Abstract
Conditional deletion of Gata4 in Sertoli cells (SCs) of adult mice has been shown to increase permeability of the blood-testis barrier (BTB) and disrupt spermatogenesis. To gain insight into the molecular underpinnings of these phenotypic abnormalities, we assessed the impact of Gata4 gene silencing in cell culture models. Microarray hybridization identified genes dysregulated by siRNA-mediated inhibition of Gata4 in TM4 cells, an immortalized mouse SC line. Differentially expressed genes were validated by quantitative RT-PCR analysis of primary cultures of Gata4flox/flox mouse SCs that had been subjected to cre-mediated recombination in vitro. Depletion of GATA4 in TM4 cells and primary SCs was associated with altered expression of genes involved in key facets of BTB maintenance, including tight/adherens junction formation (Tjp1, Cldn12, Vcl, Tnc, Csk) and extracellular matrix reorganization (Lamc1, Col4a1, Col4a5, Mmp10, Mmp23, Timp2). Western blotting and immunocytochemistry demonstrated reduced levels of tight junction protein-1, a prototypical tight junction protein, in GATA4-depleted cells. These changes were accompanied by a loss of morphologically recognizable junctional complexes and a decline in epithelial membrane resistance. Furthermore, Gata4 gene silencing was associated with altered expression of Hk1, Gpi1, Pfkp, Pgam1, Gls2, Pdk3, Pkd4, and Ldhb, genes regulating the production of lactate, a key nutrient that SCs provide to developing germ cells. Comprehensive metabolomic profiling demonstrated impaired lactate production in GATA4-deficient SCs. We conclude that GATA4 plays a pivotal role in the regulation of BTB function and lactate metabolism in mouse SCs.
Sertoli cells (SCs) provide a microenvironment that facilitates spermatogenesis, the maturation of germ cells within the seminiferous tubules. A key component of this microenvironment is the blood-testis barrier (BTB), a dynamic structure composed of tight junctions, adherens junctions, gap junctions, and other protein complexes that link adjacent SCs (1, 2). The BTB partitions the seminiferous epithelium into 2 distinct milieus: 1) a basal compartment that is in contact with the systemic circulation and harbors spermatogonial stem cells (SSCs) plus spermatogonia, and 2) an apical compartment that is isolated from the systemic circulation and contains meiotic and postmeiotic germ cells (2, 3). The BTB undergoes remodeling to permit the passage of differentiating germ cells from the basal to apical compartment (1, 4). SC-derived extracellular matrix (ECM) proteins regulate junction dynamics during spermatogenesis (5). These proteins act in concert with proteases, protease inhibitors, focal adhesion proteins, and cytokines to regulate cell-cell interactions and maintain functional barrier integrity (5).
In addition to providing a structural framework for spermatogenesis, SCs afford trophic support for germ cell development. SCs secrete growth factors and chemokines that promote SSC self-renewal and maintenance (6–9). SCs also regulate the flow of essential nutrients to germ cells in the apical compartment (10). Whereas spermatogonia use glucose as a fuel for ATP production, more developed germ cells, such as spermatocytes and spermatids, rely on SC-derived lactate as an energy source (11). To ensure adequate lactate production, SCs adopt a metabolic profile typical of cancer cells, the so-called Warburg phenotype, wherein most pyruvate generated through glycolysis is converted to lactate rather than being oxidized via the tricarboxylic acid (TCA) cycle (10, 12–15). SC lactate production is augmented further through catabolism of certain amino acids, notably glutamine (10). For their own energy needs, SCs rely on ATP derived from the β-oxidation of fatty acids (15, 16).
Studies of genetically engineered mice have implicated GATA4, a transcription factor expressed in SCs and other testicular somatic cells, in the structural and trophic support of spermatogenesis (17–19). Conditional ablation of Gata4 at embryonic day 10.5 with Wt1-creERT2 impairs SC differentiation and causes male-to-female sex reversal, whereas deletion of Gata4 at embryonic day 12.5 using Sf1-cre leads to testis cord defects and decreased expression of another sex determination gene, Dmrt1, in SCs (20). Ablation of Gata4 in fetal and neonatal SCs using Amh-cre disrupts the SSC niche and triggers germ cell depletion by impairing chemokine signaling (9). The gradual deletion of Gata4 in the SCs of adult mice using Amhr2-cre leads to increased permeability of the BTB, selective loss of late stage (haploid) germ cells, and late-onset testicular atrophy with loss of fertility (19).
Although mutant mouse studies provide compelling evidence that GATA4 regulates SC development and function, the molecular pathways involved are not well understood, particularly in SCs of the adult. This is due in part to the inherent challenges of interpreting conditional knockout studies in the mouse testis. As reviewed elsewhere (17), cellular heterogeneity, compensatory responses, and other factors confound the analysis of such experiments. To circumvent these limitations, we have assessed the impact of GATA4 deficiency on SC function in less complicated experimental systems: a mouse SC line (TM4) and primary cultures of adult mouse SCs (pSCs). Using complementary methods, including transcriptomic and metabolomic analyses, we show that Gata4 silencing disrupts specific aspects of SC function, notably BTB maintenance and lactate metabolism.
Materials and Methods
Animals and cultured cells
Experiments involving mice were approved by the Animal Studies Committee at Washington University. Gata4flox/flox mice (also termed Gata4tm1.1Sad/J) (21, 22) were purchased from The Jackson Laboratory. pSCs were isolated from 3- to 6-month-old Gata4flox/flox or wild-type (WT) 129.B6 mice using Percoll density separation (23) and maintained in DMEM/F12+GlutaMAX media supplemented with 10% fetal bovine serum, 25mM HEPES, and 100-mg/L penicillin/streptomycin (all from Life Technologies). Preparations of pSCs were determined to be 90%–95% pure on the basis of immunostaining for the SC marker reproductive homeobox 5 and the Leydig cell marker 3β-HSD (24). Mouse TM4 cells (25) were cultured in DMEM/F12+GlutaMAX media supplemented with 10% fetal bovine serum, 25mM HEPES, and 100-mg/L penicillin/streptomycin.
Knockdown of Gata4 in TM4 cells and primary adult SCs
TM4 cells (passages 12–18) and WT pSCs were transiently transfected in the absence of antibiotics with a pool of 4 small interfering RNA (siRNA) targeting Gata4 (5′-AGAGAAUAGCUUCGAACCA-3′, 5′-GGAUAUGGGUGUUCCGGGU-3′, 5′-CUGAAUAAAUCUAAGACGC-3′, 5′-GGACAUAAUCACCGCGUAA-3′) or with nontargeting control siRNA (5′-UGGUUUACAUGUCGACUAA-3′) (all from Dharmacon) using Lipofectamine RNAiMAX transfection reagent in Opti-MEM (Life Technologies) at a final concentration of 0.1μM. Conditioned media and cells were collected 72 hours after transfection for the analyses described below. pSCs from Gata4flox/flox mice were cultured in the presence of adenovirus (multiplicity of infection = 100) expressing either green fluorescent protein (Ad-GFP) or the combination of cre recombinase and GFP (Ad-cre-IRES-GFP) (Vector Biolabs). After infection, the cells were maintained in serum-free DMEM/F12+GlutaMAX (Life Technologies) for 24 hours before RNA extraction.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated using the Nucleospin RNA/Protein kit (Machrey-Nagel) and reverse transcribed using SuperScript VILO cDNA Synthesis kit (Life Technologies). qRT-PCR was performed using SYBR GREEN I (Invitrogen), and expression was normalized to the housekeeping genes Actb and L19. Primer pairs are listed in Supplemental Table 1.
Western blotting
Protein was extracted from cell cultures with the NucleoSpin RNA/Protein kit (Machrey-Nagel), and 20 μg of protein was separated by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Invitrogen). A list of antibodies used is provided in Table 1. The Immun-Star WesternC kit (Bio-Rad) was used for detection. Quantity One 1-D Analysis Software was used to determine quantitative protein signals.
