH
- dorsomedial hypothalamus
- Dyn
- dynorphin
- E
- estradiol
- EOP
- endogenous opioid
- ICC
- immunocytochemistry
- ISH
- in situ hybridization
- Kiss1r
- kisspeptin receptor
- KNDy
- kisspeptin, neurokinin B, and dynorphin
- KOR
- κ-opioid receptor
- MBH
- medial basal hypothalamus
- βME
- β-mercaptoethanol
- MOR
- μ-receptor
- NKB
- neurokinin B
- NK3R
- NKB receptor
- Nor-BNI
- norbinaltorphimine
- OVX
- ovariectomized
- P
- progesterone
- PB
- phosphate buffer
- POA
- preoptic area
- PVN
- paraventricular nucleus
- SDS
- sodium dodecyl sulfate
- SON
- supraoptic nucleus
- SSC
- saline sodium citrate
- TBS
- Tris-buffered saline
- TEN
- buffer of Tris-HCl
- EDTA
- NaCl
- VMH
- ventromedial hypothalamus.
References
- 1. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 3. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. [DOI] [PubMed] [Google Scholar]
- 4. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neuroscience letters. 2006;401:225–230. [DOI] [PubMed] [Google Scholar]
- 5. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. [DOI] [PubMed] [Google Scholar]
- 6. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. [DOI] [PubMed] [Google Scholar]
- 7. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27:624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks AN, Lamming GE, Lees PD, Haynes NB. Opioid modulation of LH secretion in the ewe. J Reprod Fertil. 1986;76:693–708. [DOI] [PubMed] [Google Scholar]
- 9. Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39:1032–1038. [DOI] [PubMed] [Google Scholar]
- 10. Ferin M, Vande Wiele R. Endogenous opioid peptides and the control of the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 1984;18:365–373. [DOI] [PubMed] [Google Scholar]
- 11. Ropert JF, Quigley ME, Yen SS. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52:583–585. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. [DOI] [PubMed] [Google Scholar]
- 13. Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. [DOI] [PubMed] [Google Scholar]
- 14. Desjardins GC, Brawer JR, Beaudet A. Distribution of μ, δ, and κ opioid receptors in the hypothalamus of the rat. Brain Res. 1990;536:114–123. [DOI] [PubMed] [Google Scholar]
- 15. Mansour A, Fox CA, Burke S, et al. μ, δ, and κ Opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. [DOI] [PubMed] [Google Scholar]
- 16. Sim LJ, Childers SR. Anatomical distribution of μ, δ, and κ opioid- and nociceptin/orphanin FQ-stimulated [35S]guanylyl-5′-O-(γ-thio)-triphosphate binding in guinea pig brain. J Comp Neurol. 1997;386:562–572. [DOI] [PubMed] [Google Scholar]
- 17. Peckys D, Landwehrmeyer GB. Expression of μ, κ, and δ opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Mestek A, Liu J, Yu L. Molecular cloning of a rat κ opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem J. 1993;295(Pt 3):625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or δ opiate receptors. Endocrinology. 1997;138:1667–1672. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of μ and κ opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport. 1997;8:3167–3172. [DOI] [PubMed] [Google Scholar]
- 21. Goodman RL, Gibson M, Skinner DC, Lehman MN. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl. 2002;59:41–56. [PubMed] [Google Scholar]
- 22. Zhang Q, Gallo RV. Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine. 2002;18:27–32. [DOI] [PubMed] [Google Scholar]
- 23. Foradori CD, Goodman RL, Lehman MN. Distribution of preprodynorphin mRNA and dynorphin-a immunoreactivity in the sheep preoptic area and hypothalamus. Neuroscience. 2005;130:409–418. [DOI] [PubMed] [Google Scholar]
- 24. Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–478. [DOI] [PubMed] [Google Scholar]
- 25. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015:156(9):3277–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen PJ, Smith AI, Evans RG, Clarke IJ. Effects of ovarian steroids on hypothalamic opioid receptor subtypes in ovariectomized ewes: regional changes in density and affinity. J Endocrinol. 1995;145:559–567. [DOI] [PubMed] [Google Scholar]
- 28. DePaoli AM, Hurley KM, Yasada K, Reisine T, Bell G. Distribution of κ opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol Cell Neurosci. 1994;5:327–335. [DOI] [PubMed] [Google Scholar]
