Abstract
Insulin resistance plays a major role in the development and progression of cardiac hypertrophy and heart failure. Heart failure in turn promotes insulin resistance and increases the risk for diabetes. The vicious cycle determines significant mortality in patients with heart failure and diabetes. However, the underlying mechanisms for the vicious cycle are not fully elucidated. Here we show that circulating levels and adipose expression of retinol-binding protein 4 (RBP4), an adipokine that contributes to systemic insulin resistance, were elevated in cardiac hypertrophy induced by transverse aortic constriction and angiotensin-II (Ang-II) infusion. Ang-II increased RBP4 expression in adipocytes, which was abolished by losartan, an Ang-II receptor blocker. The elevated RBP4 in cardiac hypertrophy may have pathophysiological consequences because RBP4 increased cell size, enhanced protein synthesis, and elevated the expression of hypertrophic markers including Anp, Bnp, and Myh7 in primary cardiomyocytes. Mechanistically, RBP4 induced the expression and activity of toll-like receptor 4 (TLR4) and myeloid differentiation primary response gene 88 (MyD88) in cardiomyocytes, resulting in enhanced inflammation and reactive oxygen species production. Inhibition or knockdown of the TLR4/MyD88 pathway attenuated inflammatory and hypertrophic responses to RBP4 stimulation. Importantly, RBP4 also reduced the expression of glucose transporter-4 and impaired insulin-stimulated glucose uptake in cardiomyocytes. This impairment was ameliorated in cardiomyocytes from TLR4 knockout mice. Therefore, RBP4 may be a critical modulator promoting the vicious cycle of insulin resistance and heart failure by activating TLR4/MyD88-mediated inflammatory pathways. Potentially, lowering RBP4 might break the vicious cycle and improve both insulin resistance and cardiac hypertrophy.
An association between diabetes and heart failure has been noted for more than a century. Mounting evidence from research over the past 30 years clearly shows that insulin resistance, the fundamental mechanism for type 2 diabetes, and heart failure are not only associated, but also they actually form a vicious cycle that leads to high mortalities in patients with heart failure and diabetes (1). Abnormalities in cardiac structure and function are very commonly observed in patients with insulin resistance, even without frank diabetes mellitus (2). Insulin resistance alters glucose and fatty acid metabolism in cardiomyocytes, induces oxidative stress and inflammation, and promotes the hyperactivity of neurohumoral system, such as the renin-angiotensin-aldosterone and the adrenergic systems (1). By the same token, heart failure is associated with systemic insulin resistance that itself predicts increased mortality (3–5). Importantly, insulin resistance is improved after the correction of heart failure with a ventricular assist device in humans, suggesting heart failure is a causative factor of insulin resistance (6). Multiple mechanisms have been implicated in the heart failure-induced insulin resistance. For example, norepinephrine is increased significantly in heart failure, which may impair insulin signaling and inhibit insulin secretion (7). Recently it is shown that p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure (8). Despite the advances in the field, the interrelationship between heart failure and insulin resistance is complex and incompletely understood. Specifically the key factor(s) linking and promoting the vicious cycle of heart failure and insulin resistance are not fully elucidated.
Chronic inflammation plays a crucial role in the development and progression of both insulin resistance and cardiac hypertrophy (9). Toll-like receptor 4 (TLR4) is an important modulator of innate immunity, which recognizes pathogen-associated molecular patterns and initiates cytokine activation (10). In insulin target organs such as adipose tissue, muscle and liver, aberrant activation of the TLR4 pathway promotes cytokine production and impairs insulin signaling (11). TLR4 is also involved in maladaptive ventricular remodeling via activating cardiac inflammation (12). Myocardium expression of TLR4 is increased in patients with heart failure (13). Genetic and pharmacological inhibition of TLR4 exerts protective effects against cardiac hypertrophy in mice (14–16). Therefore, TLR4 activation appears to be a common mechanism for insulin resistance and heart failure. Identification of factor(s) that activate the TLR4 pathway in insulin resistance and cardiac hypertrophy may lead to the discovery of novel treatments for the vicious cycle of insulin resistance and heart failure.
Retinol-binding protein 4 (RBP4) is an approximately 21-kDa secreted protein that transports vitamin A (retinol) in circulation (17). RBP4 is elevated in insulin resistant states such as obesity and type 2 diabetes in both rodents and humans. Elevated RBP4 contributes to systemic insulin resistance (18–20). Interestingly, recent studies show that RBP4 is a novel proinflammatory factor that activates TLR4-dependent signaling and causes insulin resistance (21, 22). At the same time, emerging evidences have linked elevated serum RBP4 to cardiovascular diseases, including hypertension (23–25), atherosclerosis (26–28), and coronary artery disease (29–32). Serum RBP4 levels are also increased in patients with advanced heart failure and associated with insulin resistance (33). Remarkably, serum RBP4 is decreased in response to mechanical unloading and hemodynamic correction after the implantation of a left ventricular assist device (33).
We therefore hypothesized that RBP4, as a proinflammatory factor, may serve as an important mediator in the vicious cycle of insulin resistance and heart failure by activating the TLR4 pathway. In the present study, we aimed to investigate the underlying mechanisms for the elevation of RBP4 in heart failure and study its role in inducing cardiac hypertrophy.
Materials and Methods
Cardiac hypertrophy model
For transverse aortic constriction (TAC)-induced cardiac hypertrophy, C57BL/6 male mice of 8–10 weeks old were anesthetized with 1.5%–2% isoflurane, intubated, and placed on a respirator. After thoracotomy, the thoracic aorta was isolated by blunt dissection. Partial aortic constriction with 7–0 silk sutures was then performed by ligating the aorta with a 27-gauge needle. The needle was removed before closing the thoracic cavity. Sham-operated animals underwent the same surgical procedure without aortic binding. For angiotensin-II (Ang-II)-induced cardiac hypertrophy, Ang-II (1 μg/kg · min) (A9525; Sigma) was infused via an osmotic minipump (model 1002; Alzet Osmotic Pumps) to the 6- to 8-week-old male mice for 14 days. Echocardiography was performed using the Vevo2100 imaging system (VisualSonics Inc) with a 30-MHz central frequency scan head. The investigation was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University and the University of California, Irvine.
Serum RBP4 and Ang-II measurement
Serum RBP4 levels were determined by an ELISA (R&D Systems; limit of detection, 0.021 ng/mL) and quantitative Western blotting (18) using purified mouse RBP4 for generating the standard curves. The serum Ang-II levels were measured by an ELISA kit (ENZO).
Recombinant mouse RBP4 preparation
Mouse RBP4 was expressed in Escherichia coli and purified as described previously (18). Endotoxin was removed by sequential affinity adsorption to Endotrap matrix (Hyglos GmbH) and Detoxigel (Pierce). The endotoxin level of this recombinant protein was less than 0.001 endotoxin unit per microgram, which is the same as the ambient endotoxin levels in reverse-osmosis, double-deionized water as quantitatively measured by the Limulus amoebocyte lysate test (22). Proteomic and lipidomic mass spectrometry analysis of the RBP4 preparation confirmed its purity and showed no contaminating endotoxin (lipopolysaccharide), other lipopolysaccharides, lipoproteins, lipids, or additional proteins (34). The protein was stored at −80°C and protected from exposure to light.
