Abstract
Human endometrial stromal decidualization is required for embryo receptivity, angiogenesis, and placentation. Previous studies from our laboratories established that connexin (Cx)-43 critically regulates endometrial stromal cell (ESC) differentiation, whereas gap junction blockade prevents it. The current study evaluated the plasticity of ESC morphology and Cx43 expression, as well as other biochemical markers of cell differentiation, in response to decidualizing hormones. Primary human ESC cultures were exposed to 10 nM estradiol, 100 nM progesterone, and 0.5 mM cAMP for up to 14 days, followed by hormone withdrawal for 14 days, mimicking a biphasic ovulatory cycle. Reversible differentiation was documented by characteristic changes in cell shape. Cx43 was reversibly up- and down-regulated after the estradiol, progesterone, and cAMP treatment and withdrawal, respectively, paralleled by fluctuations in prolactin, vascular endothelial growth factor, IL-11, and glycodelin secretion. Markers of mesenchymal-epithelial transition (MET), and its counterpart epithelial-mesenchymal transition, followed reciprocal patterns corresponding to the morphological changes. Incubation in the presence of 18α-glycyrrhetinic acid, an inhibitor of gap junctions, partially reversed the expression of decidualization and MET markers. In the absence of hormones, Cx43 overexpression promoted increases in vascular endothelial growth factor and IL-11 secretion, up-regulated MET markers, and reduced N-cadherin, an epithelial-mesenchymal transition marker. The combined results support the hypothesis that Cx43-containing gap junctions and endocrine factors cooperate to regulate selected biomarkers of stromal decidualization and MET and suggest roles for both phenomena in endometrial preparation for embryonic receptivity.
In women, several nonhuman primate species, the elephant shrew and fruit bats, the superficial uterine mucosa undergoes cyclic proliferation, differentiation, and if no pregnancy is conceived, shedding via menstruation. The evolutionary advantage of such a dynamic process of endometrial turnover in these species has been debated (reviewed by Martin [1]), but teleologically, this reproductive strategy is thought to create an optimal environment for successful invasive blastocyst implantation. The complex, phasic tissue transformation of the endometrium requires considerable cellular plasticity and incorporates newly discovered stem cells niches (2). The two dominant ovarian steroid hormones, estradiol (E2) and progesterone (P4), are the primary regulators of endometrial growth and differentiation; however, less well-defined protein and eicosanoid ligands, among them relaxin and prostaglandin E2, also are critical for functional differentiation of the endometrium and establishment of embryo receptivity (3). This process is referred to as decidualization (4).
Prior work from our group and others reveals that the unique endocrinology of the luteal phase induces dramatic and predictable morphological and biochemical changes in the human endometrium. Epithelial, vascular, and immune cell components all are involved, but our laboratory has focused on transformation of the stroma. Endometrial stromal cells (ESCs) that appear bipolar and fibroblastic in the proliferative phase of the cycle accumulate glycogen and lipid, reorganize cytoskeletal actin microfilaments, expand their Golgi and rough endoplasmic reticulum, increase in volume, acquire euchromatic changes in the nucleus, and display a plump, polyhedral shape in the late secretory phase (5). These intracellular changes are accompanied by a shift from the secretion of a fibronectin-based extracellular matrix, to one rich in laminin and type IV collagen (6). Under electron microscopy, decidualized ESCs express interdigitating lamellar processes within clustered microdomains called gap junctions (7). The gap junction intercellular communications (GJIC) that form among adjacent ESCs allow them to directly share ions and small molecules less than 1 kDa in mass (8), which corresponds to a Stokes radius of 1.0–1.5 nm.
Biochemical evidence of differentiation is classically monitored by production and secretion of prolactin and IGF binding protein-1 (9) (10), and morphological transformation can be quantified microscopically using shape indices that detect cellular and nuclear rounding (11–13). Stromal cell decidualization can be authentically recapitulated in vitro by exposing cultured ESCs to a combination of hormones that typically includes E2, P4, and a cAMP analog (14). By contrast, antagonists of the estrogen receptor (15), progesterone receptor (15, 16), or the protein kinase A pathway (9) abrogate the decidualized phenotype.
Mesenchymal-epithelial transition (MET) and its more commonly encountered reciprocal process, epithelial-mesenchymal transition (EMT), are phylogenetically conserved mechanisms of embryonic development. Genetic cell fate mapping in murine embryos has revealed that the Müllerian tract derives from MET involving a rostral-to-caudal proliferation of cœlomic mesodermal cells, following the path of the Wolffian duct toward the urogenital sinus. The cells that form this tube transiently express a combination of mesenchymal (eg, vimentin) and epithelial cell (eg, cytokeratin, E-cadherin) markers (17). Eventually the mature cell types of the uterus (luminal and glandular epithelium and their supporting endometrial stroma, myometrium, and vasculature) are established. Recent studies from the oncology literature indicate that reversible MET programs are manifested during tumor metastasis (18), wherein disseminated invasive tumor cells revert to an epithelial phenotype and regain rapid proliferative capacity at the metastatic site (19). Cancers also have been observed to pass through a transient or incomplete MET program, with tumor cells concomitantly expressing both mesenchymal and epithelial markers (20).
Given the remarkable plasticity of ESCs, the goals of the current studies were to validate endometrial epithelial and stromal biomarkers in situ and test the reversibility of biochemical and morphological differentiation of ESCs after hormone stimulation and withdrawal. We developed a simplified in vitro model system that approximates the secretory phase, wherein estrogen and progesterone action are manifested for up to 14 days and, in the absence of pregnancy, both hormones decline for at least 7 days. We observed that many of the phenotypic characteristics of decidualized ESCs were reminiscent of MET-like changes. The temporal and functional effects of connexin (Cx)-43 gap junctions as mediators of ESC decidualization were assessed as secondary outcomes. Our experimental findings demonstrate that human endometrial decidualization, which is critically required to sustain placentation, appears to follow a fundamental developmental MET program shared with organogenesis and tumorigenesis, wherein endocrine signals and gap junctions interact to dynamically modulate plastic, reversible ESC phenotypes.
Materials and Methods
Source of human tissues
Healthy, parous, ovulatory women, who had not received hormonal therapy for at least 3 months before surgery, were recruited. Endometrial tissue specimens were obtained from five subjects without evidence of endometriosis or other pelvic pathology, undergoing laparoscopy for tubal sterilization after providing written informed consent under a study protocol approved by the institutional review board at Wake Forest School of Medicine. Endometrial biopsies were collected under sterile conditions and transported to the laboratory on ice in DMEM/Ham's F-12 (catalog number 10-092cv; CellGro) with 10% fetal bovine serum. All endometrial biopsies were performed in the midproliferative cycle phase to avoid effects of endogenous progesterone. Histological staging (21) was consistent with the subjects' menstrual dates.
Human endometrial cell culture, hormone treatment, and in vitro decidualization
Human ESC cultures were prepared from endometrial biopsies as described previously (22, 23). After collagenase digestion, ESCs were separated from debris and glandular epithelial cells by filtration through 200-μm and 40-μm sieves, respectively. ESCs were subcultured at least twice to eliminate contamination by macrophages or other leukocytes and plated directly onto 12-well or 6-cm polystyrene dishes without exogenous extracellular matrix (ECM). The ESCs were used before the sixth passage in all experiments to avoid cellular dedifferentiation. In prior studies, our laboratory extensively characterized ESC cultures prepared using this protocol and confirmed that they are greater than 93% pure, retain functional estrogen (∼28 000/cell) and P4 receptors (∼35 000/cell), and express other phenotypic endometrial markers in vitro (22, 24). For immunocytochemistry experiments (see below), ESCs were cultured on glass Lab-Tek chamber slides (catalog number 177402; Thermo-Fisher Scientific) without ECM. Cells were grown to 60%–80% confluence in phenol red-free DMEM/Ham's F-12, supplemented with 5% charcoal-stripped fetal calf serum. To model the secretory phase of an endometrial cycle and induce decidualization, ESCs were treated with hormones (10 nM E2 + 100 nM P4 + 0.5 mM dibutyryl cAMP [E/P/c]) for up to 14 days; media containing the hormones were refreshed every 3 days. This hormone regimen has been extensively characterized (23, 25, 26) and yields reproducible differentiation effects in cells derived from individual human subjects. Although synthetic progestins (eg, medroxyprogesterone acetate [MPA]) are metabolized less rapidly in vitro, they possess androgenic and glucocorticoid activity in target cells (27), including ESCs (Yu, J., D.C. Leitman, and R.N. Taylor, unpublished observations), both of which can influence decidualization endpoints (28, 29). Replenishment of P4 every 3 days allows the maintenance of the differentiated phenotype. Subsequently, to mimic the endocrine withdrawal that occurs in the absence of pregnancy, the cells were switched to hormone-free media, allowing the decidual hormones to be withdrawn for up to 14 days to represent a simplified biphasic cycle. The morphology of the cells was assessed longitudinally by phase-contrast microscopy using a modified calculation of cell roundness (11–13), and cell supernatants, protein lysates, and RNAs were sampled from the cultures as indicated.