- 29. Maekawa K, Minami M, Yabuuchi K, et al. In situ hybridization study of μ- and κ-opioid receptor mRNAs in the rat spinal cord and dorsal root ganglia. Neurosci Lett. 1994;168:97–100. [DOI] [PubMed] [Google Scholar]
- 30. Xie GX, Meng F, Mansour A, et al. Primary structure and functional expression of a guinea pig κ opioid (dynorphin) receptor. Proc Natl Acad Sci USA. 1994;91:3779–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van de Heijning BJ, Koekkoek-Van den Herik I, Van Wimersma Greidanus TB. The opioid receptor subtypes μ and κ, but not δ, are involved in the control of the vasopressin and oxytocin release in the rat. Eur J Pharmacol. 1991;209:199–206. [DOI] [PubMed] [Google Scholar]
- 32. van Wimersma Greidanus TB, van de Heijning BJ. Opioid control of vasopressin and oxytocin release. Regul Pept. 1993;45:183–186. [DOI] [PubMed] [Google Scholar]
- 33. Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The κ opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–383. [DOI] [PubMed] [Google Scholar]
- 34. Sofroniew MV, Weindl A. Identification of parvocellular vasopressin and neurophysin neurons in the suprachiasmatic nucleus of a variety of mammals including primates. J Comp Neurol. 1980;193:659–675. [DOI] [PubMed] [Google Scholar]
- 35. Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC. Progesterone-receptive β-endorphin and dynorphin B neurons in the arcuate nucleus project to regions of high gonadotropin-releasing hormone neuron density in the ovine preoptic area. Neuroendocrinology. 2005;81:139–149. [DOI] [PubMed] [Google Scholar]
- 36. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904. [DOI] [PubMed] [Google Scholar]
- 41. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. [DOI] [PubMed] [Google Scholar]
- 42. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. [DOI] [PubMed] [Google Scholar]
- 43. Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goodman RL, Coolen LM, Lehman MN. Unraveling the mechanism of action of the GnRH pulse generator: a possible role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons. In: Conn AU-AM, ed. Cellular Endocrinology in Health and Disease. Boston: Academic Press; 2014:133–152. [Google Scholar]
- 45. Zhang X, van den Pol AN. Direct inhibition of arcuate proopiomelanocortin neurons: a potential mechanism for the orexigenic actions of dynorphin. J Physiol. 2013;591:1731–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maolood N, Meister B. Dynorphin in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus. Neuroscience. 2008;154:1121–1131. [DOI] [PubMed] [Google Scholar]
- 47. Della-Fera MA, Baile CA, Coleman BD, Miner JL, Paterson JA. Central nervous system injection of dynorphin-(1–13) overrides gastric satiety factors in sheep. Am J Physiol. 1990;258:R946–R950. [DOI] [PubMed] [Google Scholar]
- 48. Callahan P, Klosterman S, Prunty D, Tompkins J, Janik J. Immunoneutralization of endogenous opioid peptides prevents the suckling-induced prolactin increase and the inhibition of tuberoinfundibular dopaminergic neurons. Neuroendocrinology. 2000;71:268–276. [DOI] [PubMed] [Google Scholar]
- 49. Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:678–686. [PubMed] [Google Scholar]
- 50. Bart G, Borg L, Schluger JH, Green M, Ho A, Kreek MJ. Suppressed prolactin response to dynorphin A1–13 in methadone-maintained versus control subjects. J Pharmacol Exp Ther. 2003;306:581–587. [DOI] [PubMed] [Google Scholar]
- 51. Zheng SX, Bosch MA, Ronnekleiv OK. μ-Opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol. 2005;487:332–344. [DOI] [PubMed] [Google Scholar]
- 52. Pimpinelli F, Parenti M, Guzzi F, Piva F, Hokfelt T, Maggi R. Presence of δ opioid receptors on a subset of hypothalamic gonadotropin releasing hormone (GnRH) neurons. Brain Res 2006;1070:15–23. [DOI] [PubMed] [Google Scholar]
- 53. Mostari P, Ieda N, Deura C, et al. Dynorphin-κ opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhen S, Gallo RV. The effect of blockade of kappa-opioid receptors in the medial basal hypothalamus and medial preoptic area on luteinizing hormone release during midpregnancy in the rat. Endocrinology. 1992;131:1650–1656. [DOI] [PubMed] [Google Scholar]
- 55. Smith MJ, Gallo RV. The effect of blockade of κ-opioid receptors in the medial preoptic area on the luteinizing hormone surge in the proestrous rat. Brain Res. 1997;768:111–119. [DOI] [PubMed] [Google Scholar]