Table 1.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| C terminus of actin of human origin | Actin antibody (I-19) | Santa Cruz Biotechnology, Inc, sc-1616 | Goat; polyclonal | 1:5000 (WB) |
| C terminus of GATA-4 of mouse origin | GATA4 antibody (C-10) | Santa Cruz Biotechnology, Inc, sc-1237 | Goat; polyclonal | 1:1000 (WB); 1:200 (IF) |
| Amino acids 1437-1736 mapping at the C terminus of ZO-1 of human origin | ZO-1 (TJP1) antibody (H-300) | Santa Cruz Biotechnology, Inc, sc-10804 | Rabbit; polyclonal | 1:1000 (WB); 1:200 (IF); used for TM4 staining |
| ZO-1 (TJP1) antibody (H-300) | Thermo Fisher, 40-2200 | Rabbit; polyclonal | 1:200 (IF); used for pSC staining | |
| Amino acids 231-373 mapping at the C terminus of 3β-HSD type I of human origin | 3β-HSD antibody (H-143) | Santa Cruz Biotechnology, Inc, sc-28206 | Rabbit; polyclonal | 1:200 (ICH)a |
| C terminus of Pem of mouse origin | Pem antibody (M-15) | Santa Cruz Biotechnology, Inc, sc-21650 | Goat; polyclonal | 1:200 (ICH)a |
| Goat IgG | Donkey antigoat IgG-HRP secondary antibody | Santa Cruz Biotechnology, Inc, sc-2020 | Donkey; polyclonal | 1:10 000 (WB) |
| Rabbit IgG | Goat antirabbit IgG-HRP secondary antibody | Santa Cruz Biotechnology, Inc, sc-2004 | Goat; polyclonal | 1:1000 (WB) |
| Goat IgG | Alexa Fluor 594 donkey α-goat IgG secondary antibody | Life Technologies, A-11058 | Donkey; polyclonal | 1:500 (IF) |
| Rabbit IgG | Alexa Fluor 488 donkey α-rabbit IgG secondary antibody | Life Technologies, A-21206 | Donkey; polyclonal | 1:200 (TM4 IF) and 1:1000 (pSCs IF) |
ZO-1, zona occludens-1.
To check primary cell isolation purity.
Immunocytochemistry and immunofluorescence
TM4 cells and pSCs were grown on 4-well glass Lab Tek Chamber Slides (Sigma) and fixed 72 hours after transfection or 48 hours after infection with 4% paraformaldehyde (PFA) in PBS. Immunoperoxidase and indirect immunofluorescent staining were performed as described (26). See Table 1 for a list of antibodies.
Transmission electron microscopy (EM)
TM4 cells were grown on 4-well Permanox Matrigel-coated chamber slides (Sigma) and fixed 72 hours after transfection with modified Karnovsky fixative (2.5% glutaraldehyde and 2% PFA in 0.1M cacodylate buffer) for 1 hour. Samples were postfixed in 2% OsO4 in 0.1M sodium cacodylate buffer for 1 hour. The samples were then dehydrated and embedded in epon. Thick sections (1 μm) were stained with toluidine blue and examined by light microscopy to determine which blocks were to be thin sectioned (90 nm). Thin sections were stained with uranyl acetate and lead citrate and examined by EM using a Model 1400EX EM (JEOL).
Microarray expression profiling and gene set enrichment analysis
RNA was isolated from TM4 cells 72 hours after transfection with Gata4 or nontargeting siRNA (n = 3) using NucleoSpin RNA/Protein kit and purified with NucleoSpin RNA Clean-up XS kit (Machrey-Nagel). RNA quality was assessed via Bioanalyzer (Agilent). Array hybridization was performed by the Functional Genomics Unit at the University of Helsinki using an Illumina MouseWG-6 v2.0 oligonucleotide BeadChip. Data was background corrected using BeadStudio software (Illumina); quantile normalization and log2 transformation were performed using the BeadArray bioconductor package (27). Differentially expressed genes were identified using LIMMA (linear models for microarray data) (28) with Benjamini-Hochberg correction. Expression levels with at least 1.5× difference and a false discovery rate (FDR) below 5% were considered as significantly differentially expressed. Microarray data was subjected to average linkage clustering with uncentered correlation using Cluster (29). Gene set enrichment analysis of the differentially expressed genes was performed using GOstats bioconductor package (30). Hypergeometric tests with the Benjamini-Hochberg FDR were performed to adjust the P value.
Transepithelial resistance (TER) measurements
To assess barrier integrity, TM4 cells, Gata4flox/flox pSCs, and WT pSCs were treated either with siRNA or adenovirus, as described above, and then plated at a density of 0.5 × 106 cells/cm2 (TM4) or 1.2 × 106 cells/cm2 (Gata4flox/flox and WT pSCs) on Matrigel-coated bicameral culture units (Merck Millipore) (31). Cells were incubated in a humidified CO2 incubator at 37°C, and TER was measured every 12 hours using the Millicell Electrical Resistance System with Ag/AgCl electrodes as described (31).
Cell viability assay
Cell viability was assayed using CellTiter 96 Aqueous One Solution (Promega) at 24, 48, and 72 hours after transfection. Absolute absorbance (490 nm) was normalized using values obtained from wells containing nontransfected TM4 cells. To control for cell number, TM4 cells, Gata4flox/flox pSCs, and WT pSCs that had been subjected to Gata4 gene silencing were trypsinized and counted every 24 hours for 6 days using a hemocytometer.
Metabolomic profiling
Metabolites were extracted from cell samples (n = 4), separated using Acquity UPLC, and analyzed using XEVO TQ-S Triple Quadrupole liquid chromatography/mass spectrometry (Waters Corp). At 72 hours after transfection, approximately 2 million TM4 cells per sample were washed with PBS and deionized water and subsequently quenched in liquid nitrogen. Metabolites were extracted by adding 20 μL of labeled internal standard mix and 1 mL of cold extraction solvent (80/20 acetonitrile/H2O + 1% formic acid). Extracts were vacuum filtered (δ pressure 300–400 mbar for 2.5 min; Hamilton) and injected into the liquid chromatography system. A detailed description of instrument parameters is given elsewhere (see reference 42 below). A total of 110 metabolite concentrations were measured, and data were normalized and analyzed using Metaboanalyst 3.0 software.
Quantification of glucose, lactate, and ammonium concentrations in conditioned media
Conditioned cell culture media were collected 72 hours after transfection (TM4 cells) or 48 hours after infection (Gata4flox/flox pSCs), and glucose, lactate, and ammonium concentrations were measured with Konelab Arena 20 XT (n = 4–7; Thermo Electron Oy) as described (32).
Lactate dehydrogenase (LDH) activity measurements
LDH activity in TM4 cells, Gata4flox/flox pSCs, and WT pSCs was determined using the Promega CytoTox96 assay following the manufacturer's instructions. The assay was calibrated with the positive control included in the kit, and measurements (absorbance at 492 nm) were normalized to the number of cells, with values expressed as fold variation relative to the control group.
Statistical methods
mRNA levels, absorbance values for viability assays, cell counts, luminescence intensities, and metabolite concentrations in conditioned media were analyzed using the Student's t test or when appropriate, one-way ANOVA followed by Dunnett's test. Statistical significance was set at *, P < .05 and **, P < .01.
Results
GATA4-depleted SCs exhibit dysregulation of genes involved the formation and remodeling of junctional complexes
We used siRNA to inhibit Gata4 expression in mouse TM4 cells, an immortalized cell line that retains many of the properties of endogenous SCs and is easier to maintain and manipulate in culture than pSCs (33). To determine the efficiency of gene silencing, RNA and protein were isolated from TM4 cells 72 hours after siRNA transfection. Gata4 mRNA levels were reduced by 78 ± 3% in cells treated with Gata4 siRNA vs nontargeting siRNA-treated cells (n = 4; P < .01) (Figure 1A). Western blotting demonstrated only a trace of residual GATA4 protein in the Gata4 siRNA-treated cells (Figure 1B), and immunocytochemistry confirmed markedly reduced GATA4 staining in the nuclei of the targeted cells (Figure 1, C and D).
Figure 1.