Enzyme-linked immunosorbent assay
Highly sensitive and specific commercial sandwich ELISA kits were used to quantify prolactin, a lactogenic hormone (Alpha Diagnostic International), vascular endothelial growth factor (VEGF), the potent angiogenic factor (R&D Biosystems), and IL-11, an immunomodulatory cytokine involved in trophoblast invasion (R&D Biosystems). As we and others have established previously, these assays are precise and linear in ESC culture supernatants (25, 30) and are established markers of decidualization (9, 10).
Immunohistochemistry (IHC)
To validate the in situ localization of the protein biomarkers analyzed in cultured ESCs (see below), paraffin-embedded, proliferative-phase, endometrial sections were subjected to IHC as described previously (23). Briefly, after mounting 5-μm microtomed sections, the slides were deparaffinized in xylene and rehydrated in graded concentrations of ethanol. Antigen retrieval was performed by heating the slides in 10 mM sodium citrate (pH 6.0) at 100°C for 4 minutes. They were then exposed for 10 minutes to 3% hydrogen peroxide in methanol to quench endogenous hydrogen peroxide activity. Each sample was then rinsed in water. Nonspecific binding was blocked with Super Block (catalog number AAA-500; ScyTek Laboratories) overnight at 4°C. Sections were incubated with primary antibodies from Cell Signaling Technology as follows: polyclonal rabbit anti-Cx43 (catalog number 3512) at 1:100 dilution; monoclonal rabbit anti-β-catenin (catalog number 8480) at 1:100 dilution; monoclonal rabbit anti-E-cadherin (catalog number 3195) at 1:400 dilution; pan-keratin (catalog number 4545) at 1:400 dilution; monoclonal rabbit anti-N-cadherin (catalog number 13 116) at 1:125 dilution; monoclonal mouse antifibronectin (catalog number ab2413; Abcam) at 1:250–500 dilution; and monoclonal mouse anti-CD10 (catalog number M7308; Dako) at 1:50–100. The samples were incubated at room temperature for 1 hour. Staining was performed using EnVision Plus Systems (Dako) reagents, according to the manufacturer's protocol.
Western blot analyses
Western blots were performed on whole-cell extracts obtained by vortexing ESCs in cell extraction buffer (catalog number FNN0011; Life Technologies), followed by protein determination using the Thermo Scientific-Pierce BCA protein assay kit (catalog number PI-23227; Thermo-Fisher Scientific). Total protein (60 μg) was loaded onto NuPAGE Novex 4%–12% Bis-Tris protein gels and then transferred to polyvinyl difluoride membranes and blocked with 5% skim milk in PBS. Specific proteins were detected using polyclonal or monoclonal rabbit antibodies to the following human proteins: Cx43 (GJA1, 1:1000 dilution, catalog number 3512; Cell Signaling Technology); glycodelin-A (PAEP; 1:1000 dilution, catalog number ab103264; Abcam); CD10 (1:5000 dilution, catalog number ab79423; Abcam); fibronectin (1:1000 dilution, catalog number ab2413; Abcam). The following antibodies were supplied in the EMT antibody sampler kit: zonula occludens-1 (ZO1), β-catenin, N-cadherin, E-cadherin, vimentin (1:1000 dilution, catalog number 9782; Cell Signaling Technology); and discoidin domain receptor 2 (DDR2) (1:1000 dilution, catalog number 12133; Cell Signaling Technology). After overnight labeling with the primary antiserum, blots were then incubated with a secondary goat antirabbit or antimouse antibody (1:200000; catalog numbers 31460 and 31461; Pierce Biotechnology Inc) linked to horseradish peroxidase. The immunoreactive bands were visualized by the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech).
Blots were washed, reprobed with mouse monoclonal antihuman β-actin antibodies (1:1000 dilution, catalog number 31430; Sigma), and developed in an identical manner to ensure even loading. SeeBlue Plus2 prestained molecular weight standards (catalog number LC5925; Life Technologies), which are not detected by ECL, were used to calibrate the migration of proteins on the original gel and verify the identity of immunopositive bands. The size of each band is indicated in the figures. For quantification of proteins in the Western blots, the films were digitized on a flatbed scanner and Image J software (National Institutes of Health, Bethesda, Maryland) was used to integrate the density of each band. Data are presented as ratios of the MET or EMT markers normalized to β-actin band intensity.
Immunofluorescence and immunoperoxidase immunocytochemistry (ICC)
To corroborate and localize the expression of Cx43 and selected MET biomarkers observed in ESC lysates, the temporal patterns of Cx43, β-catenin (an epithelial marker), and CD10 (a stromal marker) were analyzed in intact ESCs grown under conditions of decidual hormone stimulation and withdrawal. ESCs were plated in Lab-Tek chamber sides until confluent and fixed in 4% paraformaldehyde, followed by ice-cold acetone and stained with primary antibodies whose sources, catalog numbers, and working dilutions are indicated in Table 1. Immunofluorescence ICC was performed in the Cellular Imaging Shared Resource of the Department of Pathology, Wake Forest School of Medicine, on a Zeiss confocal microscope. Immunoperoxidase ICC was performed as described previously (31).
Table 1.
Antibody Table: Antibodies for Western Blots, IHC, and ICC
| Name of Antibody | Manufacturer and Catalog Number | Species Raised, Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|
| E-cadherin | Cell Signaling Technology, 3195 | Rabbit, monoclonal | 1:1000 (W); 1:400 (IHC) |
| ZO1 | Cell Signaling Technology, 8193 | Rabbit, polyclonal | 1:1000 (W) |
| Pan-keratin | Cell Signaling Technology, 4545 | Mouse, monoclonal | 1:1000 (W); 1:500 (IHC) |
| β-Catenin | Cell Signaling Technology, 8480 | Rabbit, monoclonal | 1:1000 (W); 1:100 (IHC, ICC) |
| Glycodelin A (PAEP) | Abcam Inc, ab103264 | Rabbit, polyclonal | 1:1000 (W), 1 μg/mL |
| Cx43 | Cell Signaling Technology, 3512 | Rabbit, polyclonal | 1:1000 (W); 1:200 (IHC); 1:75 (ICC) |
| Connexin 32 | Invitrogen (Thermo Fisher Scientific), 71-0600 | Rabbit, polyclonal | 1:500 (W), 0.5 μg/mL |
| N-cadherin | Cell Signaling Technology, 13116 | Rabbit, monoclonal | 1:1000 (W); 1:125 (IHC) |
| Fibronectin | Abcam Inc, ab2413 | Rabbit, polyclonal | 1:5000 (W); 1:250 (IHC, ICC) |
| CD10 | Abcam Inc, ab79423 | Rabbit, monoclonal | 1:2500 (W), 0.2 μg/mL |
| CD10 | Dako, M7308 | Mouse, monoclonal | 1:50) (IHC) |
| β-Actin | Sigma-Aldrich, A4700 | Mouse, monoclonal | 1:1000 (W) |
| β-Actin | Sigma-Aldrich, A2066 | Rabbit, monoclonal | 1:1000 (W) |
| β-Actin | Cell Signaling Technology, 3700 | Mouse, monoclonal | 1:600 (ICC) |
| TCF8/ZEB1 | Cell Signaling Technology, 3396 | Rabbit, monoclonal | 1:1000 (W) |
| Vimentin | Cell Signaling Technology, 5741 | Rabbit, monoclonal | 1:1000 (W) |
| DDR2 | Cell Signaling Technology, 3396 | Rabbit, polyclonal | 1:1000 (W) |
| Antirabbit IgG (H + L) F(ab′)2 fragment | Cell Signaling Technology, 4412 | Alexa Fluor488 conjugate | 1:250 (ICC) |
| Antimouse IgG (H + L) F(ab′)2 fragment | Cell Signaling Technology, 4409 | Alexa Fluor555 conjugate | 1:250 (ICC) |
| Goat antimouse IgG (H + L) peroxidase-conjugated antibody | Thermo Scientific Pierce, 31430 | Goat | 1:30 000 (W) |
| Goat antirabbit IgG (H + L) peroxidase-conjugated antibody | Thermo Scientific Pierce, 31460 | Goat | 1:30 000 (W) |
Abbreviations: TCF8, transcription factor-like 8; W, Western (blots); ZEB1, zinc finger E-box binding homeobox 1.
Interruption and reversal of hormone-induced differentiation by gap junction blockade
These studies used 18α-glycyrrhetinic acid (AGA) to block gap junctions in ESCs. Although this compound has other reported biochemical effects, including the inhibition of 11β-hydroxysteroid dehydrogenase (32) and induction of peroxisomal proliferator-activated receptor-γ (33), our previous studies validated that 50 μM AGA blocked gap junction coupling by approximately 50% and prevented differentiation in E/P/c-treated ESCs. The cytological and biochemical effects of AGA in E/P/c-treated ESCs fully recapitulated the effects of gap junction inhibition using specific genetic ablation strategies with Cx43 small interfering RNA (34) and short hairpin RNA (25). Control cells received 3–7 days of decidualizing hormones in this protocol.