Isolation and culture of primary neonatal cardiomyocytes
We used cardiomyocytes isolated from mouse neonatal ventricles. Although there are limitations including a more fetal gene expression profile, neonatal cardiomyocytes are commonly used for studying cardiac hypertrophy (35, 36). Tlr4 knockout (Tlr4−/−) mice (B6.B10ScN-Tlr4lps-del/JthJ, C57BL/6 background, stock number 007227), myeloid differentiation primary response gene 88 (Myd88) knockout (Myd88−/−) mice (B6.129P2[SJL]-Myd88tm1.1Defr/J, C57BL/6 background, stock number 009088), and control wild-type (WT) C57BL/6 mice (stock number 000664) were purchased from Jackson Laboratory. Neonatal mouse ventricular cardiomyocytes were isolated from 1- to 2-day-old mice as described previously with minor modifications (37, 38). In brief, ventricles were obtained after decapitation, immersed in a buffer of 116 mM NaCl, 18 mM HEPES, 845 μM NaH2PO4, 5.55 mM glucose, 5.37 mM KCl, and 831 μM MgSO4 (pH 7.35) and minced with scissors. The minced ventricular tissue was then digested with 0.4 mg/mL type II collagenase (LS004177; Worthington Biochemical Corp) and 0.6 mg/mL pancreatin (P3292; Sigma) in a buffer of 116 mM NaCl, 18 mM HEPES, 845 μM NaH2PO4, 5.55 mM glucose, 5.37 mM KCl, and 831 μM MgSO4 (pH 7.35) at 37°C. The isolated cells were first preplated for 30 minutes at 37°C to allow fibroblasts to adhere. The unadhered cells were collected and further purified on a discontinuous Percoll (GE Healthcare) density gradient. The gradient consisted of a 40.5% Percoll layer over a layer of 58.5% Percoll. After centrifugation at 3000 rpm for 40 minutes with no deceleration brake, the purified cardiomyocytes were collected and maintained in DMEM/F12 containing 10% fetal bovine serum (FBS), 5% horse serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). Before treatment, the cells were first serum starved overnight and then treated with Ang-II (A9525; Sigma) or recombinant RBP4 for 48 hours. For experiments of the antioxidant and the TLR4 inhibitor, the cells were treated with N-acetylcysteine (NAC) (Sigma) for 1 hour or TAK242 (InvivoGen) for 6 hours prior to the stimulation of the recombinant RBP4.
Measurement of the cell size
Cardiomyocytes were washed with PBS and fixed in 4% paraformaldehyde for 30 minutes at room temperature. Then the cells were permeabilized with 0.5% Triton X-100 made in PBS for 30 minutes, followed by blocking with 2% BSA for 60 minutes. The cells were stained with anti-α-actinin (1:200, A7811; Sigma) overnight at 4°C. After being washed in PBS, the cells were incubated with Alexa Fluor 594-conjugated secondary antibody (1:200, A21205; Life Technologies) and 4′,6′-diamino-2-phenylindole (1 μg/mL, D9542; Sigma). Images were captured using EVOS FL auto cell imaging system (Life Technologies). The cell surface area was analyzed by ImageJ (National Institutes of Health, Bethesda, Maryland).
Protein to DNA ratio measurement
Total protein and DNA contents were analyzed as previously described (39). In brief, cells were washed with PBS and incubated with 1 mL of 0.2 N perchloric acid for 5 minutes. The samples were then centrifuged for 10 minutes at 10 000 × g. The precipitates were incubated for 20 minutes at 60°C with 250 μL of 0.3 N KOH. Protein content was analyzed by the Bradford method using BSA as a standard. DNA content was detected using Hoechst dye 33258 (Life Technologies) with calf thymus DNA (Sigma) as a standard.
Glucose uptake measurement
Glucose uptake was measured using 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose (2-NBDG) (Invitrogen) as previously described (40). Briefly, cells were starved in Krebs Ringer HEPES buffer (140 mM NaCl; 5 mM KCl; 2.5 mM CaCl2; 1 mM MgSO4; 1 mM KH2PO4; 10 mM HEPES, pH 7.40) for 3 hours, followed by stimulation with or without 100 nM insulin for 30 minutes in the presence of 100 μM 2-NBDG at 37°C. After the incubation, the cells were washed three times with ice-cold PBS, and the resultant fluorescence was measured using a fluorescent microplate reader at excitation and emission wavelengths of 485 and 528 nm, respectively.
Intracellular reactive oxygen species (ROS) measurement
Intracellular ROS production was measured based on the oxidation of 2′,7′-dichlorofluoresce diacetate (Invitrogen) as previously described (37). Cardiomyocytes were seeded into a black 96-well plate at a density of 1 × 105/well. After treatment, the cells were incubated with 2′,7′-dichlorofluoresce diacetate at 37°C for 20 minutes, followed by washing three times with PBS. The resultant fluorescence was measured using a fluorescent microplate reader at excitation and emission wavelengths of 485 and 528 nm, respectively.
Measurement of proinflammatory cytokine secretion
Cardiomyocyte culture media were collected and centrifuged at 16 000 × g for 10 minutes to remove cell debris and stored at −80°C until measurement. Secretion of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, were measured by ELISA kits from eBioscience (TNF-α, catalog number 88-7324; IL-6, catalog number 88-7064; IL-1β, catalog number 88-7013). Secretion of monocyte chemotactic protein-1 (MCP-1) was measured by an ELISA kit from Biolegend (catalog number 432704).
Isolation and culture of primary adipocytes
Subcutaneous white adipose tissue excised from 4-week-old male C57BL/6 mice was minced and digested in a PBS digestion buffer containing 1.5 U/mL collagenase D (11088882001; Sigma), 2.4 U/mL Dispase II (4942078001; Sigma), and 10 mM CaCl2 at 37°C in a shaking water bath for 20–30 minutes. The digested tissue was filtered through 100-μm nylon cell strainers (BD Biosciences). The stromal vascular fraction cells containing preadipocytes were separated from floating primary adipocytes by centrifugation at 600 × g for 5 minutes. The resulting pellets were resuspended and further filtered using 40-μm nylon cell strainers (BD Biosciences). Stromal vascular fraction cells were maintained in DMEM/F12 containing 15% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. For differentiation, confluent preadipocytes were treated with medium containing 10% FBS, 0.5 mM isobutylmethylxanthine (I7018; Sigma), 1 μM dexamethasone (D4902; Sigma), 2 μg/mL insulin (I0546; Sigma), and 1 μM rosiglitazone (R2408; Sigma) for 48 hours. Adipocytes were then maintained in DMEM/F12 containing 10% FBS and 2 μg/mL insulin. Before treatment, cells were first serum starved overnight and then treated with Ang-II in the presence or absence of losartan (R&D Systems) for 48 hours.