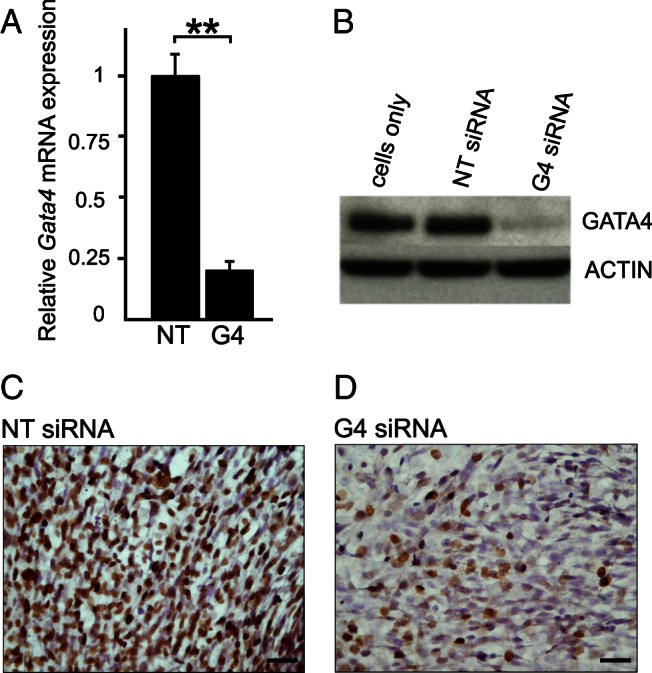
siRNA mediated inhibition of Gata4 expression in TM4 cells. Cells were treated with nontargeting (NT) siRNA or Gata4 (G4) siRNA for 72 hours (A–D). The efficiency of gene silencing was determined using qRT-PCR, Western blotting, and immunocytochemistry. A, qRT-PCR results, normalized to Actb and L19 mRNA, presented as mean relative expression values ± SD; **, P < .01, n = 4. B, Western blot analysis with actin antibody as a control. C and D, GATA4 immunoperoxidase staining of TM4 cells exposed to NT siRNA or G4 siRNA, respectively. Cells were counterstained with hematoxylin. Scale bars, 50 μm.
Microarray hybridization was used to assess the impact of Gata4 silencing on the TM4 transcriptome (complete results are available via GEO accession number GSE74471). A total of 2414 probes were differentially expressed (1230 up-regulated, 1184 down-regulated). Results were ranked according to their log2 fold change values. Unsupervised hierarchical clustering of the top 50 differentially expressed probes is shown in Figure 2. To identify biological processes affected by inhibition of Gata4 in TM4 cells, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) analysis with all differentially expressed probes (Table 2). Among the terms identified in the KEGG pathway and GO analyses were processes linked to BTB function, such as focal adhesion, ECM receptor interaction, adherens junctions, and extracellular structure organization (1, 4, 5).
Figure 2.
Gene expression profile of GATA4-deficient TM4 cells. Microarray analysis (n = 3 per group) was performed using the Illumina Mouse WG-6 v2.0 BeadChip. After background correction, quantile normalization, and log2 transformation, differentially expressed genes were identified using LIMMA with the Benjamini-Hochberg correction. Only probes with expression levels with at least 1.5-fold difference and a FDR below 5% were considered significantly differentially expressed. A heatmap showing the top 50 DEGs (sorted according to their log2 fold changes) was generated with R. Red represents down-regulation and green signifies up-regulation of the particular probes.
Table 2.
Gene Set Enrichment Analysis of Microarray Data
| Analysis | Term | Size | Number of Genes | P Value |
|---|---|---|---|---|
| KEGG pathway | Focal adhesion | 200 | 42 | 2.20E-06 |
| Metabolic pathways | 1184 | 163 | 3.70E-06 | |
| ECM-receptor interaction | 86 | 19 | 7.10E-04 | |
| D-glutamine and D-glutamate metabolism | 3 | 2 | 2.82E-02 | |
| Adherens junction | 75 | 13 | 3.50E-02 | |
| Glycolysis/gluconeogenesis | 62 | 11 | 4.27E-02 | |
| GO | Cadherin binding | 23 | 2 | 4.00E-03 |
| Extracellular structure organization | 161 | 4 | 4.60E-03 | |
| L-lactate-dehydrogenase activity | 3 | 1 | 1.20E-02 | |
| Lactate metabolic process | 7 | 1 | 2.90E-02 | |
| Protein localization to extracellular region | 3 | 1 | 1.20E-02 | |
| Extracellular region | 1813 | 14 | 1.20E-02 | |
| Proteinaceous ECM | 333 | 5 | 1.10E-02 |
KEGG GO pathway analysis results are arranged on the basis of P values. Size describes the overall number of genes related to 1 specific term, and number of genes represents the number of genes within this group that were significantly changed in microarray analysis (n = 3).
To confirm the microarray results, qRT-PCR analysis was performed on RNA isolated from TM4 cells transfected with Gata4 siRNA or nontargeting siRNA. As further validation, we assessed the impact of Gata4 inhibition on pSCs from adult mice. Gata4 expression in pSCs was inhibited either via siRNA or through cre-mediated recombination. For the latter approach, SCs isolated from Gata4flox/flox mice were infected in vitro with the cre-expressing adenoviral vector Ad-cre-IRES-GFP or the control vector Ad-GFP. Based on GFP expression, the infection efficiency of the adrenoviral vectors was determined to be more than 90%. qRT-PCR analysis showed that infection of Gata4flox/flox pSCs with Ad-cre-IRES-GFP vs Ad-GFP resulted in 58 ± 10% inhibition of Gata4 at 48 hours after infection (n = 4; P < .01) (Supplemental Figure 1A). Treatment of WT pSCs with Gata4 siRNA resulted in 81 ± 6% inhibition of Gata4 (n = 4; P < .01) (Supplemental Figure 1A). The loss of GATA4 protein in cre-recombined or siRNA-treated pSCs was confirmed by Western blotting (Supplemental Figure 1B). As shown in Figure 3, the changes in gene expression observed in response to deletion of Gata4 in Gata4flox/flox pSCs were strikingly similar to those seen in Gata4 siRNA-treated TM4 cells, implying that TM4 cells are a reasonable model to study the consequences of GATA4 deficiency on SC function.
Figure 3.
Impact of Gata4 silencing on gene expression in TM4 cells and primary mouse SCs. Each panel shows the relative mRNA expression results for a specific gene, as determined by 3 different methods: (method 1, array) microarray analysis of mRNA derived from TM4 cells 72 hours after siRNA treatment with nontargeting (NT) or Gata4 (G4) siRNA (n = 3); (method 2, TM4) qRT-PCR analysis of mRNA derived from TM4 cells 72 hours after siRNA treatment with NT or G4 siRNA (n = 4); and (method 3, pSC) qRT-PCR analysis of mRNA derived from primary Gata4flox/flox SCs (Gata4F/F pSC) 48 hours after infection with adenovirus expressing either cre + GFP (Cre) or GFP alone (GFP) (n = 4). Microarray results are presented as relative fold changes in mRNA expression. qRT-PCR results, normalized to Actb and ribosomal protein L19 mRNA, are presented as relative expression values of the mean ± SD; **, P < .01; *, P < .05. Tight junction associated genes: Tjp1 (A) and Cldn12 (B). AES associated genes: Vcl (C), Tnc (D), and Csk (E). Gap junction associated genes: Cx30.2 (F). Components of ECM: Lamc1 (G), Col4a1 (H), and Col4a5 (I). Metalloproteinases and protease inhibitors: Mmp10 (J), Mmp23 (K), and Timp2 (L). Cytokine and chemokine signaling: Tnf (M), C-X-C motif chemokine 1 (Cxcl1) (N), C-X-C motif chemokine 12 (Cxcl12) (O), C-C motif ligand 9 (Ccl9) (P), and C-C motif ligand 25 (Ccl25) (Q). Transcription factors: Sox9 (R), Kruppel-like factor 4 (Klf4) (S), and Rhox5 (T). Note that Cx30.2, Tnf, and Rhox5 were not represented on the microarray. N.A., not available.