RNA isolation and real-time quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from cells using the TRIzol Plus RNA purification kit (Life Technologies) following the manufacturer's protocol. Total RNA concentrations were measured by the NANODROP 2000 method. cDNA was synthesized from mRNA samples and subsequently used as template for real-time quantitative PCR assays. For real-time quantitative PCR, we used the Bio-Rad SsoAdvanced SYBR Green supermix (catalog number 172-5261; Bio-Rad Laboratories) and followed the vendor guidelines with some modifications. A total reaction volume of 20 μL contained 10 μL SYBR, 1 μL primer mix, and 0.5 μL 50 mM MgCl2. For amplification of all the target genes, 5 μL cDNA were used, and 3 μL was used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; control). All the primers were ordered from QIAGEN QuantiTect Primer Assays, with the exception of GAPDH primers, which have been previously reported (23). The PCR was set for 40 cycles in a CFX Manager 3.1 real-time thermocycler (Bio-Rad Laboratories) under the following conditions: one denaturation cycle at 95°C for 5 minutes followed by 32 amplification cycles at 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 40 seconds. The data were analyzed after normalization with GAPDH mRNA levels, using the formula 2ΔΔct, where ct is the cycle threshold.
Transfection and overexpression of recombinant Cx43 in ESCs
To determine whether the correlative findings of the preceding descriptive studies in fact reflected a Cx43-mediated mechanism, we genetically modified ESC cultures by introducing a plasmid that allowed precise overexpression of recombinant Cx43 or Cx36 proteins (GJA1, catalog number SC125548 and GJD2, catalog number SC122104, respectively; Origene) and an empty vector control according to the manufacturer's protocol. After 48 hours, prolactin, VEGF, and IL-11 were quantified in the spent cell culture media by an ELISA, and E-cadherin, glycodelin-A (GdA), ZO1, and N-cadherin were assessed by Western blot.
Statistical analysis
All of the data presented are representative examples of assays replicated in a minimum of three independent ESC isolates. The data were normally distributed (Kolmogorov-Smirnov test) and are expressed as means ± SEM. Significant differences between individual groups were accepted when Student's t tests or ANOVA (two tailed analyses) with Scheffé's post hoc tests yielded P < .05.
Results
Reversible changes in ESC morphology are induced by decidual hormone stimulation and withdrawal
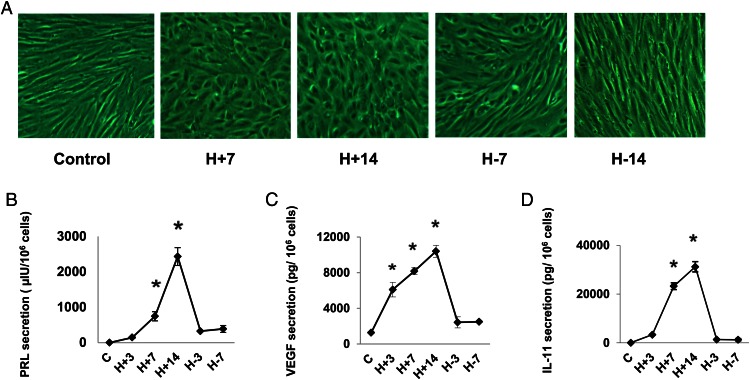
Freshly isolated human ESCs prepared from independent proliferative phase biopsies of five normal subjects were treated with hormones (10 nM E2 + 100 nM P4 + 0.5 mM dibutyryl cAMP [E/P/c]) for up to 14 days; media containing the hormones were refreshed every 3 days. Subsequently, hormone-free media were substituted, allowing the decidual hormones to be withdrawn for up to 14 days, mimicking a biphasic ovulatory cycle. The ESCs derived from proliferative-phase biopsies show classical, bipolar fibroblastic morphology when grown under standard culture conditions in the absence of decidualizing hormones (Figure 1A, control). The same cells exposed to hormones (E/P/c) demonstrated characteristic rounding by 7 days (Figure 1A, H+7) and showed pronounced epithelioid changes after 14 days of hormone exposure (Figure 1A, H+14). Within 7 days of E/P/c withdrawal, the ESCs had begun to show visibly reduced rounding (Figure 1A, H-7) and by 14 days of hormone withdrawal they had resumed their basal fibroblastic appearance (Figure 1A, H-14). Similar morphological transformation kinetics were noted in ESCs derived from the other four subjects. Morphometric analyses (11–13) demonstrated a significant and reversible change in cell shape index (1.00 = round) over the 28-day cycle of hormone stimulation and withdrawal. Shape index values varied from 0.17 ± 0.06 under basal conditions, increasing to 0.83 ± 0.04 after 14 days of hormone stimulation and reverting to 0.33 ± 0.12 within 7 days after hormone withdrawal (P < .05, ANOVA).
Figure 1.
A, Hormone-induced changes in ESC morphology. ESCs were cultured without added hormones (control) or with 10 nM E2, 100 nM P4, and 0.5 mM dibutyryl cAMP (E/P/c) for 7 days (H+7). ESCs incubated with E/P/c for 14 days showed maximal decidualization morphology (H+14). When ESCs were incubated for 14 days with E/P/c and then withdrawn from the hormones for 7 days, the cells became more fibroblastic in appearance (H-7). After 7 further days of hormone withdrawal, the cell shape approached that of controls (H-14). B–D, Hormone-induced changes in biomarker secretion (ELISA). ESCs were cultured for up to 14 days (H+14) with E/P/c as in panel A and then withdrawn from the decidualizing stimulus for up to 7 days (H-7). The secretion of prolactin (panel B), VEGF (panel C), and IL-11 (panel D), all determined by ELISA, had similar patterns. *, P < .05, ANOVA with Scheffé's post hoc tests (different from controls).
Reversible changes in classical and nonclassical decidualization biomarkers parallel morphological changes associated with hormone stimulation and withdrawal
Decidual transformation of ESCs in vitro is associated with differential secretion of hormones, growth factors, and cytokines. Decidual prolactin, a classical biomarker of ESC differentiation, was measured by an ELISA across the 14-day treatment period with E/P/c and during a 7-day withdrawal interval. Prolactin secretion peaked at day 14 and fell rapidly after hormone withdrawal (Figure 1B). Similar findings were observed in all five patients' samples. VEGF, a decidual angiogenic growth factor that we showed was required for implantation in the mouse (34), demonstrated a biphasic pattern of secretion similar to prolactin during hormone stimulation and withdrawal. Peak secretion was noted after 14 days of hormone treatment, and levels fell close to basal within 3 days of E/P/c withdrawal (Figure 1C). This result was replicated in three independent cultures. IL-11 is an immunomodulatory cytokine associated with ESC decidualization that regulates trophoblast invasion in the mouse and human (30, 35). The kinetics of IL-11 secretion also paralleled those of prolactin and VEGF, with peak levels detected on day 14, decreasing rapidly to control concentrations within 3 days of hormone withdrawal (Figure 1D). These findings also were replicated in three independent ESC cultures and indicate that both classical and nonclassical secretory markers of decidualization are reversibly regulated by ovarian steroids. It should be noted that hormone withdrawal did not induce cellular apoptosis (ie, no activation of caspase-3 or terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling staining), nor did cells slough from the culture dishes during the 14-day, hormone-free interval (data not shown).
Immunohistochemical confirmation of epithelial and stromal biomarkers in endometrial tissues in situ
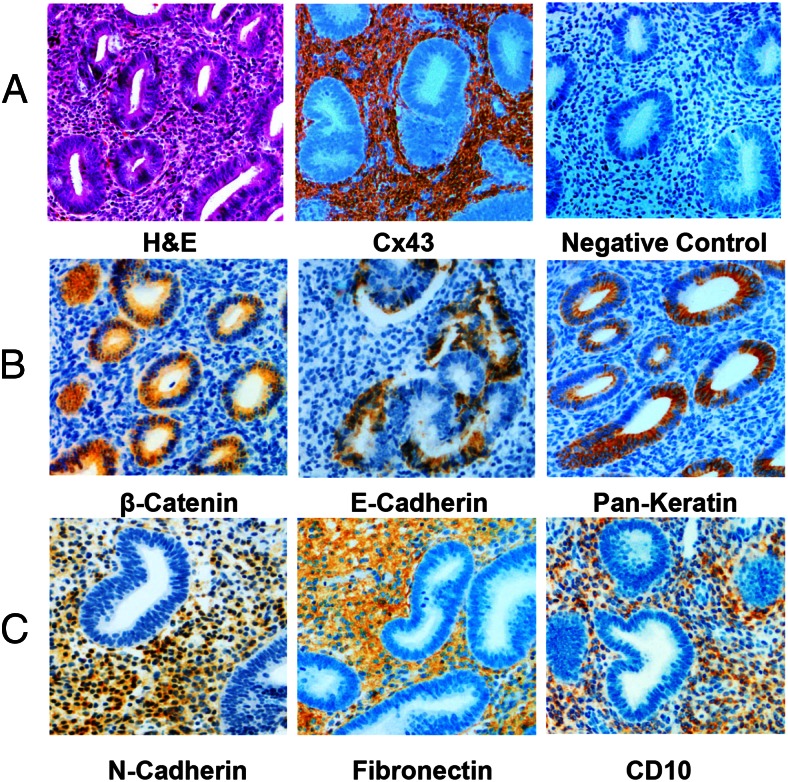
Undifferentiated ESCs derived from proliferative phase endometrial tissue can be hormonally induced to transition from mesenchymal to epithelioid decidual morphology. Our experiments indicated that in vitro cytological and biochemical markers are reversible and mirror endometrial cell characteristics in vivo. Thus, we performed a series of IHC experiments to validate expression of defined epithelial and stromal cell biomarkers in intact proliferative phase endometrium that confirm the in vivo situation can be mirrored in vitro. A hematoxylin and eosin-stained section is provided for orientation (Figure 2A). As we have reported before (23), specific Cx43 immunostaining is almost exclusively localized to the endometrial stroma, as shown in comparison with nonimmune rabbit serum as a negative control (Figure 2A). β-Catenin, E-cadherin, and pan-keratin are mostly confined to the epithelial glands (Figure 2B), whereas N-cadherin, fibronectin, and CD10 are exclusively localized in the stromal compartment (Figure 2C). These findings were replicated in three endometrial biopsies and corroborate reports in the literature (36, 37).