RNA extraction and quantitative PCR
Total RNA was extracted using the RNeasy minikit from QIAGEN. cDNA was synthesized using the SuperScript III first-strand synthesis supermix for quantitative RT-PCR (qRT-PCR; Invitrogen) and used in real-time PCRs with Power SYBR Green PCR master mix (Applied Biosystems) on a 7900HT real-time PCR system (Applied Biosystems). The relative gene expression levels were calculated by the 2-δδCt method using Tata-binding protein (Tbp) as an internal control. Primer sequences are shown in Supplemental Table 1.
Western blot
Tissues or cells were lysed in radioimmunoprecipitation assay buffer containing 1 mM NaF, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride. Lysates were then subjected to immunoblotting with the indicated antibodies. The following antibodies were used (Table 1): RBP4 (A0040; Dako); glucose transporter-4 (GLUT4) (07-1404; Millipore); and β-actin (8457; Cell Signaling). Band densitometry measurements were performed using Image J (National Institutes of Health).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used | DOI or Publication Data |
|---|---|---|---|---|---|---|
| RBP4 | Antiretinol-binding protein | Dako, A0040 | Rabbit, polyclonal | 1:1000 | ||
| GLUT4 | Anti-GLUT-4 antibody, C terminus | Millipore, 07-1404 | Rabbit, polyclonal | 1:1000 | ||
| β-Actin | β-Actin (D6A8) rabbit mAb | Cell Signaling, 8457 | Rabbit, monoclonal | 1:1000 |
Abbreviation: mAb, monoclonal antibody.
Statistical analysis
All data were expressed as mean ± SEM. Statistical differences were assessed using unpaired Student's two-tailed t tests for two groups and a one-way ANOVA for three groups or more. Spearman correlation analysis was used for the correlations of RBP4 with Ang-II and homeostasis model assessment index of insulin resistance (HOMA-IR). Statistical significance was assumed at P < .05.
Results
Serum and adipose RBP4 levels are increased in cardiac hypertrophy
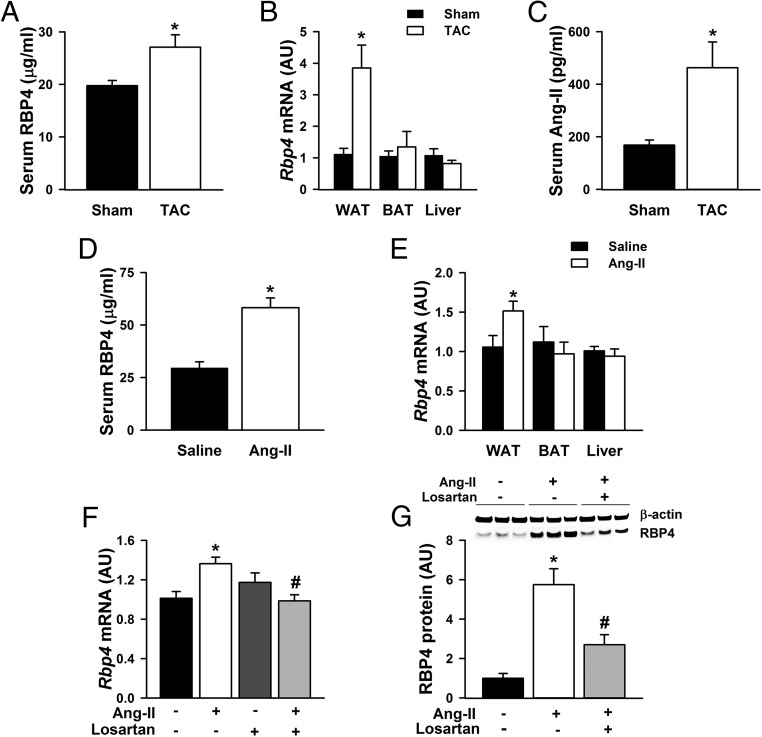
To determine whether RBP4 may potentially play a role in the vicious cycle of insulin resistance and heart failure, we first established a cardiac hypertrophic mouse model using TAC procedure and measured RBP4 levels in serum and tissues. Four weeks after the TAC procedure, echocardiography confirmed cardiac hypertrophy showing increased left ventricular wall thickness and mass in the TAC mice (Supplemental Table 2). Serum RBP4 levels were significantly higher in the TAC mice compared with the sham controls (Figure 1A). RBP4 was not detected in the heart (41) or in the cultured primary cardiomyocytes (Supplemental Figure 1) but was highly expressed in the adipose tissue and liver (42). We found that the Rbp4 mRNA levels were selectively increased in the white adipose tissue (WAT), but not in the brown adipose tissue (BAT) or the liver of TAC mice (Figure 1B).
Figure 1.
Increased serum and adipose RBP4 levels in cardiac hypertrophy. Serum RBP4 levels (A); Rbp4 mRNA expression in WAT, BAT, and liver (B); and serum Ang-II levels (C) in mice with TAC or sham procedures (n = 6 per group). *, P < .05 vs sham. Serum RBP4 levels (D) and Rbp4 mRNA expression (E) in WAT, BAT, and liver of mice with Ang-II or saline infusion (n = 6 per group). *, P < .05 vs saline. F and G, Primary adipocytes were pretreated with Ang-II receptor inhibitor losartan (100 μM for 6 h), followed by stimulation with Ang-II (1 μM for 48 h). F, Rbp4 mRNA levels. G, RBP4 protein levels (n = 6 per group). *, P < .05 vs control; #, P < .05 vs Ang-II alone; AU, arbitrary units.
The increased Rbp4 levels were at least partially caused by the activated renin-angiotensin system (RAS) in cardiac hypertrophy. Serum Ang-II levels were elevated in the TAC mice compared with the sham controls (Figure 1C) and were positively correlated with serum RBP4 levels (R = 0.875, P < .001). In mice treated with Ang-II infusion, serum RBP4 was increased by approximately 2-fold compared with the controls (Figure 1D). Serum RBP4 levels were overall higher in the Ang-II- and saline-infused mice (58.24 ± 4.64 μg/mL and 29.62 ± 2.89 μg/mL, respectively, Figure 1D) than that in the TAC and sham-operated mice (27.08 ± 2.36 μg/mL and 19.76 ± 0.99 μg/mL, respectively, Figure 1A). This is likely due to the more extensive surgery for the TAC procedure than that for osmotic minipump insertion. Our data show that even the sham TAC operation reduced circulating RBP4 by nearly 60% (Supplemental Figure 2).
Consistent with the selective induction of Rbp4 expression in the WAT of TAC mice, Rbp4 mRNA was also increased in the WAT, but not in the BAT or liver of Ang-II-treated mice compared with the controls (Figure 1E). The direct effect of Ang-II on RBP4 expression was further confirmed in the in vitro study. Incubating primary adipocytes with Ang-II stimulated RBP4 mRNA and protein expression, and the effects were abolished by losartan, a specific Ang-II receptor antagonist (Figure 1, F and G). These data reveal that enhanced RAS activity is a novel mechanism for adipose RBP4 induction in cardiac hypertrophy.