Notably, GATA4 depletion in TM4 cells and Gata4flox/flox pSCs was associated with dysregulation of genes involved the formation and remodeling of junctional complexes. Silencing of Gata4 was accompanied by decreased expression of 2 tight junction genes: tight junction protein-1 (Tjp1) and claudin-12 (Cldn12) (Figure 3, A and B). GATA4 depletion also led to aberrant expression of vinculin (Vcl), tenascin C (Tnc), and c-src tyrosine kinase (Csk) (Figure 3, C–E), genes involved in formation of the apical ectoplasmic specialization (AES), a distinct actin-based adherens junction restricted to the Sertoli-spermatid interface (1, 34–36). Although not detected in the microarray, connexin 30.2 (Cx30.2), a component of gap junctions, was down-regulated in GATA4-deficient TM4 cells and pSCs (Figure 3F). GATA4 depletion was associated with altered expression of genes encoding ECM proteins, proteases, and protease inhibitors implicated in the regulation of BTB remodeling and integrity. Among these were laminin 1 (Lamc1), type IV collagen α1 (Col4a1) and (Col4a5), matrix metalloproteinase 10 (Mmp10), matrix metalloproteinase 23 (Mmp23), and tissue inhibitor of metalloproteinases 2 (Timp2) (Figure 3, G–L). The gene encoding TNF, a cytokine released when ECM proteins are degraded (37), was up-regulated in GATA4-deficient TM4 cells and SCs (Figure 3M). Collectively, these findings implicate GATA4 in the regulation of genes involved in BTB dynamics.
As further confirmation of our gene silencing models, we used qRT-PCR to examine the expression of certain other markers, including established targets of GATA4. In agreement with a recent report linking GATA4 to chemokine signaling in SCs (9), we found that silencing Gata4 in TM4 cells and primary adult SCs led to decreased expression of the chemokines C-X-C motif chemokine 12 (Cxcl12) and C-C motif ligand 9 (Ccl9) (Figure 3, N and O). Additionally, the mRNA levels of C-C motif ligand 25 (Ccl25) and C-X-C motif chemokine 1 (Cxcl1) were altered after GATA4 depletion (Figure 3, P and Q). Silencing of Gata4 in TM4 cells and pSCs altered the expression of sex determining region Y-box 9 (Sox9) (Figure 3R), a transcription factor known to be regulated by GATA4/friend of GATA 2 (17). Krüppel family like protein 4 (Klf4), a transcription factor previously linked to claudin gene expression (19, 38), was also dysregulated in the knockdown cells (Figure 3S). Expression of another transcription factor gene, reproductive homeobox X-linked protein 5 (Rhox5), an established SC marker (39), was not altered by GATA4 depletion in either TM4 cells or pSCs (Figure 3T).
Decreased TJP1 protein levels in GATA4-deficient SCs
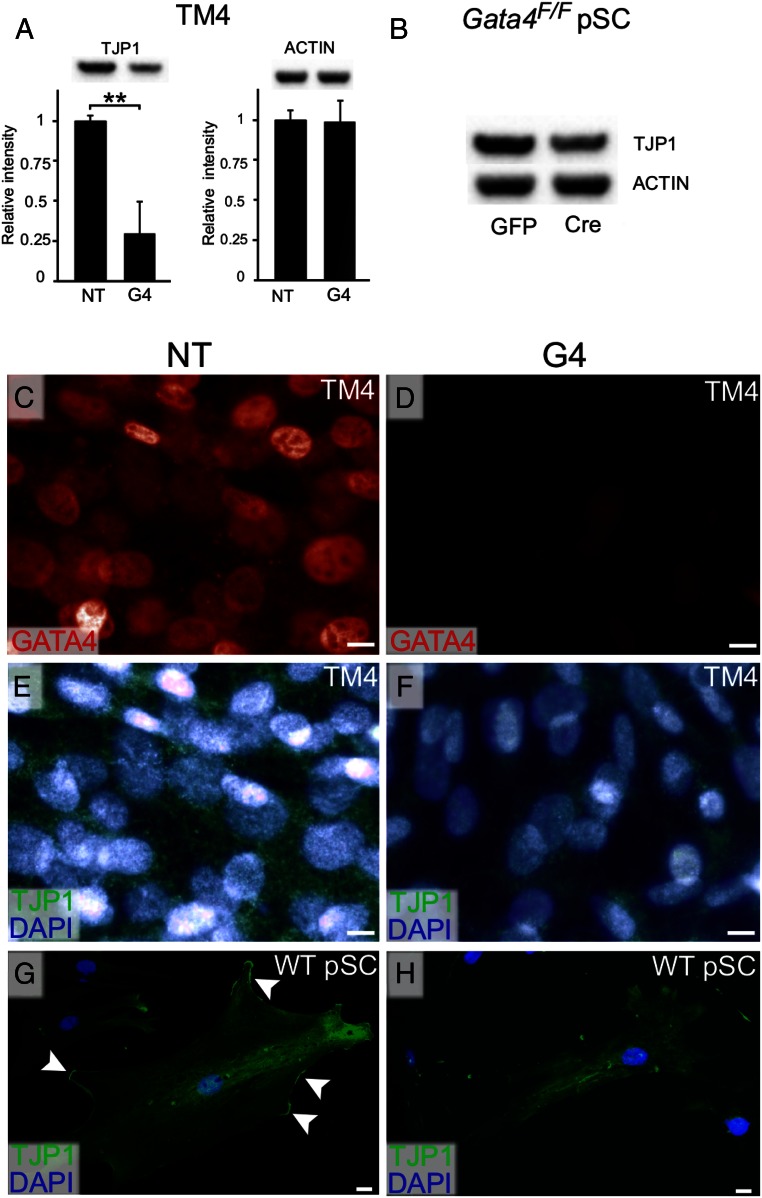
Spurred by the results of the transcriptomic analysis, we investigated the expression of Tjp1, a prototypical tight junction marker (40), in more detail. TM4 cells were transfected with Gata4 siRNA or nontargeting siRNA and analyzed 72 hours later. Western blotting (Figure 4A) demonstrated a significant decrease (68 ± 23%) in the level of TJP1 protein in the GATA4-depleted cells (n = 4; P < .01), whereas the level of the housekeeping protein actin was unchanged. Using the same method, a slight reduction in TJP1 protein was also observed in Gata4flox/flox pSCs treated with cre-recombinase when compared with controls (Figure 4B). Immunofluorescence staining showed a concomitant decrease in GATA4 and TJP1 immunoreactivity in TM4 cells treated with Gata4 siRNA vs nontargeting siRNA (Figure 4, C–F). Similarly, immunostaining of pSCs showed a loss of TJP1 protein from the cell surface in response to Gata4 silencing (Figure 4, G and H).
Figure 4.
Reduced TJP1 protein levels in GATA4-depleted SCs. Cells were treated with either siRNA for 72 hours (A and C–H) or adenovirus for 48 hours (B) and grown in the absence of Matrigel. A and B, Western blot analysis of TJP1 protein levels with ACTIN antibody as control. Quantitative protein signal was determined using Quantity One 1-D Analysis Software (Bio-Rad). Data are presented as mean relative expression values ± SD; **, P < .01, n = 4. C–H, TM4 cells and WT pSC were fixed in 4% PFA and subjected to double immunofluorescence staining. GATA4 (red) and TJP1 (green); DAPI (blue) was used as nuclear stain. Note the disappearance of TJP1 protein from the cell surface after GATA4 loss in WT pSCs. Scale bars, 2 μm.