Figure 2.
A–C, Histology and IHC of proliferative-phase endometrium. Biopsies of proliferative human endometrium were collected as described and stained with hematoxylin and eosin (H&E), Cx43 antibodies or nonimmune rabbit serum as a negative control (panel A). Epithelial proteins were identified by antibodies against β-catenin, E-cadherin, and pan-keratin (panel B). Stromal proteins were localized using antibodies against N-cadherin, fibronectin, and CD10 (panel C).
Cx43 levels are phasically modulated during decidual hormone stimulation and withdrawal
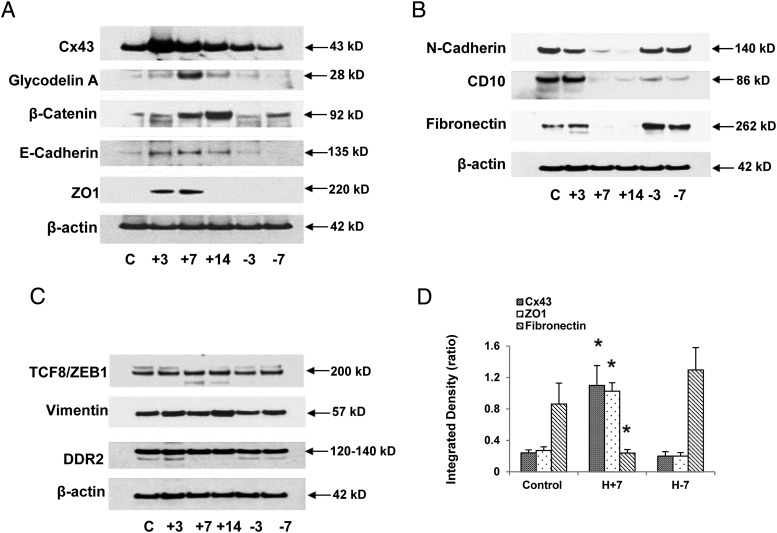
We previously observed that in vivo tissue concentrations of the gap junction protein Cx43 were 2-fold higher in secretory endometrial biopsies relative to those procured in the proliferative phase of the cycle and that in vitro decidualization of ESCs with E/P/c also afforded an approximately 2-fold increase (P < .02) in Cx43 levels (23). To assess the reversibility of the Cx43 response, we subjected ESCs to 14 days of hormone stimulation, followed by 7 days of hormone withdrawal and measured Cx43 in the cell lysates by Western blotting. As shown in Figure 3A (top panel), a biphasic response pattern was observed, with peak Cx43 levels noted 3–7 days after E/P/c exposure. Some interindividual variability was noted and this is described further (see below).
Figure 3.
A, Hormone-induced changes in epithelial protein expression (Western blots). ESCs were cultured for up to 14 days (+14) with E/P/c and then withdrawn from the decidualizing stimulus for up to 7 days (−7) as in Figure 1. The individual kinetics of several different proteins were assessed by Western blotting with specific antibodies to each. The molecular mass of each band is denoted at the right and the common protein names or abbreviations are indicated at the left. Expression of β-actin was constant across the time course. B, Hormone-induced changes in stromal protein expression (Western blots). ESCs were cultured for up to 14 days (+14) with E/P/c and hormone withdrawn for up to 7 days (−7) as in Figure 1. The molecular mass of each band is denoted at the right and the common protein names or abbreviations are indicated at the left. C, Some EMT biomarkers remain stable despite decidual hormone stimulation and withdrawal. ESCs were cultured for up to 14 days (+14) with E/P/c and hormone withdrawn for up to 7 days (−7) as in Figure 1. The molecular mass of each band is denoted at the right and the common protein names or abbreviations of EMT markers are indicated at the left. D, Quantitative changes in Cx43, ZO1, and fibronectin. Densitometry of Western blot bands from at least three biological replicates at each time point (control, no hormones; H+7, E/P/c stimulation for 7 d; H-7, E/P/c stimulation for 7 d followed by 7 d hormone withdrawal) was performed. The densitometry of each protein was normalized to the intensity of corresponding β-actin bands was calculated as an integrated density (ratio) and subjected to statistical analysis. *, P < .05, ANOVA with Scheffé's post hoc tests (different from control). C, control.
GdA is biphasically regulated in decidualized ESCs
GdA cannot be detected in proliferative endometrium, but within 6 days after ovulation, at the initiation of uterine receptivity to the blastocyst, its expression is up-regulated in the endometrial glands (38, 39) in which it is localized in pinopods (40). After the embryo implantation, GdA expression redistributes to decidualized stromal cells (41) and persists there until term (42). As expected, untreated ESCs had barely detectable GdA, but the expression of this glycoprotein increased maximally after 7 days of hormone stimulation and returned to baseline by 7 days after E/P/c withdrawal (Figure 3A, second panel).
Epithelial and stromal biomarkers reveal biphasic, but obverse, response profiles during decidual hormone stimulation and withdrawal
Based on our observation that decidualized ESCs manifest epithelioid changes, we evaluated a panel of validated epithelial biomarkers to directly test cell phenotype transition. Western blots using β-catenin, E-cadherin, and ZO1 antibodies all demonstrated a hormone-induced up-regulation in ESCs, followed by a fall in their concentrations after hormone withdrawal (Figure 3A, panels 3–5). β-Actin showed no endocrine responsiveness (Figure 3A, last panel). Each epithelial marker demonstrated slightly different kinetics in response to hormone manipulation.
In distinction to the epithelial markers above, the stromal biomarkers N-cadherin, CD10, and fibronectin showed an obverse pattern of expression, with nadirs after 7 and 14 days of E/P/c stimulation, and a rebound to baseline after hormone withdrawal (Figure 3B, panels 1–3). Again, β-actin showed no endocrine responsiveness (Figure 3B, panel 4). Three markers characterized in other cell types as indicators of mesenchymal transition, transcription factor-like 8/zinc finger E-box binding homeobox1, vimentin, and DDR2, failed to change dynamically during ESC decidualization and withdrawal, similar to β-actin, the constitutive control (Figure 3C). Also, neither Snail nor Slug were modulated in our experiments (data not shown), although a reduction in the former marker was noted by Zhang et al (43) using a similar decidualization model. Using densitometry to quantify Western blot band intensity normalized to β-actin, Figure 3D represents the means ± SEM of selected MET and EMT markers expressed in three biological replicates, analyzed under control, H+7 and H-7 conditions and time points, showing that Cx43 and ZO1 are significantly up-regulated, whereas fibronectin is significantly reduced at H+7 relative to control expression (ANOVA, P = .02 with Scheffé's post hoc tests, P < .05 for all three markers).
Immunofluorescence ICC shows changes in Cx43 expression and distribution with hormone stimulation and withdrawal
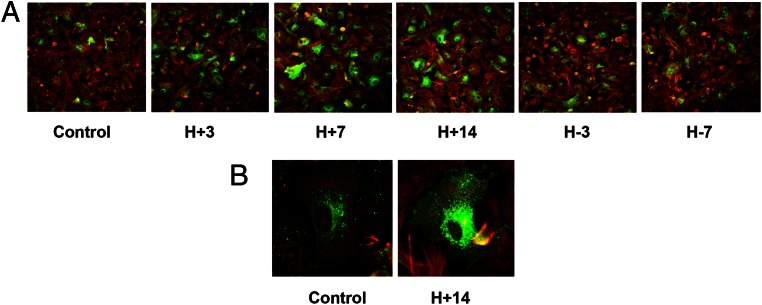
Immunofluorescence ICC of ESCs grown in chamber slides was performed. As shown in Figure 4 (panel A), the number of ESCs showing Cx43 immunopositivity (green fluorescence) increased with the duration of E/P/c treatment, peaking after 7–14 days of hormone exposure, and diminished with the duration of hormone withdrawal. The percentage of Cx43-positive cells was increased by hormone treatment (21% ± 3% in controls to 60% ± 8% at H+7 and back to 17% ± 3% at H-7 days, ANOVA, P = .02 with post hoc Scheffé's test, P < .05 for control vs H+7). Red fluorescence (Figure 4A) reflects β-actin immunostaining. Under higher magnification (Figure 4B), Cx43 appeared to be concentrated within the endoplasmic reticulum under control conditions, whereas after 14 days of E/P/c stimulation, expression extending throughout the plasma membranes was prominent.
Figure 4.