RBP4 stimulates hypertrophic responses of cardiomyocytes
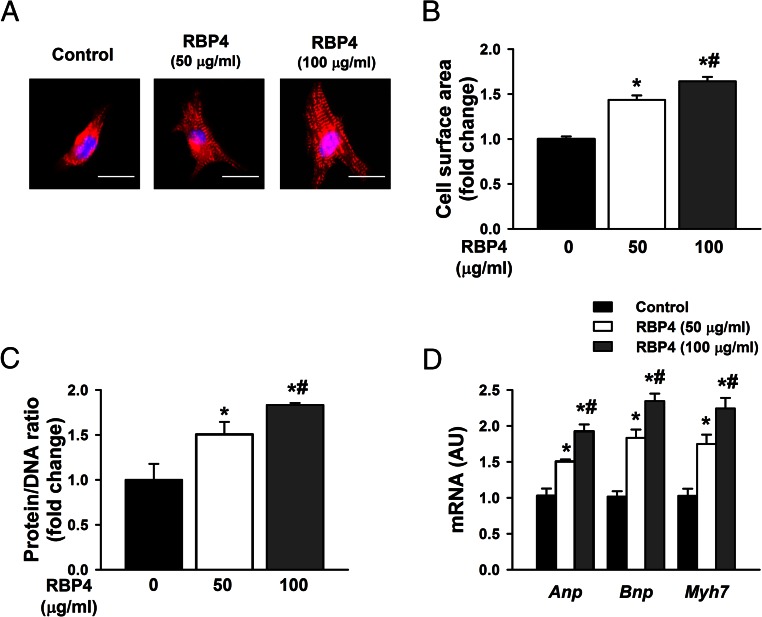
Because we found that RBP4 levels were elevated in cardiac hypertrophy, we sought to determine whether RBP4 may have direct effects on cardiomyocytes to induce hypertrophy. Three methods including cell surface measurements, protein to DNA ratio, and gene markers were used to assess cardiomyocyte hypertrophy in vitro. Incubation of primary cardiomyocytes with recombinant RBP4 at concentrations of 50 and 100 μg/mL dose dependently increased cell size (Figure 2, A and B), protein to DNA ratio (Figure 2C), and hypertrophic gene markers including atrial natriuretic peptide (Anp), brain natriuretic peptide (Bnp), and myosin heavy chain 7 (Myh7) (Figure 2D). A lower dose of RBP4 at 25 μg/mL significantly increased the expression of Bnp and Myh7 but not Anp (Supplemental Figure 3). These data indicate that RBP4 can directly stimulate cardiomyocytes to induce hypertrophy.
Figure 2.
RBP4 induces hypertrophic responses in primary cardiomyocytes. A–D, Cardiomyocytes were treated with recombinant mouse RBP4 or vehicle control for 48 hours. Hypertrophy was assessed by cell size (A), cell surface area measurement (B), protein to DNA ratio (C), and analysis of the mRNA expression for Anp, Bnp, and Myh7 by qRT-PCR (D) (n = 6 per group). *, P < .05 vs control; #, P < .05 vs RBP4 with 50 μg/mL. Bar, 20 μm. AU, arbitrary units.
RBP4 induces inflammation and oxidative stress in cardiomyocytes
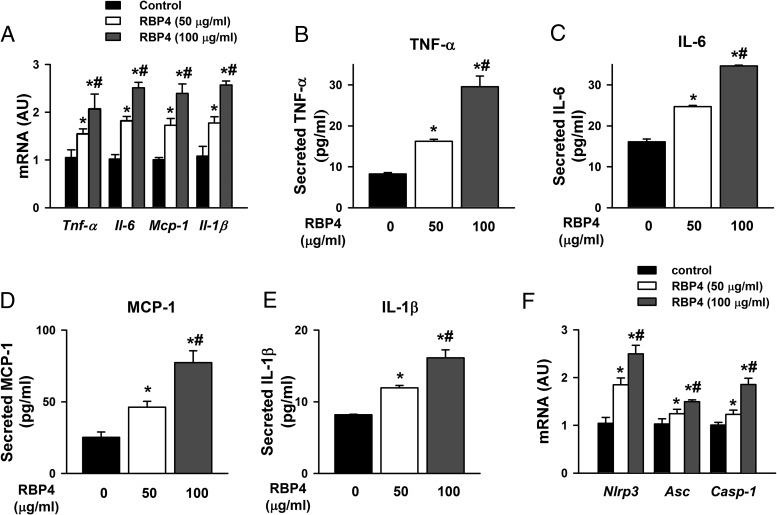
Chronic inflammation plays a crucial role in the development of cardiac hypertrophy, and RBP4 has been shown to be a proinflammatory adipokine (21, 22). Treating primary cardiomyocytes with RBP4 at concentrations of 50 and 100 μg/mL increased the mRNA expression of proinflammatory cytokines, including TNF-α, IL-6, MCP-1, and IL-1β (Figure 3A). Consistently, the protein production of these cytokines was elevated in the supernatant of the cultured cardiomyocytes treated with RBP4 (Figure 3, B–E). A lower dose of RBP4 (25 μg/mL) also increased the cytokine production from cardiomyocytes but to a lesser degree (Supplemental Figure 4, A–D). Interestingly, the mRNA levels of the inflammasome components including nucleotide-binding oligomerization domain-like receptor (NLR) family pyrin domain-containing 3 (Nlrp3), apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain (Asc), and caspase-1 (43) were also increased after RBP4 treatment (Figure 3F). These results suggest that RBP4-induced inflammasome and inflammation may be involved in cardiac hypertrophy.
Figure 3.
RBP4 promotes inflammation in primary cardiomyocytes. A, mRNA expression levels of Tnf-α, Il-6, Mcp-1, and Il-1β. B–E, Secretion of TNF-α, IL-6, MCP-1, and IL-1β protein in the supernatant of cultured cardiomyocytes. F, mRNA expression of the components of NLRP3 inflammasome (n = 6 per group). *, P < .05 vs control; #, P < .05 vs RBP4 with 50 μg/mL. AU, arbitrary units.