GATA4-depleted SCs cells exhibit impaired junctional complex formation and barrier function
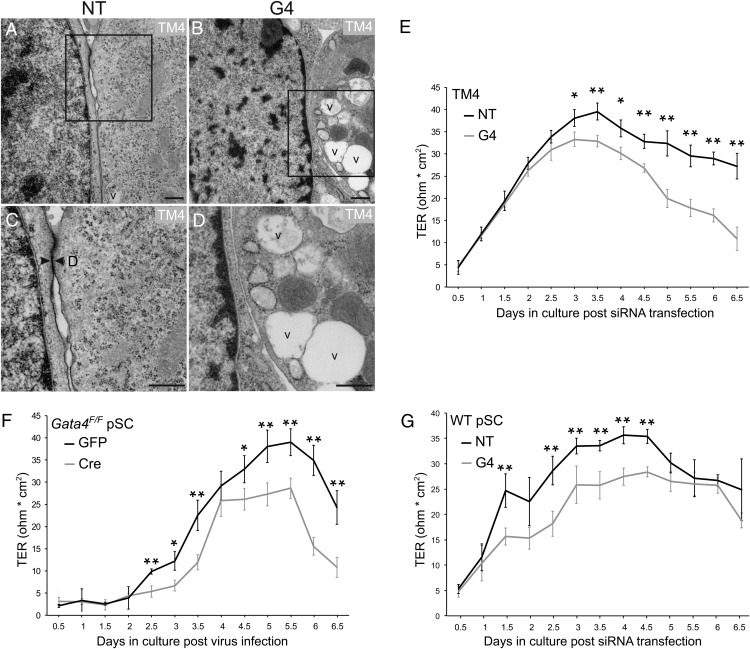
To further probe the role of GATA4 in the formation of junctional complexes, TM4 cells were cultured on Matrigel-coated slides, treated with Gata4 siRNA or nontargeting siRNA, and then processed for EM (Figure 5, A–D). Junctional complexes with the ultrastructural hallmarks of desmosomes (Figure 5C, arrowheads) were detected readily in cells treated with nontargeting siRNA but not in cells treated with Gata4 siRNA (Figure 5D). The GATA4-depleted TM4 cells contained increased number of vacuoles (Figure 5, B and D), a phenotypic feature previously reported in the SCs of Gata4 conditional knockout mice generated with Amhr2-cre (9, 19).
Figure 5.
Decreased junctional complexes and epithelial barrier resistance in GATA4-depleted SCs. A–D, Shown are representative electron micrographs of TM4 cells grown on Matrigel-coated chamber slides. Cells were transfected with Gata4 siRNA (G4) or nontargeting (NT) siRNA and then processed for EM 72 hours later. Higher magnification views of the boxed areas in panels A and B are shown in C and D, respectively. Note the presence of large vacuoles in the GATA4-deficient cells (B and D). Even though 2 adjacent cells are aligned over a long distance and their membranes are juxtaposed, junctional complexes with ultrastructural hallmarks of desmosomes are absent in GATA4-depleted cells (B and D). No morphological features of apoptosis, such as homogenous chromatin condensation within nuclei, were evident after GATA4 depletion. D, desmosome; v, vacuole. Scale bars, 500 nm. E–G, Indicated cells were either transfected with siRNA or infected with adenovirus (gray line, Gata4 knockdown cells; black line, control cells) and cultured at a density of 0.5 × 106 cells/cm2 (TM4) or 1.2 × 106 cells/cm2 (Gata4F/F and WT pSC) in Matrigel-coated bicameral culture units. The establishment of a tight junction permeability barrier was assessed by measurement of TER. Values are expressed as the mean ± SD; *, P < .05; **, P < .01; n = 3.
To assess the consequences of GATA4 deficiency on epithelial barrier function, we measured TER, an indicator of the paracellular barrier to ion conductance (31). For this analysis TM4 cells, WT pSCs, or Gata4flox/flox pSCs were grown as monolayers on Matrigel-coated bicameral units and treated with siRNA or adenovirus (Figure 5, E–G). Beginning 3 days after siRNA transfection, a significantly lower TER was observed in Gata4 siRNA-treated TM4 cells than in TM4 cells treated with nontargeting siRNA (Figure 5E). Gata4 silencing in pSCs was associated with a significantly lower TER at even earlier time points, ie, at day 2.5 in Gata4flox/flox pSCs subjected to adrenoviral-mediated cre-recombination (Figure 5F) or day 1.5 in WT pSC treated with siRNA (Figure 5G).
In theory, changes in cell viability or number could account for the observed differences in TER. We found that cell viability, measured with an MTS-based assay, was not significantly altered in either Gata4 siRNA-treated TM4 cells (Supplemental Figure 2A) or GATA4-deficient Gata4flox/flox pSCs subjected to cre-mediated recombination (Supplemental Figure 2B). Gata4 silencing led to significantly reduced cell numbers only after day 5 (TM4 cells) or day 6 (Gata4flox/flox pSCs and WT pSCs) (Supplemental Figure 2, C–E). Thus, the early (day 1–4) differences in TER between GATA4-depleted SCs and controls cannot be attributed to reduced cell viability or number.
GATA4-depleted SCs exhibit aberrant expression of genes involved in lactate metabolism
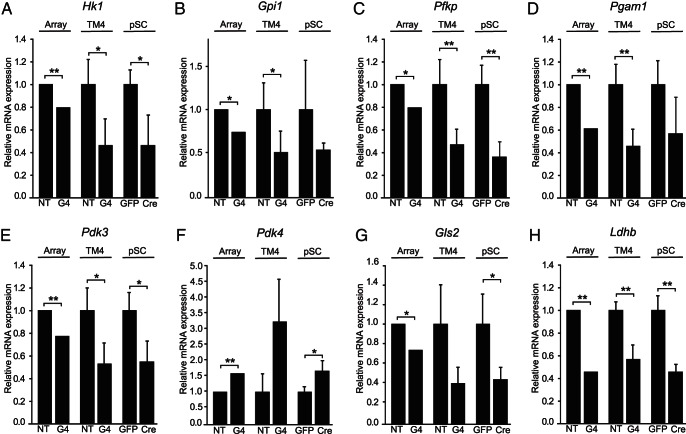
The production of lactate via glycolysis and glutaminolysis, to fulfill the energy needs of developing spermatocytes and spermatids, is a crucial function of SCs (11). KEGG pathway and GO analyses of GATA4-depleted TM4 cells identified significant changes in pathways controlling lactate and glutamine metabolism (glycolysis, L-lactate-dehydrogenase activity, lactate metabolic process, as well as D-glutamine and D-glutamate metabolism) (Table 2). Subsequent qRT-PCR analysis of GATA4-depleted TM4 and Gata4flox/flox pSCs confirmed the down-regulation of glycolytic enzymes hexokinase 1 (Hk1), glucose phosphate isomerase 1 (Gpi1), phosphofructokinase (Pfkp), and phosphoglycerate mutase 1 (Pgam1) (Figure 6, A–D).
Figure 6.
Altered expression of genes impacting lactate metabolism in GATA4-depleted SCs. A–H, Each panel shows the relative mRNA expression results for a specific gene, as determined by 3 different methods: (method 1, array) microarray analysis of mRNA derived from TM4 cells 72 hours after siRNA treatment with nontargeting (NT) or Gata4 (G4) siRNA (n = 3); (method 2, TM4) qRT-PCR analysis of mRNA derived from TM4 cells 72 hours after siRNA treatment with NT or G4 siRNA (n = 4); and (method 3, pSC) qRT-PCR analysis of mRNA derived from primary Gata4flox/flox SCs (Gata4F/F pSC) 48 hours after infection with adenovirus expressing either cre + GFP (Cre) or GFP alone (GFP) (n = 4). Microarray results are presented as relative fold changes in mRNA expression. qRT-PCR results, normalized to Actb and ribosomal protein L19 mRNA, are presented as relative expression values of the mean ± SD; **, P < .01; *, P < .05. A Hk1; B, Gpi1; C, Pfkp; D, Pgam1; E, Pdk3; F, Pdk4; G, Gls2; H, Ldhb.
Two other genes of importance in SC lactate metabolism, pyruvate dehydrogenase kinase (PDK), isoenzyme 3 (Pdk3) and Pdk4, encode kinases that modulate the activity of pyruvate dehydrogenase complex (PDC), determining whether pyruvate is converted to lactate or alternatively oxidized via the TCA cycle (41–43). In SCs, the FSH-induced up-regulation of Pdk3 with concomitant down-regulation of PDK, isoenzyme 4 (Pdk4) was recently shown to increase lactate production (44). Interestingly, depletion of GATA4 in TM4 and Gata4flox/flox pSCs elicited the reciprocal changes of decreased Pdk3 and increased Pdk4 expression (Figure 6, E and F). Glutaminase 2 (Gls2), which catalyzes the first step in the conversion of glutamine to lactate via glutaminolysis, was down-regulated in Gata4 siRNA-treated TM4 and Gata4flox/flox pSCs (Figure 6G). In addition, altered expression of LDH B (Ldhb) was also evident in the GATA4-deficient cells (Figure 6H). Taken together, the above mentioned changes in gene expression suggest that GATA4 influences the production of lactate.