A and B, Immunofluorescence ICC confirms hormone-induced changes in Cx43 protein expression. Immunofluorescence ICC in ESC monolayers showed Cx43 up- and down-regulation after the E/P/c-induced decidualization (H+3, H+7, H+14) and withdrawal (H-3, H-7, panel A). Green fluorescence represents Cx43 and red fluorescence is β-actin. Panel B shows a high-magnification view (×480) demonstrating intracellular distribution of Cx43 (green) under control conditions and after 14 days of hormone stimulation.
ICC confirms dynamic shifts in Cx43 and MET biomarkers with hormone stimulation and withdrawal
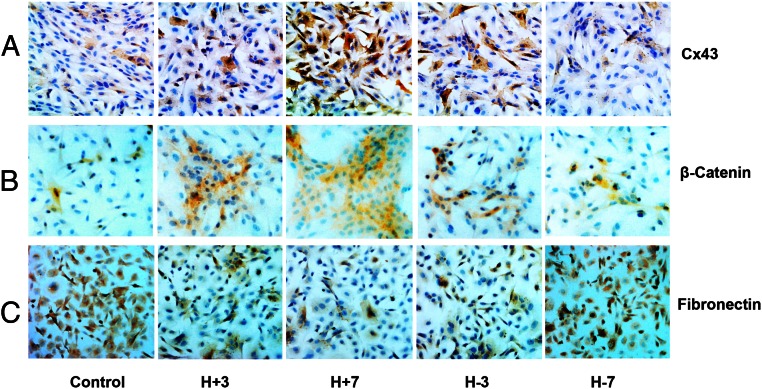
Immunoperoxidase ICC showed similar patterns of Cx43 (Figure 5A) and β-catenin (Figure 5B) after exposure to E/P/c. An opposite pattern of fibronectin staining in the cultures was noted, with an overall diminution of fibronectin-expressing cells in the hormone-differentiated state (H+7 d), with a subsequent rebound after hormone withdrawal (H-7 d) (Figure 5C).
Figure 5.
Immunoperoxidase ICC confirms hormone-induced changes in Cx43, MET, and EMT protein expression. Immunoperoxidase ICC in ESC monolayers showed Cx43 up- and down-regulation after E/P/c-induced decidualization (H+3, H+7) and withdrawal (H-3, H-7, panel A). Sequential up- and down-regulation of the epithelial marker β-catenin was associated with hormone exposure and withdrawal (panel B). Conversely, the stromal marker fibronectin showed concomitant down- and up-regulation, respectively (panel C). Magnification, ×200.
AGA rapidly reverses selected markers of decidualized phenotype and MET
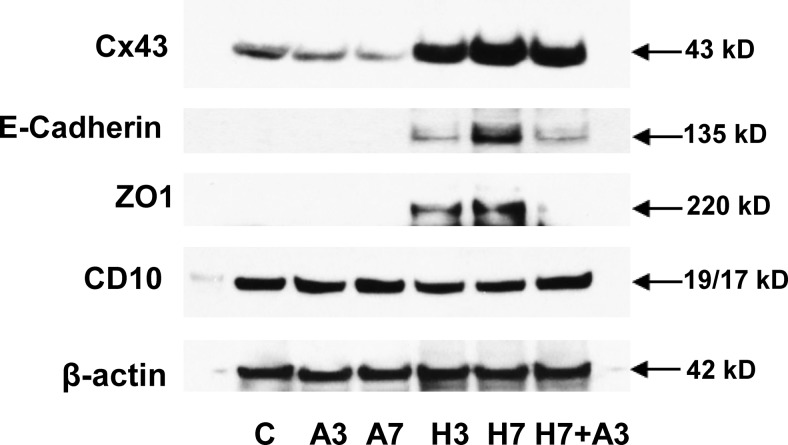
Given our current and prior findings that decidualization increased Cx43 expression and function (25) and based on the biphasic temporal induction and reversal of cell morphology, Cx43, prolactin, VEGF, and IL-11 protein production (Figures 1 and 3, respectively) and expression of MET markers (Figure 3), we postulated that Cx43 gap junctions might be direct mediators of differentiated ESC function. To test this hypothesis, we used the well-characterized gap junction blocker AGA under optimal dose and time conditions that we previously established (25). Incubation with 50 μM AGA time dependently suppressed basal Cx43 expression and addition of AGA for the last 3 days of 7 days of E/P/c treatment partially reversed the hormone-induced increase in Cx43 (Figure 6, top panel). Addition of the gap junction inhibitor for 3 days also reversed the decidualization-mediated up-regulation of E-cadherin and ZO1 (Figure 6, panels 2 and 3). Normalizing to β-actin signals showed that addition of AGA significantly reduced Cx43 by 43% ± 15%, E-cadherin by 62% ± 22% and ZO1 by 74% ± 21% (P < .05, t tests). As we reported previously, 50 μM AGA inhibited Cx43, prolactin, and VEGF secretion by 50%, 55%, and 50%, respectively (25, 26). Decidualization subtly suppressed CD10, with AGA partially reversing this inhibition, but these changes did not reach statistical significance (ANOVA, P = .28).
Figure 6.
Gap junction inhibition reverses decidualized phenotype and MET changes. Incubation in the presence of a GJIC inhibitor (50 μM 8α-AGA [A]) for 3 or 7 days resulted in a decrease in Cx43 under basal (A3, A7) conditions. E/P/c treatment for 3 or 7 days (H3, H7) increased Cx43, but the latter effect was reversed when AGA also was present from day 4 to day 7 (H7+A3). Similarly, the up-regulation of E-cadherin and ZO1 after 3 and 7 days of E/P/c was blocked when AGA also was present from day 4 to day 7 (H7+A3). CD10 was reduced subtly after 3 or 7 days of E/P/c, with some reversal when AGA also was present from day 4 to day 7 (H7+A3). Expression of β-actin was stable across time and treatments. C, control.
Endocrine modulation of decidualized ESC proteins and MET biomarkers appears to occur at the transcriptional or posttranscriptional levels
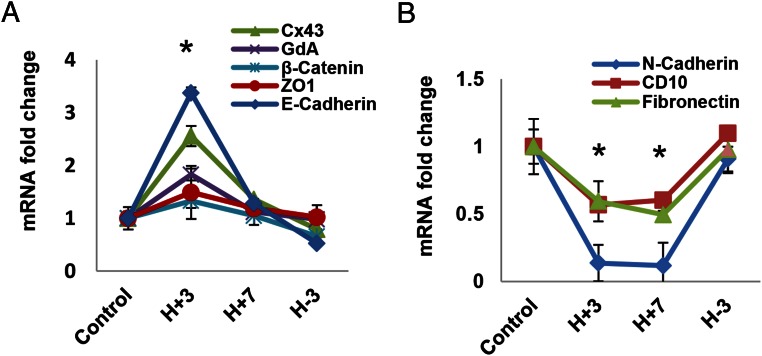
The data presented above indicate that E/P/c treatment increases the steady-state concentrations of Cx43, GdA, β-catenin, E-cadherin, and ZO1, whereas N-cadherin, CD10, and fibronectin levels all are reduced during decidualization. The changes were reversed after E/P/c withdrawal. To assess whether these changes also occur at the mRNA level, we performed real-time qRT-PCR with specific primers optimized for faithful transcript quantification. Our findings showed that the steady-state mRNA levels closely paralleled those of the proteins, although the magnitude of change in the former was more subtle, and as expected, pilot experiments indicated that the kinetics were more rapid. Figure 7 shows that Cx43, GdA, β-catenin, and E-cadherin mRNA levels all peaked 3 days after E/P/c treatment (H+3). These concentrations were all significantly higher than control levels (ANOVA, P < .01 with Scheffé's post hoc tests, P < .05) and returned to baseline by 3 days after hormone withdrawal (H-3). ZO1 levels had similar trends but were not statistically different (Figure 7A). The EMT stromal marker transcripts (N-cadherin, CD10, and fibronectin) were significantly reduced at 3 and 7 days after E/P/c treatment (H+3 and H+7) (ANOVA, P < .01 with Scheffé's post hoc tests, P < .05) and returned (increased) to baseline within 3 days of hormone withdrawal (H-3) (Figure 7B). Assessments of endocrine stimulation on the stability of these mRNA transcripts have not yet been performed.
Figure 7.
Hormone-induced changes in mRNA expression (qRT-PCR). ESCs were cultured for 3 or 7 days (H+3, H+7) with E/P/c and then withdrawn from the decidualizing stimulus for up to 3 days (H-3), similar to the conditions above but for shorter intervals, to examine kinetics of accumulation of mRNAs corresponding to the proteins in Figure 3. A, Cx43, GdA, β-catenin, and E-cadherin were all different from control at H+3 days. ZO1 showed a similar trend but did not meet significance. B, N-cadherin, CD10, and fibronectin all differed from control at H+3 and H+7 days. *, P < .05, ANOVA with Scheffé's post hoc test (different from control).