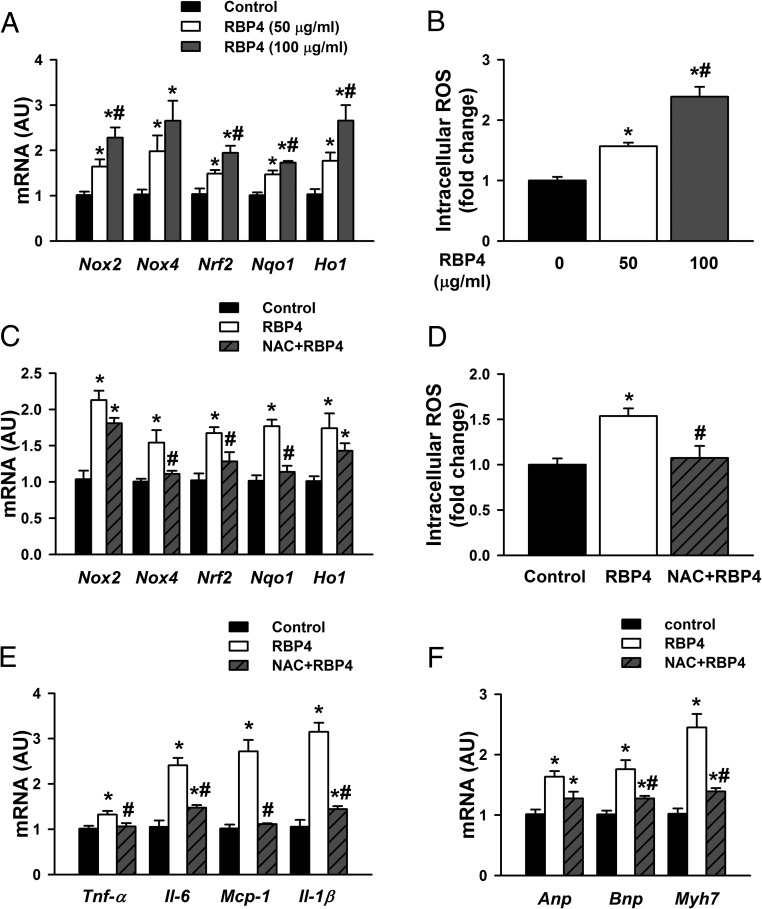
Increased oxidative stress has been identified as one of the key contributing factors to the development of cardiac hypertrophy (44). In cardiomyocytes, RBP4 induced the expression of genes involved in oxidative stress, including nicotinamide adenine inucleotide phosphate oxidase 2 (Nox2), Nox4, nuclear factor erythroid 2 (Nrf2), nicotinamide adenine dinucleotide phosphate oxidase dehydrogenase, quinone 1 (Nqo1), and heme oxygenase-1 (Ho1) (Figure 4A and Supplemental Figure 5A). Consistently, intracellular ROS production was elevated in RBP4-treated cardiomyocytes (Figure 4B and Supplemental Figure 5B). The elevated levels of both ROS gene markers and production were attenuated by antioxidant NAC (Figure 4, C and D). Moreover, NAC pretreatment mitigated RBP4-induced cytokine expression and hypertrophic responses in cardiomyocytes (Figure 4, E and F). These results indicate that the interaction between inflammation and ROS production may play an important role in RBP4-induced cardiac hypertrophy.
Figure 4.
RBP4 enhances oxidative stress in primary cardiomyocytes. A and B, Cardiomyocytes were treated with recombinant mouse RBP4 or vehicle control for 48 hours. Oxidative stress was assessed by mRNA expression of Nox2, Nox4, Nrf2, Nqo1, and Ho1 (A) and intracellular ROS production (B) (n = 6 per group). *, P < .05 vs control; #, P < .05 vs RBP4 with 50 μg/mL. C–F, Cardiomyocytes were pretreated with antioxidant NAC (10 mM for 1), and then recombinant mouse RBP4 was added (50 μg/mL for 48 h). C, mRNA expression of Nox2, Nox4, Nrf2, Nqo1, and Ho1. D. Intracellular ROS production. E, mRNA expression of Tnf-α, Il-6, Mcp-1, and Il-1β. F, mRNA expression of Anp, Bnp, and Myh7 (n = 6 per group). *, P < .05 vs control; #, P < .05 vs RBP4 alone. AU, arbitrary units.
RBP4 induces cardiac inflammation and hypertrophy through TLR4/MyD88 pathway
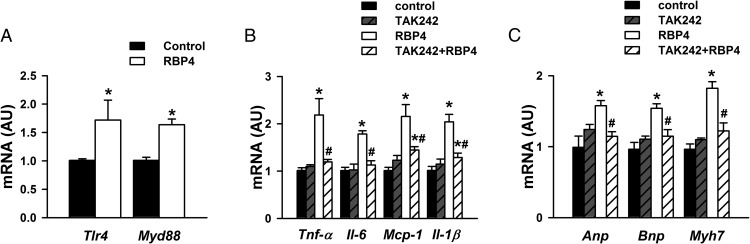
Previous studies indicated that the TLR4/MyD88 pathway was involved in the proinflammatory role of RBP4 in macrophages (21, 22, 45). More importantly, the TLR4/MyD88 mediated proinflammatory pathway plays a major role in cardiac hypertrophy and heart failure (14, 15, 39, 46). We therefore investigated whether RBP4 may affect the TLR4/MyD88 pathway in cardiomyocytes. Treatment of cardiomyocytes with RBP4 increased the expression of Tlr4 and Myd88 (Figure 5A). TLR4 inhibitor TAK242 abolished RBP4-induced expression of proinflammatory cytokines Tnf-α, Il-6, Mcp-1, and Il-1β (Figure 5B) as well as hypertrophic markers Anp, Bnp, and Myh7 (Figure 5C).
Figure 5.
RBP4 induces inflammation and hypertrophy in cardiomyocytes via activating TLR4. A, Tlr4 and Myd88 mRNA expression after treatment with RBP4 (50 μg/mL) or vehicle control for 48 hours. B and C, Cardiomyocytes were pretreated with TLR4 inhibitor (3 μM for 6 h), and then recombinant mouse RBP4 was added (50 μg/mL for 48 h). B, mRNA expression of Tnf-α, Il-6, Mcp-1, and Il-1β. C, mRNA expression of Anp, Bnp, and Myh7 (n = 6 per group). *, P < .05 vs control; #, P < .05 vs RBP4 alone. AU, arbitrary units.
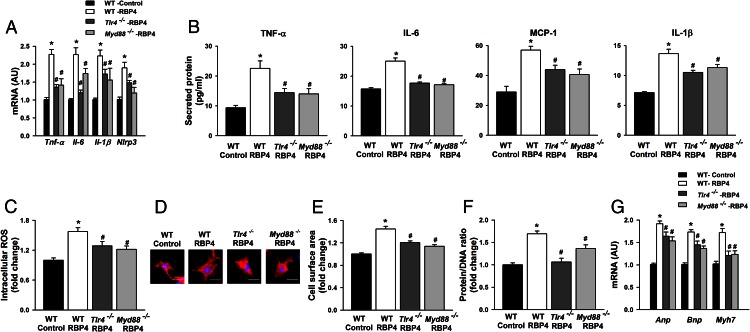
To further investigate the roles of TLR4 and MyD88 in RBP4-induced inflammation and hypertrophy in cardiomyocytes, we isolated cardiomyocytes from Tlr4 and Myd88 knockout mice. Similar to the effects of the TLR4 inhibitor, both Tlr4 and Myd88 knockdown markedly attenuated RBP4-stimulated cytokine expression and secretion as well as ROS production in cardiomyocytes (Figure 6, A–C). Importantly, the increased cell size, protein to DNA ratio, and hypertrophic markers induced by RBP4 were mitigated in cardiomyocytes isolated from Tlr4 and Myd88 knockout mice compared with those from WT mice (Figure 6, D–G). Furthermore, RBP4 was induced by Ang-II treatment similar to the levels in Tlr4 knockout and control mice (Supplemental Figure 6A). However, cardiac hypertrophy was attenuated in Tlr4 knockout mice compared with controls (Supplemental Figure 6B). These results together indicate that the TLR4/MyD88 proinflammatory pathway is critical for RBP4-induced inflammation and hypertrophy in cardiomyocytes.