Metabolomic analysis of GATA4-depleted TM4 cells demonstrates impaired lactate production
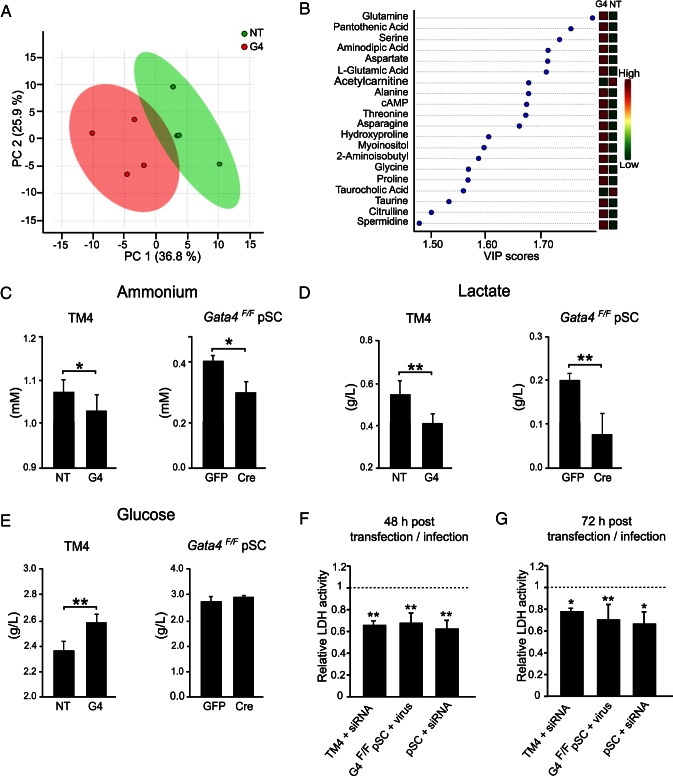
The physiological consequences of treatment of TM4 cells with Gata4 siRNA vs nontargeting siRNA were determined by comprehensive metabolomic profiling using liquid chromatography tandem mass spectrometry. Unsupervised principle component analysis demonstrated a separation of the sample set into 2 groups based on the metabolic profiles (Figure 7A), thus confirming that the metabolic profile of GATA4-depleted TM4 cells differed from that of control cells.
Figure 7.
Altered metabolic profile and lactate metabolism in GATA4-depleted SCs. A and B, For metabolic profiling of TM4 cells a total of 110 metabolites were analyzed 72 hours after siRNA transfection using mass spectrometry (n = 4). A, Principle component (PC) analysis revealed the unsupervised separation between of the 2 sample groups (Gata4 [G4] siRNA vs nontargeting [NT] siRNA treated) based on differences in their metabolic profiles. B, Partial least squares discriminant analysis identified the 20 most significantly changed metabolites and their relative abundance in control and GATA4-depleted cells. The components were sorted according to the variable of importance in the projection (VIP) for the first component. Relative magnitudes of each metabolite disturbance are listed on the right as high (red) and low (green). C–E, Concentrations of ammonium, lactate, and glucose in conditioned media from TM4 cells and Gata4flox/flox pSCs (Gata4F/F pSC) were quantified with Konelab Arena 20 XT analyzer (n = 4–7) 72 hours after siRNA transfection or 48 hours after infection with adenovirus, respectively. F and G, 48 and 72 hours after transfection or infection cells of all 3 Gata4 knockdown, experiments were trypsinized and counted; 5000 cells of each sample were lysed and LDH activity was determined. Results are presented as fold variation to control. C–G, Values are expressed as the mean ± SD; *, P < .05; **, P < .01.
To identify the most significantly changed metabolites, we performed a partial least squares discriminant analysis followed by quantitative enrichment analysis. The top 20 changed metabolites are presented in Figure 7B. A heatmap of all the analyzed metabolites and a list of the pathways identified by quantitative enrichment analysis are shown in Supplemental Figure 3 and 4, respectively. The metabolite exhibiting the largest change was glutamine, which was enriched 2.3-fold in the GATA4-depleted TM4 cells (Figure 7B), suggesting an impaired conversion of this amino acid to lactate via glutaminolysis (10). Glutamine is known to inhibit the incorporation of alanine into protein (45), and indeed alanine levels were increased in the GATA4-deficient cells (Figure 7B). Gata4 silencing also led to increased levels of several other amino acids (serine, glycine, threonine, asparagine, and aspartate) that, like alanine, normally function as alternative fuel sources in SCs (Figure 7B) (10, 45). The concentration of the metabolic waste product ammonium, a byproduct of glutaminolysis, was lower in conditioned media from GATA4 siRNA-treated TM4 cells and cre-treated Gata4flox/flox pSCs when compared with controls (Figure 7C).
In keeping with the aforementioned metabolic changes, the concentration of lactate was significantly lower in conditioned media from GATA4-deficient SCs as compared with controls (Figure 7D), whereas the concentration of glucose was significantly higher (TM4 cells) or unchanged (Gata4flox/flox pSCs) (Figure 7E).
Finally, LDH activity was measured in all 3 Gata4 knockdown cell preparations (Figure 7, F and G). Reduced enzyme activity was evident in cells lacking GATA4.
Collectively, these findings are indicative of both an overall reduced metabolic activity and significantly impaired lactate production in the GATA4-depleted cells. Thus, silencing of Gata4 in SCs cells was associated with a loss of the Warburg phenotype that normally typifies the metabolism of this cell type. Although there are known differences between the biology of immortalized SC cell lines and pSCs (46) the results obtained from the TM4 cell line and pSCs are strikingly similar, reinforcing the notion that TM4 cells are a useful model to study SC metabolism.
Discussion
Multiple lines of evidence support the notion that GATA4 plays a key role in the differentiation and function of SCs (17, 47). GATA4 is expressed in SCs throughout fetal and adult life (18, 48–56). Promoter analyses and related studies have identified groups of putative target genes for GATA4 in SCs, including genes involved in sex determination (Sry, Sox9, Dmrt1) (20, 57–60), FSH signaling (Fshr) (61, 62), cell-cell interactions (Clmp, Cldn11, Cx30.2) (19, 38, 63), and peptide hormone production (Inha, Inhba, Amh) (64). Studies of genetically-engineered mice have shown that GATA4 is required for early testicular development, germ cell licensing, maintenance of the SSC niche, and spermatogenesis (9, 19, 20, 58–60, 65, 66). Mouse fibroblasts can be efficiently reprogrammed into embryonic Sertoli-like cells using Gata4 in combination with Nr5a1, Wt1, Dmrt1, and Sox9 (67). Although genetic studies in the mouse provide strong evidence that GATA4 is essential for SC development and function, the molecular pathways regulated by this transcription factor have not been fully elucidated, especially in SCs of the adult animal. The results described herein provide new insights into the downstream targets of GATA4 in adult SCs. Specifically, our findings suggest that GATA4 plays a pivotal role in the regulation of BTB function and lactate metabolism in mouse SCs.
Silencing of Gata4 in TM4 cells and pSCs was associated with decreased expression of the tight junction genes Tjp1 and Cldn12. Tjp1 encodes phosphoprotein that localizes to the cytoplasmic membrane surface at sites of cell-cell contact (68), and Cldn12 encodes a transmembrane protein implicated in barrier function (69). The down-regulation of Tjp1 and Cldn12 in GATA4-depleted TM4 cells was accompanied by a loss of morphologically recognizable junctional complexes. TM4 cells and pSCs deficient for GATA4 further showed a decline in transepithelial membrane resistance. Previous studies have implicated GATA factors in the regulation of other genes important for BTB integrity, including the tight junction associated genes Cldn2 (70) and Cldn11 (63).
Cx30.2, encoding a gap junction protein hypothesized to mediate interactions between SCs and germ cells (71), was down-regulated in GATA4-deficient TM4 cells and pSCs. Cx30.2 is a known target of GATA4 in the heart (72), and decreased expression of Cx30.2 has been reported in whole testis extracts from conditional knockout mice lacking GATA4 in adult SCs (19).
GATA4 depletion in TM4 cells and pSCs also led to dysregulated expression of genes involved in the AES (Vcl, Tnc, Csk). One of the functions of the AES is to prevent the release of immature spermatozoa into the lumen of the seminiferous epithelium (34). Interestingly, mice harboring a conditional deletion of Gata4 in SCs exhibit premature release of spermatocytes and spermatids into the tubule lumen (19).