Cx43 protein overexpression directly regulates expression of MET marker and modulates VEGF and IL-11 secretion in ESCs in the absence of added hormones
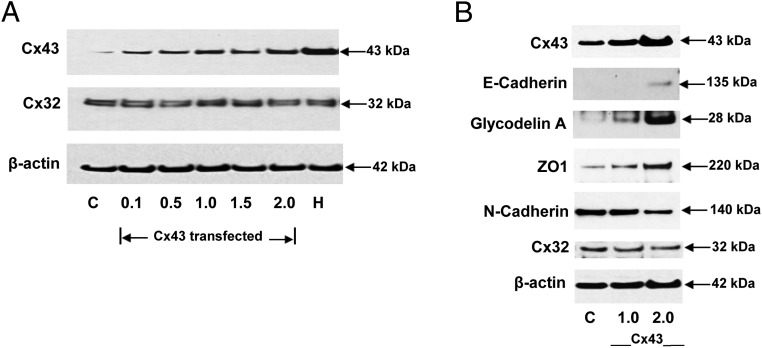
To extend our hypothesis that Cx43 gap junctions might directly mediate differentiated ESC function, even in the absence of decidualizing hormones, we overexpressed Cx43 mRNA in ESCs via transfection of a recombinant expression vector. Dose-response experiments showed progressive Cx43 protein accumulation, but no change in an unrelated connexin (Cx32) or β-actin protein accumulation was noted, nor did E/P/c treatment have any effect on Cx32 or β-actin expression (Figure 8A). As indicated by Western blotting, decidual hormone treatment was more effective than transgenic overexpression at increasing Cx43 levels. In addition to the expected increase in Cx43 protein itself, Cx43 overexpression was sufficient to increase the MET markers, E-cadherin (3.3- ± 0.2-fold), GdA (2.7- ± 0.1-fold) and ZO1 (3.8- ± 0.2-fold). By contrast, forced expression of Cx43 was sufficient to inhibit ESC production of the EMT indicator N-cadherin by 48% ± 2%. These effects were all statistically different from controls (t tests, P < .05) and appeared to be Cx43 plasmid dose dependent (Figure 8B).
Figure 8.
A, Transfection of Cx43 expression vectors dose responsively induces specific gap junction proteins. To optimize Cx43 transfection, dose-response experiments with increasing vector concentrations (0.1–2.0 μg per 6 cm dish) were performed. The EC50 was determined to be approximately 1.0 μg per 6-cm dish, which yielded less Cx43 protein accumulation than that achieved after 7 days of E/P/c treatment (H). Note that neither Cx43 overexpression nor E/P/c treatment had any effect on Cx32 or β-actin expression. B, Transfection of Cx43 expression vectors dose responsively increases MET markers and decreases N-cadherin in the absence of hormone treatment. Transfection of 1.0 and 2.0 μg per 6 cm dish Cx43 vectors resulted in increases in Cx43. Western blots of MET markers (E-cadherin, GdA, and ZO1) also showed increases. By contrast, the stromal marker N-cadherin was reduced when Cx43 was overexpressed. All experiments were performed in the absence of hormones. No effects on Cx32 or β-actin were observed. C, control.
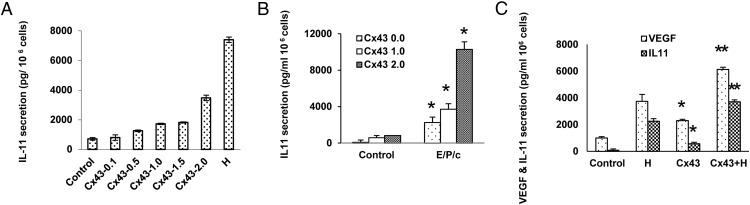
Although no effect on prolactin production was noted, dose-response effects of the transfected Cx43 vector showed an EC50 for VEGF (data not shown) and IL-11 secretion of 1.0 μg DNA per 6-cm dish; at that concentration approximately 25% of the full, decidual hormone-induced increase in IL-11 was achieved (Figure 9A). IL-11 secretion was more than additively stimulated in Cx43-overexpressing ESCs after the addition of E/P/c, suggesting that Cx43 overexpression enhanced sensitivity to decidual hormone stimulation (Figure 9B, P < .05, t tests compared with control). Under these experimental conditions, Cx43 overexpression induced a 2.3-fold increase in VEGF production and a 7.2-fold increase in IL-11 secretion in the absence of any exogenous decidualizing hormones (Figure 9C, P < .05, t tests compared with control). Control experiments, in which empty vectors or those encoding the related Cx36 protein replaced overexpressed Cx43, failed to alter VEGF or IL-11 secretion (data not shown), indicating the selective effects were Cx43 specific.
Figure 9.
A and B, Overexpression of Cx43 induces ESC up-regulation of IL-11 in the absence of hormone treatment. Transgenic expression of Cx43 dose responsively up-regulates IL-11 secretion, with 2.0 μg per 6-cm dish approaching approximately 50% the magnitude of secretion after 7 days of exposure to E/P/c (panel A). The combination of recombinant Cx43 and decidual hormones have a more than additive effect on IL-11 secretion (panel B). *, P < .05, t tests (different from corresponding controls). C, Overexpression of Cx43 up-regulates VEGF and IL-11 production in ESCs. Transfection of 1.0 μg Cx43 vector DNA per 6-cm dish increased VEGF secretion by 2.3-fold and IL-11 by 7.2-fold, but these responses were lower than those induced by E/P/c for 7 days (H), *, P < .05, t tests (different from control). Additive effects were noted when transfection was combined with E/P/c treatment (Cx43+H), **, P < .05, t tests (different from corresponding Cx43 transfection controls [Cx43]).
Discussion
Human endometrial stroma cells acquire epithelioid structure and function, accumulating glycogen, lipids, and subcellular organelles in response to decidualization (7) and secrete proteins that are characteristically epithelial, rather than mesenchymal, products. Prolactin, for example, although expressed at low levels by some lymphocytes, is predominantly synthesized by pituitary lactotrophs, epithelial cells of the adenohypophysis (44). In this example an alternate gene promoter 5.7 kb upstream of the pituitary promoter drives prolactin expression in nonpituitary cells (45). VEGF is highly expressed in villous cytotrophoblasts (46) and endometrial epithelium (47, 48), whereas IL-11 is abundant in nasal, bronchial, (49) and gastric epithelia (50). Under the influence of the same endocrine factors, ESCs also up-regulate connexin proteins and acquire new gap junctions, facilitating polarization and cellular coordination (25). Others established that Cx43 is predominantly a stromal protein in human endometrium (51), but Cx43 is highly expressed in human reproductive epithelia, including the seminiferous tubules (52) and trophoblast (53).
The rapid induction of Cx43 and its prompt return to baseline after hormone withdrawal (Figure 3A) are consistent with the 1-hour half-life of Cx43 (54) and support the hypothesis that gap junctions comprised of Cx43 subunits directly mediate ESC differentiation. This hypothesis was functionally tested by genetic (small interfering RNA and short hairpin RNA) and pharmacological abrogation of gap junctions (25, 34), which blocked hormone-induced differentiation and angiogenesis in vivo and in vitro. It should be noted that in the current experiments, hormone withdrawal alone did not induce apoptosis or sloughing of the ESCs from the culture dishes during the 14-day, hormone-free interval in these experiments, indicating that our decidualized cells are not in a terminally differentiated state but rather have reversible features. Similar findings were reported by Pohnke et al (55) in ESCs decidualized for 6 days with cAMP and withdrawn for 6 days. However, in alternate models, hormone withdrawal has been shown to induce ESC apoptosis and cell death. Labied et al (56) showed that when MPA was withdrawn from ESCs decidualized with cAMP and MPA for 3 days, activation of proapoptotic Bim expression and flow cytometric evidence of increased sub-G1 ploidy were observed. Also, using telomerase-immortalized ESCs grown in three-dimensional collagen I hydrogels, estrogen and progesterone withdrawal resulted in the activation of matrix metalloproteinase-2, collagenase, and demonstrable matrix degradation (12). We speculate that the extent of differentiation may dictate reversibility. For example, cells grown in three-dimensional matrices or decidualized with a more potent steroid (MPA) may achieve a terminally differentiated state and undergo apoptosis after hormone withdrawal.
The primary source of GdA is the endometrial epithelium, in which it is a major progesterone-regulated secretory product of human uterine glands and is expressed only in the secretory phase. Other tissues that express glycodelin include the fallopian tube, breast, seminal vesicle, ovary, and eccrine glands (57). GdA is not expressed in mid or late secretory endometrial stroma (58), but it is expressed in early pregnancy decidua in vivo (42). It is possible that this selective induction in decidual cells reflects contributions from the implanting conceptus, which may be mimicked by our decidualization cocktail in vitro (Figure 3A). GdA potently inhibits immune cell function, including natural killer cells (59), monocytes (60), and T cells (61), at concentrations present in endometrial tissue. In addition, data support GdA as a proangiogenic (62) and trophoblast stimulating (63) factor, which we postulate contributes to neovascularization and placental invasion at the site of embryo attachment. Other human endometrial epithelial proteins whose expression is redistributed to stroma during decidualization include the tight junction proteins β-catenin, E-cadherin, and ZO1 (Figure 3A). Curiously, the kinetics of their expression patterns differ subtly in response to hormone administration in vitro, even within the same subject (eg, ZO1 peaks at H+7, whereas β-catenin peaks at H+14 [Figure 3A]). These effects are likely related to differences in gene regulation and/or protein turnover and require further evaluation. In addition to MET markers previously noted to be up-regulated during secretory transformation in vivo (36, 64), a number of genes up-regulated during stromal decidualization (monoamine oxidase-b, clusterin, and the forkhead transcription factor Forkhead box O1) are expressed constitutively in endometrial epithelium (28).