Figure 6.
RBP4-induced inflammation, oxidative stress and hypertrophy are TLR4 and MyD88 dependent. Primary cardiomyocytes were isolated from Tlr4 and Myd88 knockout (Tlr4−/− and Myd88−/−) or control mice and then treated with recombinant mouse RBP4 or vehicle control for 48 hours. A, mRNA expression of Tnf-α, Il-6, Mcp-1, and Il-1β. B, Secretion of TNF-α, IL-6, MCP-1, and IL-1β in the supernatant of cultured cardiomyocytes. C, Intracellular ROS production. D–G, Hypertrophy was assessed by cell size (D), cell surface area measurement (E), protein to DNA ratio (F), and analysis of the mRNA expression for Anp, Bnp, and Myh7 by qRT-PCR (G) (n = 6 per group). *, P < .05 vs control-WT; #, P < .05 vs RBP4-WT. Bar, 20 μm. AU, arbitrary units.
RBP4 impairs insulin-stimulated glucose uptake in cardiomyocytes
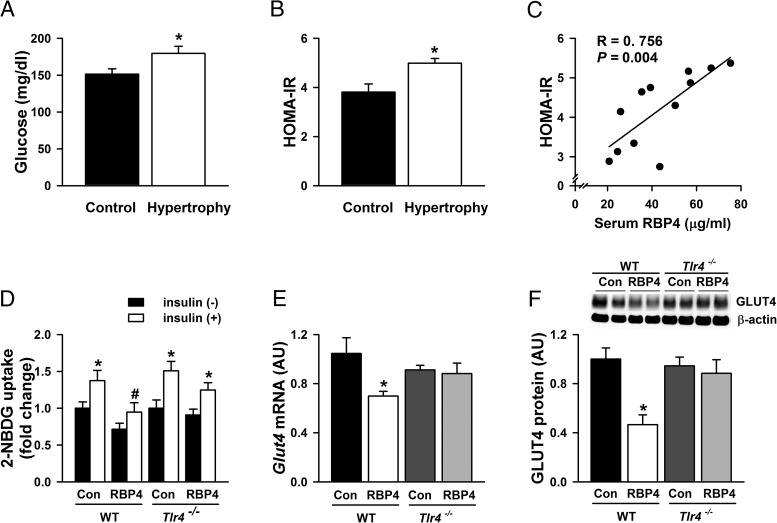
Cardiac hypertrophy/heart failure induces systemic insulin resistance and increases the risk for diabetes (47). Consistently, mice with Ang-II induced hypertrophy had higher glucose levels (Figure 7A) and were more insulin resistant as assessed by HOMA-IR (Figure 7B) compared with the controls. Interestingly, the serum RBP4 levels were positively correlated with HOMA-IR (Figure 7C). These results strongly suggest that elevated RBP4 levels may be involved in systemic insulin resistance in cardiac hypertrophy/heart failure. We then investigated whether RBP4 may also have a direct effects on insulin action in cardiomyocytes. RBP4 significantly impaired the insulin-stimulated glucose uptake in the cardiomyocytes isolated from WT mice. The impairment was attenuated in the cardiomyocytes from the Tlr4 knockout mice (Figure 7D). Insulin-stimulated glucose disposal in cardiomyocytes is mainly medicated by GLUT4 (48). Consistently, RBP4 decreased the mRNA and protein levels of GLUT4 in cardiomyocytes. Remarkably, Tlr4 knockdown normalized the GLUT4 expression in cardiomyocytes treated with RBP4 (Figure 7, E and F). The results indicate that the elevated RBP4 in cardiac hypertrophy/heart failure may play a major role in systemic and cardiac insulin resistance.
Figure 7.
RBP4 and insulin resistance in cardiac hypertrophy. Serum glucose levels (A); HOMA-IR (B); and correlation of serum RBP4 with HOMA-IR (C) in Ang-II-induced cardiac hypertrophy and controls (n = 6 per group). *, P < .05 vs control. D, Insulin-stimulated glucose uptake in primary cardiomyocytes isolated from Tlr4 knockout (Tlr4−/−) or WT mice. RBP4-treated cardiomyocytes were stimulated with 2-NBDG together with or without insulin (100 nM) for 30 minutes (n = 8 per group). *, P < .05 vs no insulin in each group; #, P < .05 vs control with insulin stimulation in WT mice. E and F, Glut4 mRNA expression (E) and GLUT4 protein levels (F) in cardiomyocytes treated with or without RBP4 in Tlr4−/− and WT mice (n = 6 per group). *, P < .05 vs control in WT mice. AU, arbitrary units.
Discussion
Insulin resistance and heart failure form a vicious cycle that determines high mortalities in patients with heart failure and diabetes (1). RBP4 has been shown to be an important adipokine that contributes to systemic insulin resistance in obesity and type 2 diabetes (18, 19). Now we show that adipose RBP4 expression and serum RBP4 levels are elevated in cardiac hypertrophy. RBP4 exerts direct effects on promoting hypertrophy of cardiomyocytes, as evidenced by increased cell size, enhanced protein synthesis, and elevated expression of hypertrophic markers. These results complement nicely with a previous report showing that Rbp4 knockout mice are protected from Ang-II-induced cardiac hypertrophy (41). Furthermore, our data show that elevated RBP4 in cardiac hypertrophy is involved in both systemic and cardiac insulin resistance. RBP4 levels are associated with HOMA-IR in Ang-II-induced hypertrophy. RBP4 also directly impairs GLUT4 expression and insulin-stimulated glucose uptake in cardiomyocytes. Therefore, RBP4 may be a key mediator that promotes the vicious cycle of insulin resistance and cardiac hypertrophy/heart failure.
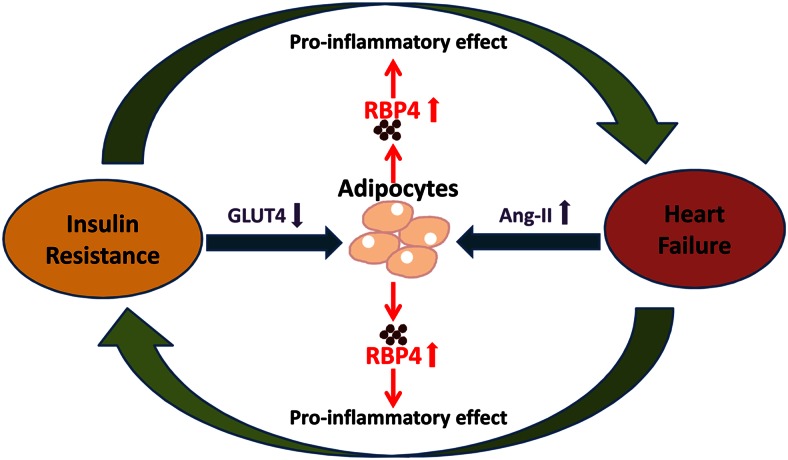
RBP4-induced inflammation may be a common mechanism for this vicious cycle (Figure 8). Chronic inflammation in insulin-responsive organs including adipose tissue plays a major role in the development of insulin resistance (49). In obesity and type 2 diabetes, proinflammatory macrophages produce cytokines that in turn impair insulin action in adipocytes. The TLR4 pathway is critical for the proinflammatory macrophage activation in adipose tissue in obesity (11). RBP4 appears to be an initiating factor to activate TLR4 and promote cytokine production in macrophages (21, 22, 34). Interestingly, direct RBP4 treatment in adipocytes does not affect insulin signaling, whereas coculturing adipocytes with RBP4-stimulated macrophages impair insulin-induced Akt phosphorylation. Therefore, RBP4 indirectly inhibits insulin signaling in adipocytes by stimulating proinflammatory cytokine secretion from macrophages (22). In cardiomyocytes, however, RBP4 directly stimulates proinflammatory cytokine production and promotes cardiac hypertrophy and insulin resistance through the TLR4-mediated proinflammatory pathway.