As reviewed in detail elsewhere (5), SC-derived ECM proteins have been shown to regulate tight junction remodeling during spermatogenesis, and genes encoding basement membrane components (Lamc1, Col4a1, Col4a5) were down-regulated in GATA4-depleted TM4 cells and pSCs. Providing independent evidence that GATA4 impacts the expression of ECM genes, decreased levels of laminin and type IV collagen have been reported in embryoid bodies derived from Gata4−/− embryonic stem cells (73).
Metalloproteinases, protease inhibitors, and cytokines are known to impact BTB integrity and remodeling (5, 74). Mmp10 was up-regulated in GATA4-deficient TM4 cells and pSCs, whereas Mmp23 and Timp2 were down-regulated. These findings are consistent with a previous report showing that tissue-remodeling genes, including Mmp23, are dysregulated in the ovaries of Gata4/6 double conditional knockout mice (75).
Taken together, these marked derangements in the expression profile of genes important for BTB integrity and SC-ECM interactions underline the crucial role of GATA4 for physiological SC function. One additional up-regulated factor in GATA4-depleted SCs is TNF, a cytokine released when ECM proteins are degraded (37). Earlier studies revealed that TNF perturbs SC tight junction formation in a dose-dependent manner and that Gata4 conditional knockout mice, suffering from a leaky BTB, have elevated TNF mRNA levels (19, 76). Interestingly, TNF is known to stimulate lactate production in SCs (77), suggesting a link between deranged ECM-SC interactions and lactate production in these cells.
The profound metabolic changes observed in GATA4-depleted TM4 cells are summarized in Figure 8, A and B, and are a probable attempt to maintain lactate production. Glutamine, the most elevated metabolite in GATA4-depleted TM4 cells, is one of the most important substrates for the production of lactate. Indeed, conversion of this amino acid to lactate via glutaminolysis has been reported to yield much of the energy required by SCs (13, 45). The decreased expression of Gls2 in GATA4-deficient TM4 cells and pSCs offers a plausible explanation for the elevation of intracellular glutamine levels. Glutamine is known to inhibit the incorporation of alanine during protein anabolism (45). This is of importance in SCs since alanine can be converted into pyruvate, thus serving as a substrate to maintain lactate production. GATA4-deficient TM4 cells accumulated other amino acids (serine, threonine, asparagine, glycine, proline, aspartate) normally used as alternative fuel sources to support lactate production (10). These changes are indicative of an overall reduced metabolic activity in TM4 cells lacking GATA4. Consistent with this notion of reduced metabolic activity, conditioned media from GATA4-depleted TM4 cells and pSCs contained decreased concentrations of lactate and the metabolic waste product ammonium.
Figure 8.
Impact of GATA4 depletion on lactate metabolism in SCs. Shown are metabolic pathways important for production of lactate, the principal source of energy for postmeiotic germ cells. A, Normal SCs exhibit a Warburg phenotype and use glucose and glutamine for the production of lactate. Key reactions in shunting glucose to lactate within SCs are catalyzed by LDH and PDC. PDC activity is inhibited through phosphorylation by PDK3 and PDK4. Glutaminase 2 (GLS2) converts glutamine into glutamate, which can be further metabolized to lactate via glutaminolysis. B, SCs lacking GATA4 no longer exhibit the Warburg-like state, as evidenced by changes in mRNA levels (italic letters) or metabolite concentrations. The most important alterations identified in this study are indicated with white (elevated) or black (decreased) arrows, and impaired catalytic steps after GATA4 depletion are highlighted in red.
The 3 major steps in shunting glucose to lactate within SCs are regulated by glycolytic enzymes (converting glucose to pyruvate), PDC (regulating the entry of pyruvate into the TCA cycle), and LDH (converting pyruvate to lactate) (Figure 8A). The glycolytic activity of GATA4-depleted SCs was attenuated as evidenced by reduced expression of key glycolytic genes (Hk1, Gpi1, Pfkp, Pgam1) and decreased glucose utilization. This is in accordance with our recent findings in Leydig cells, which exhibit diminished glycolytic activity in response to GATA4 depletion (24).
PDC activity is tightly regulated through a reversible phosphorylation/dephosphorylation mechanism (41). Phosphorylation of PDC by PDK isoforms leads to an inactive state of PDC, whereas dephosphorylation by pyruvate dehydrogenase phosphatase leads to an active state. Consequently, the relative activities of PDK and pyruvate dehydrogenase phosphatase determine the flux of pyruvate into the TCA cycle (42, 43). A recent study showed that FSH increases lactate production in rodent SCs by regulating this balance; after FSH exposure, Pdk3 mRNA levels increased, whereas Pdk4 mRNA levels decreased (44). We observed the opposite effect (↓Pdk3, ↑Pdk4) in GATA4-depleted pSCs and an attendant decrease in lactate production. Hence, Gata4 silencing in SCs mimics the phenotypic changes associated with FSH withdrawal. GATA4-deficient TM4 cells have elevated intracellular levels of succinate (Supplemental Figure 4), consistent with increased flow of pyruvate into the TCA cycle.
The final step of lactate production, the conversion of pyruvate to lactate (Figure 8), is catalyzed by LDH, a tetramer composed of LDH A and/or LDH B subunits. The random combination of these subunits into tetramers results in 5 LDH isoenzymes: LDH-1 (B4), LDH-2 (A1 B3), LDH-3 (A2 B2), LDH-4 (A3 B1), and LDH-5 (A4) (78). Recent reports suggest that LDH A may be the most important LDH subunit in SCs (15), although LDH B is also present in this cell type (78, 79). We observed reduced Ldhb expression accompanied with a decreased LDH activity in GATA4-depleted TM4 cells and pSCs.
SC-derived lactate is the principal energy source for mature germ cells, and lactate has a stimulating effect on RNA and protein synthesis in spermatids (80). Impaired lactate production in SCs can have profound effects on spermatogenesis. Concentrations of lactate are low in the testes of the cryptorchid rat, and intratesticular infusion of lactate into these animals improves spermatogenesis (81). In addition to serving as an energy source, lactate functions as an antiapoptotic factor in mature testicular germ cells (82). We surmise that the apoptosis of haploid germ cells seen in Gata4 conditional knockout mice generated with Amhr2-cre (19) reflects in part an insufficient supply of SC-derived lactate.
In summary, we propose that GATA4 functions physiologically to regulate the integrity of BTB and AESs and to maintain SCs in a Warburg-like state, thereby ensuring a robust supply of lactate for nourishment of germ cells. GATA4 exerts its metabolic effects by transmitting signals of the FSH pathway and regulating the expression and activity of key enzymes involved in lactate production. The concept that transcription factor GATA4 can govern intracellular metabolism was recently demonstrated in another testicular somatic cell type, the Leydig cell (24, 83, 84). Manipulation of Gata4 expression in SCs affords a means to study the Warburg effect in a noncancerous cell type. These findings come on the heels of a report demonstrating that GATA4 plays a central role in senescence; in a variety of cell types the DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4 (85). Thus, GATA4 has emerged as a pivotal regulator of a diverse array of biological processes, including stem cell maintenance, cell-cell interactions, intermediary metabolism, inflammation, and senescence.
Acknowledgments
We thank Dr Hélder A. Santos, Head of the Pharmaceutical Nanotechnology and Chemical Microsystems Research Unit, Faculty of Pharmacy, University of Helsinki, Finland for providing the equipment to measure TER. We also thank members of the EM core, the histology core, and Rebecca Cochran from the Washington University in St Louis, St Louis, MO for their expert assistance as wee as Dr René Handrick from the Institute of Applied Biotechnology, University of Applied Sciences Biberach, Germany for technical assistance.