Reorganization of the actin cytoskeleton and altered production of extracellular matrix proteins reduce the invasive properties of ESCs during decidualization. In primates, collagens III and VI (65) and fibronectin (66) predominate in proliferative-phase endometrial stroma, whereas collagen IV and laminin are the dominant ECM proteins deposited around individual decidualized stromal cells (67), replicating an epithelial pattern. Indeed, these ECM components are shared with basement membranes of corneal, mammary (68), and ovarian (69) epithelia. We observed that fibronectin is down-regulated during ESC decidualization (Figure 3B).
Three classic epithelial biomarkers, β-catenin, E-cadherin, and ZO1, were studied previously in cycling human endometrium (36, 37), in which their transcripts were reported to be highest during secretory transformation. Using a similar model of human ESCs decidualized with E2 and P4 for 5–8 days, Zhang et al (43) reported up-regulated E-cadherin and cytokeratin, and a concomitant reduction in N-cadherin, vimentin, and Snail. Earlier studies from our team established that β-catenin signaling was critical for ESC decidualization and was mediated via a pathway comprising bone morphogenetic protein 2, WNT4, β-catenin, Forkhead box O1, CCAAT/enhancer binding protein-β, and the P4 receptor, which converge to transcriptionally regulate genes associated with ESC differentiation, including prolactin and IGF binding protein-1 (70). Our current observations raise the interesting possibility that the cyclic monthly, secretory transformation of ESCs recapitulates the embryological MET program as suggested (43) but that this process is remarkably dynamic and reversible.
MET and its reciprocal counterpart, EMT, are well-established features of embryological reproductive tract development, allowing formation of the genital ridge after gastrulation (71). Genetic cell fate mapping in the mouse embryo indicates that the Müllerian tract derives from a rostral-to-caudal proliferation of cœlomic mesodermal cells that transiently express a combination of mesenchymal (vimentin) and epithelial cell (cytokeratin) markers (17). Eventually, through MET, the mature cell types of the uterus (luminal and glandular epithelium, surrounded by endometrial stroma and myometrium) are established. This cellular transformation appears to correspond to transient β-catenin expression along the Müllerian mesenchyme (72). Proteomic analyses of human endometrium in vivo revealed a doubling of keratin 8 and a 2-fold decrease in vimentin expression associated with decidualization (73).
Recent studies of tissue repair in the murine endometrium after parturition offer a further example of MET, in which subpopulations of endometrial stromal progenitor cells were found to contribute to endometrial epithelial healing (74, 75). In a mouse model of menstruation, Cousins et al (76) demonstrated that MET contributes to endometrial epithelium after P4 withdrawal-induced tissue breakdown. Their findings are consistent with our observations that luteal hormone concentration and/or duration thresholds confer plasticity and reversibility of endometrial stromal phenotype via MET. Epigenetic mechanisms have been proposed recently to promote the expression of epithelial genes during MET and may provide clues to the ultimate regulation of this phenomenon (77).
In summary, our study supports the hypothesis that under the conditions of these experiments, decidualized human ESCs are not terminally differentiated but in fact demonstrate reversible plasticity of morphology and function in response to decidual endocrine stimulation and withdrawal. Transformation of fibroblastic proliferative phase ESCs to plump, decidualized cells follows characteristics of the MET program. By contrast, reversion to the stromal fibroblast phenotype after hormone withdrawal tends to recapitulate the EMT process. Based on our current and prior studies, Cx43-containing ESC gap junctions appear to exert a central role in the permissiveness of endometrial stromal differentiation and, by extension, successful embryonic implantation and pregnancy outcome. Genetic or pharmacological disruption of Cx43 gap junction communication inhibits human ESC decidualization (25) and can lead to apoptosis (26). Conditional gene deletion of Cx43 in the mouse uterus prevents implantation (34). These data indicated that Cx43 was necessary for successful endometrial differentiation.
In the current experiments, we demonstrate that overexpression of Cx43 in ESCs is sufficient for the up-regulation of MET markers and VEGF and IL-11 secretion in the absence of exogenous E/P/c. Whereas the magnitude of changes in these markers is less robust than that observed after hormone-induced differentiation, we note that even at the highest plasmid concentration (2.0 μg DNA per 6 cm dish) the transgenic Cx43 levels achieved are only approximately 50% of the full, decidual hormone-induced increase in Cx43 (Figure 8A). Moreover, overexpression of Cx43 was sufficient to up-regulate the MET markers E-cadherin, GdA, and ZO1, even in the absence of hormones, whereas it was sufficient to inhibit the EMT indicator N-cadherin (Figure 8B). We conclude that Cx43 gap junctions are critical mediators of reversible endometrial differentiation and may represent a precise target for fertility disorders, including endometriosis and recurrent miscarriage that are associated with implantation failure and subfecundity. Efforts to pharmacologically modulate Cx43 expression, for example, via retinoic acid supplementation (78, 79), provide clues to potential clinical interventions for women with infertility related to GJIC.
Acknowledgments
We thank Kenneth W. Grant, PhD (Manager of the Cellular Imaging Shared Resource, Department of Pathology, Wake Forest School of Medicine) for his help with immunofluorescence immunocytochemistry and photography and the patients who generously contributed specimens for this study.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Cooperative Agreements U54 HD55787 (Specialized Cooperative Centers Program in Reproduction and Infertility Research, to I.C.B., M.K.B., and R.N.T.) and U01 HD66439 (Cooperative Research Partnerships to Promote Workforce Diversity in the Reproductive Sciences, to J.Y., N.S., and R.N.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGA
- 18α-glycyrrhetinic acid
- Cx
- connexin
- DDR2
- discoidin domain receptor 2
- E2
- estradiol
- ECL
- enhanced chemiluminescence
- ECM
- extracellular matrix
- EMT
- epithelial-mesenchymal transition
- E/P/c
- E2 + P4 + dibutyryl cAMP
- ESC
- endometrial stromal cell
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GJIC
- gap junction intercellular communications
- ICC
- immunocytochemistry
- IHC
- immunohistochemistry
- MET
- mesenchymal-epithelial transition
- MPA
- medroxyprogesterone acetate
- P4
- progesterone
- qRT-PCR
- quantitative RT-PCR
- VEGF
- vascular endothelial growth factor
- ZO1
- zonula occludens-1.