Figure 8.
Potential role of RBP4 in the vicious cycle of insulin resistance and heart failure. Adipokine RBP4 is regulated by the systemic activation of Ang-II in cardiac hypertrophy/heart failure and by the down-regulation of adipose GLUT4 in insulin resistance (18). RBP4 in turn promotes insulin resistance and cardiac hypertrophy/heart failure through its proinflammatory effects.
Both the biological knockdown and pharmacological inhibition of TLR4 attenuated RBP4-induced cytokine production and hypertrophy. TLR4 is critical for inducing the priming signals that are necessary for upregulation of NLRP3 inflammasome components (50). Recent studies indicate that activation of NLRP3 inflammasome is implicated in the development of heart failure (51). Consistently, we found that RBP4 increases the expression of NLRP3, apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain (ASC), caspase-1, and IL-1β in cardiomyocytes. Moreover, RBP4 increases the cardiac ROS production, which is attenuated by the antioxidant NAC. NAC is a synthetic precursor of glutathione, and its antioxidant property results from its free radical scavenging effects either directly via thiol-disulphide exchange activity or indirectly via increasing intracellular glutathione levels (52). NAC has an optimal thiol redox state, which is of great importance to optimize the protective ability of the cell to counterbalance oxidative stress and inflammation (53). Consistently, NAC treatment also improves RBP4-induced inflammation in cardiomyocytes (Figure 4E). Antioxidants may potentially be beneficial for inhibiting ROS and inflammation-positive feedback loop in the development of RBP4-stimulated hypertrophy (54).
The mechanism by which RBP4 stimulates the TLR4 pathway is unclear. The known RBP4 receptor stimulated by retinoic acid 6 (STRA6) does not appear to be involved because STRA6 is not expressed in the macrophage (22), primary cardiomyocyte, or HL-1 cardiac muscle cell line (data not shown). Our data show that Tlr4 and Myd88 mRNA levels are increased by RBP4 stimulation, which may at least partially explain the enhanced activity of the TLR4 pathway. It is also possible that RBP4 may directly interact with TLR4 or the components of the TLR4 complex such as myeloid differentiation protein-2 (MD-2) and CD14 (55). Alternatively, RBP4 may bind to an unidentified receptor in macrophages and cardiomyocytes to activate TLR4.
The molecular signals controlling RBP4 gene expression in adipocytes remain to be fully elucidated. GLUT4 and all-trans retinoic acid may be involved in regulating the RBP4 expression in adipocytes (18, 56). Here we show that Ang-II, a key player of the RAS, is a novel regulator of adipose RBP4 expression. Systemic RAS activation involves multiple organs including the kidney, liver, lung, and adrenal gland. Interestingly, RAS components including angiotensinogen, renin, and angiotensin-converting enzyme are also expressed in adipocytes (57). The up-regulation of the adipose RAS promotes inflammation, lipogenesis, and ROS generation and impairs insulin signaling (57). In our TAC-induced cardiac hypertrophy, Ang-II is elevated (Figure 1C), likely due to systemic RAS activation because the expression of angiotensinogen and angiotensin-converting enzyme was not altered in the adipose tissue of TAC mice (not shown). RAS is traditionally known for its role in the regulation of blood pressure, fluid, and electrolyte balance (58). Emerging evidence shows RAS also plays an important role in systemic insulin resistance (59). In heart failure, RAS is significantly activated, which not only worsens heart function but also induces insulin resistance (59). We now provide one mechanistic explanation for RAS-induced heart failure and insulin resistance in that it increases RBP4 levels. Consistent with this, blocking RAS using losartan decreases RBP4 expression in adipocytes (Figure 1F) and lower serum RBP4 in humans (60).
In summary, our data reveal a novel role of RBP4 in promoting hypertrophy and insulin resistance in cardiomyocytes. RBP4 induces proinflammatory responses and ROS production in cardiomyocytes by activating the TLR4/MyD88 pathway. Therefore, RBP4 may be a central component in the vicious cycle of insulin resistance and heart failure (Figure 8). Lowering RBP4 might break the vicious cycle and benefit both insulin sensitivity and heart function.
Acknowledgments
We thank Pratik Aryal and Barbara B. Kahn (Beth Israel Deaconess Medical Center, Boston, Massachusetts) for kindly providing the recombinant RBP4 that was used for cell culture studies.
This work was supported by grants from the National Science Foundation of China Grants 81270428 and 81470501 (to X.L.); National Institutes of Health Grant R01 DK100385 (to Q.Y.); and the Postgraduate Research and Innovation Project in Jiangsu Province, China (Grant JX22013318) and China Scholarship Council Grant 1410150039 (to W.G.).
Disclosure Summary: Q.Y. is one of the inventors on a patent on RBP4 and insulin resistance (US patent 7553631). The other authors have nothing to disclose.
Footnotes
- Ang-II
- angiotensin-II
- BAT
- brown adipose tissue
- FBS
- fetal bovine serum
- GLUT4
- glucose transporter-4
- HOMA-IR
- homeostasis model assessment index of insulin resistance
- MCP-1
- monocyte chemotactic protein-1
- NAC
- N-acetylcysteine
- 2-NBDG
- 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose
- NLR
- nucleotide-binding oligomerization domain-like receptor
- qRT-PCR
- quantitative RT-PCR
- RAS
- renin-angiotensin system
- RBP4
- retinol-binding protein 4
- ROS
- reactive oxygen species
- TAC
- transverse aortic constriction
- TLR4
- Toll-like receptor 4
- WAT
- white adipose tissue
- WT
- wild type.
References
- 1. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin. 2012;8:619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paolisso G, Tagliamonte MR, Rizzo MR, et al. Prognostic importance of insulin-mediated glucose uptake in aged patients with congestive heart failure secondary to mitral and/or aortic valve disease. Am J Cardiol. 1999;83:1338–1344. [DOI] [PubMed] [Google Scholar]
- 4. Paolisso G, De Riu S, Marrazzo G, Verza M, Varricchio M, D'Onofrio F. Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metabolism. 1991;40:972–977. [DOI] [PubMed] [Google Scholar]
- 5. Doehner W, Rauchhaus M, Ponikowski P, et al. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. [DOI] [PubMed] [Google Scholar]
- 6. Uriel N, Naka Y, Colombo PC, et al. Improved diabetic control in advanced heart failure patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uriel N, Gonzalez-Costello J, Mignatti A, et al. Adrenergic activation, fuel substrate availability, and insulin resistance in patients with congestive heart failure. JACC Heart Fail. 2013;1:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimizu I, Yoshida Y, Katsuno T, et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15:51–64. [DOI] [PubMed] [Google Scholar]
- 9. Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. [DOI] [PubMed] [Google Scholar]
- 10. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 11. Shah PK. Innate immune pathway links obesity to insulin resistance. Circ Res. 2007;100:1531–1533. [DOI] [PubMed] [Google Scholar]
- 12. Timmers L, Sluijter JP, van Keulen JK, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. [DOI] [PubMed] [Google Scholar]
- 13. Birks EJ, Felkin LE, Banner NR, Khaghani A, Barton PJ, Yacoub MH. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant. 2004;23:228–235. [DOI] [PubMed] [Google Scholar]
- 14. Ha T, Li Y, Hua F, et al. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–234. [DOI] [PubMed] [Google Scholar]
- 15. Ehrentraut H, Weber C, Ehrentraut S, et al. The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. Eur J Heart Fail. 2011;13:602–610. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda S, Umemoto S, Yoshimura K, et al. Angiotensin activates MCP-1 and induces cardiac hypertrophy and dysfunction via Toll-like receptor 4. J Atheroscler Thromb. 2015;22(8):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blaner WS. Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev. 1989;10:308–316. [DOI] [PubMed] [Google Scholar]
- 18. Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. [DOI] [PubMed] [Google Scholar]
- 19. Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. [DOI] [PubMed] [Google Scholar]
- 20. Kloting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. [DOI] [PubMed] [Google Scholar]
- 21. Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norseen J, Hosooka T, Hammarstedt A, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solini A, Santini E, Madec S, Rossi C, Muscelli E. Retinol-binding protein-4 in women with untreated essential hypertension. Am J Hypertens. 2009;22:1001–1006. [DOI] [PubMed] [Google Scholar]
- 24. Chiba M, Saitoh S, Ohnishi H, et al. Associations of metabolic factors, especially serum retinol-binding protein 4 (RBP4), with blood pressure in Japanese—the Tanno and Sobetsu study. Endocr J. 2010;57:811–817. [DOI] [PubMed] [Google Scholar]
- 25. Deng W, Zhang Y, Zheng Y, et al. Serum retinol-binding protein 4 levels are elevated but do not contribute to insulin resistance in newly diagnosed Chinese hypertensive patients. Diabetol Metab Syndr. 2014;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bobbert T, Raila J, Schwarz F, et al. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. 2010;213:549–551. [DOI] [PubMed] [Google Scholar]
- 27. Dessein PH, Tsang L, Norton GR, Woodiwiss AJ, Solomon A. Retinol binding protein 4 concentrations relate to enhanced atherosclerosis in obese patients with rheumatoid arthritis. PloS One. 2014;9:e92739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kadoglou NP, Lambadiari V, Gastounioti A, et al. The relationship of novel adipokines, RBP4 and omentin-1, with carotid atherosclerosis severity and vulnerability. Atherosclerosis. 2014;235:606–612. [DOI] [PubMed] [Google Scholar]
- 29. Sun Q, Kiernan UA, Shi L, et al. Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the Nurses' Health Study. Circulation. 2013;127:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cubedo J, Padro T, Cinca J, Mata P, Alonso R, Badimon L. Retinol-binding protein 4 levels and susceptibility to ischaemic events in men. Eur J Clin Invest. 2014;44:266–275. [DOI] [PubMed] [Google Scholar]
- 31. Lambadiari V, Kadoglou NP, Stasinos V, et al. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol. 2014;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Wang D, Chen H, Xia M. Circulating retinol binding protein 4 is associated with coronary lesion severity of patients with coronary artery disease. Atherosclerosis. 2015;238:45–51. [DOI] [PubMed] [Google Scholar]
- 33. Chavarria N, Kato TS, Khan R, et al. Increased levels of retinol binding protein 4 in patients with advanced heart failure correct after hemodynamic improvement through ventricular assist device placement. Circ J. 2012;76:2148–2152. [DOI] [PubMed] [Google Scholar]
- 34. Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19:512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirtschink P, Krishnan J, Grimm F, et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature. 2015;522:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin Sci (Lond). 2015;128:325–335. [DOI] [PubMed] [Google Scholar]
- 38. Deng W, Leu HB, Chen Y, et al. Protein kinase B (PKB/AKT1) formed signaling complexes with mitochondrial proteins and prevented glycolytic energy dysfunction in cultured cardiomyocytes during ischemia-reperfusion injury. Endocrinology. 2014;155:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang K, Liu F, Zhou LY, et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014;114:1377–1388. [DOI] [PubMed] [Google Scholar]
- 40. Arora A, Dey CS. SIRT2 negatively regulates insulin resistance in C2C12 skeletal muscle cells. Biochim Biophys Acta. 2014;1842:1372–1378. [DOI] [PubMed] [Google Scholar]
- 41. Kraus BJ, Sartoretto JL, Polak P, et al. Novel role for retinol-binding protein 4 in the regulation of blood pressure. FASEB J. 2015;29(8):3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. [DOI] [PubMed] [Google Scholar]
- 44. Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blaner WS. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 2007;5:164–166. [DOI] [PubMed] [Google Scholar]
- 46. Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012;52:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heck PM, Dutka DP. Insulin resistance and heart failure. Curr Heart Fail Rep. 2009;6:89–94. [DOI] [PubMed] [Google Scholar]
- 48. Steinbusch LK, Schwenk RW, Ouwens DM, Diamant M, Glatz JF, Luiken JJ. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol Life Sci. 2011;68:2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta-cell dysfunction. Transl Res. 2016;167(1):228–256. [DOI] [PubMed] [Google Scholar]
- 50. Gurung P, Li B, Subbarao Malireddi RK, Lamkanfi M, Geiger TL, Kanneganti TD. Chronic TLR stimulation controls NLRP3 inflammasome activation through il-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci Rep. 2015;5:14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butts B, Gary RA, Dunbar SB, Butler J. The importance of NLRP3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Andrade KQ, Moura FA, Dos Santos JM, de Araujo OR, de Farias Santos JC, Goulart MO. Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-acetylcysteine. Int J Mol Sci. 2015;16:30269–30308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mercader J, Granados N, Bonet ML, Palou A. All-trans retinoic acid decreases murine adipose retinol binding protein 4 production. Cell Physiol Biochem. 2008;22:363–372. [DOI] [PubMed] [Google Scholar]
- 57. Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136–149. [DOI] [PubMed] [Google Scholar]
- 58. Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. [DOI] [PubMed] [Google Scholar]
- 59. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Derosa G, Maffioli P, Ferrari I, et al. Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients. Hypertens Res. 2011;34:145–151. [DOI] [PubMed] [Google Scholar]