References
- 1. Martin RD. The evolution of human reproduction: a primatological perspective. Am J Phys Anthropol Suppl. 2007;45:59–84. [DOI] [PubMed] [Google Scholar]
- 2. Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. [DOI] [PubMed] [Google Scholar]
- 3. Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–241. [DOI] [PubMed] [Google Scholar]
- 4. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. [DOI] [PubMed] [Google Scholar]
- 6. Aplin JD, Charlton AK, Ayad S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988;253:231–240. [DOI] [PubMed] [Google Scholar]
- 7. Lawn AM, Wilson EW, Finn CA. The ultrastructure of human decidual and predecidual cells. J Reprod Fertil. 1971;26:85–90. [DOI] [PubMed] [Google Scholar]
- 8. Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151–159. [DOI] [PubMed] [Google Scholar]
- 9. Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–307. [DOI] [PubMed] [Google Scholar]
- 10. Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann NY Acad Sci. 1994;734:7–18. [DOI] [PubMed] [Google Scholar]
- 11. Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. [DOI] [PubMed] [Google Scholar]
- 12. Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nikoo S, Ebtekar M, Jeddi-Tehrani M, et al. Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol Hum Reprod. 2014;20:905–918. [DOI] [PubMed] [Google Scholar]
- 14. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. [DOI] [PubMed] [Google Scholar]
- 15. Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–873. [DOI] [PubMed] [Google Scholar]
- 16. Wang JD, Zhu JB, Shi WL, Zhu PD. Immunocytochemical colocalization of progesterone receptor and prolactin in individual stromal cells of human decidua. J Clin Endocrinol Metab. 1994;79:293–297. [DOI] [PubMed] [Google Scholar]
- 17. Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. [DOI] [PubMed] [Google Scholar]
- 19. Brabletz T, Hlubek F, Spaderna S, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. [DOI] [PubMed] [Google Scholar]
- 20. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. [DOI] [PubMed] [Google Scholar]
- 21. Noyes RW, Hertig A, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- 22. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. [DOI] [PubMed] [Google Scholar]
- 23. Yu J, Boicea A, Barrett KL, et al. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod. 2014;20:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tseng JF, Ryan IP, Milam TD, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–1122. [DOI] [PubMed] [Google Scholar]
- 25. Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol. 2011;344:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu J, Berga SL, Zou W, et al. Gap junction blockade induces apoptosis in human endometrial stromal cells. Mol Reprod Dev. 2014;81:666–675. [DOI] [PubMed] [Google Scholar]
- 27. Poulin R, Baker D, Poirier D, Labrie F. Androgen and glucocorticoid receptor-mediated inhibition of cell proliferation by medroxyprogesterone acetate in ZR-75–1 human breast cancer cells. Breast Cancer Res Treat. 1989;13:161–172. [DOI] [PubMed] [Google Scholar]
- 28. Cloke B, Fusi L, Brosens JJ. Decidualization. In: Pijnenborg R, Brosens I, Romero R, eds. Placental Bed Vascular Disorders—Basic Science and Its Translation to Obstetrics. Cambridge (UK): Cambridge Press; 2010:29–40. [Google Scholar]
- 29. Whirledge SD, Oakley RH, Myers PH, Lydon JP, DeMayo F, Cidlowski JA. Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc Natl Acad Sci USA. 2015;112:15166–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. [DOI] [PubMed] [Google Scholar]
- 31. Tee MK, Vigne JL, Yu J, Taylor RN. Natural and recombinant human glycodelin activate a proapoptotic gene cascade in monocyte cells. J Leukoc Biol. 2008;83:843–852. [DOI] [PubMed] [Google Scholar]
- 32. Shimojo M, Stewart PM. Apparent mineralocorticoid excess syndromes. J Endocrinol Invest. 1995;18:518–532. [DOI] [PubMed] [Google Scholar]
- 33. Zong L, Qu Y, Xu MY, Dong YW, Lu LG. 18α-glycyrrhetinic acid extracted from Glycyrrhiza radix inhibits proliferation and promotes apoptosis of the hepatic stellate cell line. J Dig Dis. 2013;14:328–336. [DOI] [PubMed] [Google Scholar]
- 34. Laws MJ, Taylor RN, Sidell N, et al. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. [DOI] [PubMed] [Google Scholar]
- 36. Buck VU, Windoffer R, Leube RE, Classen-Linke I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem Cell Biol. 2012;137:777–790. [DOI] [PubMed] [Google Scholar]
- 37. Fujimoto J, Ichigo S, Hori M, Tamaya T. Alteration of E-cadherin, α- and β-catenin mRNA expression in human uterine endometrium during the menstrual cycle. Gynecol Endocrinol. 1996;10:187–191. [DOI] [PubMed] [Google Scholar]
- 38. Li TC, Dalton C, Hunjan KS, Warren MA, Bolton AE. The correlation of placental protein 14 concentrations in uterine flushing and endometrial morphology in the peri-implantation period. Hum Reprod. 1993;8:1923–1927. [DOI] [PubMed] [Google Scholar]
- 39. Taylor RN, Vigne JL, Zhang P, Hoang P, Lebovic DI, Mueller MD. Effects of progestins and relaxin on glycodelin gene expression in human endometrial cells. Am J Obstet Gynecol. 2000;182:841–847. [DOI] [PubMed] [Google Scholar]
- 40. Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, Seppala M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril. 2006;85:1803–1811. [DOI] [PubMed] [Google Scholar]
- 41. Jeschke U, Kuhn C, Mylonas I, et al. Development and characterization of monoclonal antibodies for the immunohistochemical detection of glycodelin A in decidual, endometrial and gynaecological tumour tissues. Histopathology. 2006;48:394–406. [DOI] [PubMed] [Google Scholar]
- 42. Jeschke U, Kunert-Keil C, Mylonas I, et al. Expression of glycodelin A in decidual tissue of preeclamptic, HELLP and intrauterine growth-restricted pregnancies. Virchows Arch. 2005;446:360–368. [DOI] [PubMed] [Google Scholar]
- 43. Zhang XH, Liang X, Liang XH, et al. The mesenchymal-epithelial transition during in vitro decidualization. Reprod Sci. 2013;20:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Molitch M. Prolactin in human reproduction. In: Strauss JF, III, Barbieri RL, eds. Yen and Jaffe's Reproductive Endocrinology. Philadelphia: Saunders-Elsevier; 2013:45–50. [Google Scholar]
- 45. Gellersen B, Kempf R, Telgmann R, DiMattia GE. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol. 1994;8:356–373. [DOI] [PubMed] [Google Scholar]
- 46. Demir R, Kayisli UA, Seval Y, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25:560–572. [DOI] [PubMed] [Google Scholar]
- 47. Shifren JL, Tseng JF, Zaloudek CJ, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–3118. [DOI] [PubMed] [Google Scholar]
- 48. Charnock-Jones DS, Sharkey AM, Rajput-Williams J, et al. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. [DOI] [PubMed] [Google Scholar]
- 49. Elias JA, Zheng T, Einarsson O, Landry M, Trow T, Rebert N, Panuska J. Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J Biol Chem. 1994;269:22261–22268. [PubMed] [Google Scholar]
- 50. Chivu EM, Necula LG, Dragu D, et al. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453–1464. [PubMed] [Google Scholar]
- 51. Jahn E, Classen-Linke I, Kusche M, et al. Expression of gap junction connexins in the human endometrium throughout the menstrual cycle. Hum Reprod. 1995;10:2666–2670. [DOI] [PubMed] [Google Scholar]
- 52. Steger K, Tetens F, Bergmann M. Expression of connexin 43 in human testis. Histochem Cell Biol. 1999;112:215–220. [DOI] [PubMed] [Google Scholar]
- 53. Cronier L, Defamie N, Dupays L, et al. Connexin expression and gap junctional intercellular communication in human first trimester trophoblast. Mol Hum Reprod. 2002;8:1005–1013. [DOI] [PubMed] [Google Scholar]
- 54. Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pohnke Y, Schneider-Merck T, Fahnenstich J, et al. Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:5233–5244. [DOI] [PubMed] [Google Scholar]
- 56. Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. [DOI] [PubMed] [Google Scholar]
- 57. Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23:401–430. [DOI] [PubMed] [Google Scholar]
- 58. Mueller MD, Vigne JL, Vaisse C, Taylor RN. Glycodelin: a pane in the implantation window. Semin Reprod Med. 2000;18:289–298. [DOI] [PubMed] [Google Scholar]
- 59. Okamoto N, Uchida A, Takakura K, et al. Suppression by human placental protein 14 of natural killer cell activity. Am J Reprod Immunol. 1991;26:137–142. [DOI] [PubMed] [Google Scholar]
- 60. Vigne JL, Hornung D, Mueller MD, Taylor RN. Purification and characterization of an immunomodulatory endometrial protein, glycodelin. J Biol Chem. 2001;276:17101–17105. [DOI] [PubMed] [Google Scholar]
- 61. Rachmilewitz J, Riely GJ, Tykocinski ML. Placental protein 14 functions as a direct T-cell inhibitor. Cell Immunol. 1999;191:26–33. [DOI] [PubMed] [Google Scholar]
- 62. Park JK, Song M, Dominguez CE, et al. Glycodelin mediates the increase in vascular endothelial growth factor in response to oxidative stress in the endometrium. Am J Obstet Gynecol. 2006;195:1772–1777. [DOI] [PubMed] [Google Scholar]
- 63. Lee CL, Lam KK, Koistinen H, et al. Glycodelin-A as a paracrine regulator in early pregnancy. J Reprod Immunol. 2011;90:29–34. [DOI] [PubMed] [Google Scholar]
- 64. MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF., III Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn. 1996;206:201–211. [DOI] [PubMed] [Google Scholar]
- 65. Jussila T, Kauppila S, Bode M, et al. Synthesis and maturation of type I and type III collagens in endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol. 2004;115:66–74. [DOI] [PubMed] [Google Scholar]
- 66. Fazleabas AT, Bell SC, Fleming S, Sun J, Lessey BA. Distribution of integrins and the extracellular matrix proteins in the baboon endometrium during the menstrual cycle and early pregnancy. Biol Reprod. 1997;56:348–356. [DOI] [PubMed] [Google Scholar]
- 67. Iwahashi M, Muragaki Y, Ooshima A, Yamoto M, Nakano R. Alterations in distribution and composition of the extracellular matrix during decidualization of the human endometrium. J Reprod Fertil. 1996;108:147–155. [DOI] [PubMed] [Google Scholar]
- 68. Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. [DOI] [PubMed] [Google Scholar]
- 69. Auersperg N, Maines-Bandiera SL, Dyck HG, Kruk PA. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest. 1994;71:510–518. [PubMed] [Google Scholar]
- 70. Li Q, Kannan A, Das A, et al. WNT4 acts downstream of BMP2 and functions via β-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. MacLaughlin DT, Teixeira J, Donahoe PK. Perspective: reproductive tract development—new discoveries and future directions. Endocrinology. 2001;142:2167–2172. [DOI] [PubMed] [Google Scholar]
- 72. Stewart CA, Wang Y, Bonilla-Claudio M, et al. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Mullerian duct and postnatal uterine development. Mol Endocrinol. 2013;27:1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rai P, Kota V, Sundaram CS, Deendayal M, Shivaji S. Proteome of human endometrium: identification of differentially expressed proteins in proliferative and secretory phase endometrium. Proteomics Clin Appl. 2010;4:48–59. [DOI] [PubMed] [Google Scholar]
- 74. Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22:964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9:e86378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bedi U, Mishra VK, Wasilewski D, Scheel C, Johnsen SA. Epigenetic plasticity: a central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget. 2014;5:2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu J, Taylor RN, Sidell N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J Cell Physiol. 2013;228:903–910. [DOI] [PubMed] [Google Scholar]
- 79. Taylor RN, Kane MA, Sidell N. Pathogenesis of endometriosis: roles of retinoids and inflammatory pathways. Semin Reprod Med. 2015;33:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]