This work was supported by the Sigrid Jusélius Foundation (M.H.), the Academy of Finland (M.H.), National Institutes of Health Grants DK52574 (to D.B.W.) and DK075618 (to D.B.W.), the American Heart Association Grant 13GRNT16850031 (to D.B.W.), the Department of Defense Grant PC141008 (to D.B.W.), the Jalmari and Rauha Ahokas Foundation (A.S.), the Biomedicum Helsinki Foundation (A.S.), Orion Pharma Foundation (A.S.), the Oskar Öflund Foundation (A.S.), Doctoral Programme in Biomedicine, University of Helsinki (A.S.), the Finnish Cultural Foundation Grant 00150083 (to O.A.), and the Ida Montin Foundation (O.A.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad-cre-IRES-GFP
- adenovirus expressing the combination of cre recombinase and GFP
- Ad-GFP
- adenovirus expressing green fluorescent protein
- AES
- apical ectoplasmic specialization
- BTB
- blood-testis barrier
- Cldn12
- claudin-12
- Col4a1
- type IV collagen α1
- Csk
- c-src tyrosine kinase
- Cx30.2
- connexin 30.2
- ECM
- extracellular matrix
- EM
- electron microscopy
- FDR
- false discovery rate
- Gls2
- glutaminase 2
- GO
- Gene Ontology
- Gpi1
- glucose phosphate isomerase 1
- Hk1
- hexokinase 1
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- Lamc1
- laminin 1
- LDH
- lactate dehydrogenase
- Mmp10
- matrix metalloproteinase 10
- Mmp23
- matrix metalloproteinase 23
- PDC
- pyruvate dehydrogenase complex
- PDK
- pyruvate dehydrogenase kinase
- Pdk3
- PDK, isoenzyme 3
- Pdk4
- PDK, isoenzyme 4
- PFA
- paraformaldehyde
- Pfkp
- phosphofructokinase
- Pgam1
- phosphoglycerate mutase 1
- pSC
- primary cultures of adult mouse SC
- qRT-PCR
- quantitative RT-PCR
- Rhox5
- reproductive homeobox X-linked protein 5
- SC
- Sertoli cell
- siRNA
- small interfering RNA
- Sox9
- sex determining region Y-box 9
- TCA
- tricarboxylic acid
- TER
- transepithelial resistance
- Timp2
- tissue inhibitor of metalloproteinases 2
- Tjp1
- tight junction protein-1
- Tnc
- tenascin C
- Vcl
- vinculin
- WT
- wild type.
References
- 1. Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. [DOI] [PubMed] [Google Scholar]
- 2. Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. [DOI] [PubMed] [Google Scholar]
- 3. Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. [DOI] [PubMed] [Google Scholar]
- 4. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. [DOI] [PubMed] [Google Scholar]
- 5. Siu MK, Cheng CY. Extracellular matrix and its role in spermatogenesis. Adv Exp Med Biol. 2008;636:74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng X, Lindahl M, Hyvönen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. [DOI] [PubMed] [Google Scholar]
- 7. Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J Cell Sci. 2013;126:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen SR, Tang JX, Cheng JM, et al. Loss of Gata4 in Sertoli cells impairs the spermatogonial stem cell niche and causes germ cell exhaustion by attenuating chemokine signaling. Oncotarget. 2015;6:37012–37027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveira PF, Martins AD, Moreira AC, Cheng CY, Alves MG. The Warburg effect revisited–lesson from the Sertoli cell. Med Res Rev. 2015;35:126–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab. 2004;15:345–350. [DOI] [PubMed] [Google Scholar]
- 12. Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. [DOI] [PubMed] [Google Scholar]
- 13. Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil. 1986;77:109–118. [DOI] [PubMed] [Google Scholar]
- 14. Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981;24:1032–1041. [DOI] [PubMed] [Google Scholar]
- 15. Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–338. [DOI] [PubMed] [Google Scholar]
- 16. Xiong W, Wang H, Wu H, Chen Y, Han D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction. 2009;137:469–479. [DOI] [PubMed] [Google Scholar]
- 17. Tevosian SG. Transgenic mouse models in the study of reproduction: insights into GATA protein function. Reproduction. 2014;148:R1–R14. [DOI] [PubMed] [Google Scholar]
- 18. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development. 1998;125:2665–2675. [DOI] [PubMed] [Google Scholar]
- 19. Kyrönlahti A, Euler R, Bielinska M, et al. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol. 2011;333:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol. 2011;353:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oka T, Maillet M, Watt AJ, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. [DOI] [PubMed] [Google Scholar]
- 22. Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA. 2004;101:12573–12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang YF, Lee-Chang JS, Panneerdoss S, MacLean JA, 2nd, Rao MK. Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. BioTechniques. 2011;51:341–342, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schrade A, Kyrönlahti A, Akinrinade O, et al. GATA4 is a key regulator of steroidogenesis and glycolysis in mouse Leydig cells. Endocrinology. 2015;156:1860–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rebois RV. Establishment of gonadotropin-responsive murine leydig tumor cell line. J Cell Biol. 1982;94:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krachulec J, Vetter M, Schrade A, et al. GATA4 is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology. 2012;153:2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunning MJ, Smith ML, Ritchie ME, Tavaré S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics. 2007;23:2183–2184. [DOI] [PubMed] [Google Scholar]
- 28. Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, eds, Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York, NY: Springer; 2005:397–420. [Google Scholar]
- 29. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. [DOI] [PubMed] [Google Scholar]
- 31. Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stojanović N, Rogić D, Stavljenić-Rukavina A. Evaluation of the Konelab 20XT clinical chemistry analyzer. Clin Chem Lab Med. 2005;43:646–653. [DOI] [PubMed] [Google Scholar]
- 33. Mather JP, Zhuang LZ, Perez-Infante V, Phillips DM. Culture of testicular cells in hormone-supplemented serum-free medium. Ann NY Acad Sci. 1982;383:44–68. [DOI] [PubMed] [Google Scholar]
- 34. Berruti G, Paiardi C. The dynamic of the apical ectoplasmic specialization between spermatids and Sertoli cells: the case of the small GTPase Rap1. Biomed Res Int. 2014;2014:635979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004;71:375–391. [DOI] [PubMed] [Google Scholar]
- 36. Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. [DOI] [PubMed] [Google Scholar]
- 37. Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. [DOI] [PubMed] [Google Scholar]
- 38. Sze KL, Lee WM, Lui WY. Expression of CLMP, a novel tight junction protein, is mediated via the interaction of GATA with the Kruppel family proteins, KLF4 and Sp1, in mouse TM4 Sertoli cells. J Cell Physiol. 2008;214:334–344. [DOI] [PubMed] [Google Scholar]
- 39. Hu Z, Dandekar D, O'Shaughnessy PJ, De Gendt K, Verhoeven G, Wilkinson MF. Androgen-induced Rhox homeobox genes modulate the expression of AR-regulated genes. Mol Endocrinol. 2010;24:60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chung SS, Lee WM, Cheng CY. Study on the formation of specialized inter-Sertoli cell junctions in vitro. J Cell Physiol. 1999;181:258–272. [DOI] [PubMed] [Google Scholar]
- 41. Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. [DOI] [PubMed] [Google Scholar]
- 42. Kolobova E, Tuganova A, Boulatnikov I, Popov KM. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem J. 2001;358:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–149. [DOI] [PubMed] [Google Scholar]
- 44. Regueira M, Artagaveytia SL, Galardo MN, et al. Novel molecular mechanisms involved in hormonal regulation of lactate production in Sertoli cells. Reproduction. 2015;150:311–321. [DOI] [PubMed] [Google Scholar]
- 45. Kaiser GR, Monteiro SC, Gelain DP, Souza LF, Perry ML, Bernard EA. Metabolism of amino acids by cultured rat Sertoli cells. Metabolism. 2005;54:515–521. [DOI] [PubMed] [Google Scholar]
- 46. Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007;236:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazaud-Guittot S, Prud'homme B, Bouchard MF, et al. GATA4 autoregulates its own expression in mouse gonadal cells via its distal 1b promoter. Biol Reprod. 2014;90:25. [DOI] [PubMed] [Google Scholar]
- 50. Ketola I, Rahman N, Toppari J, et al. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999;140:1470–1480. [DOI] [PubMed] [Google Scholar]
- 51. Ketola I, Anttonen M, Vaskivuo T, Tapanainen JS, Toppari J, Heikinheimo M. Developmental expression and spermatogenic stage specificity of transcription factors GATA-1 and GATA-4 and their cofactors FOG-1 and FOG-2 in the mouse testis. Eur J Endocrinol. 2002;147:397–406. [DOI] [PubMed] [Google Scholar]
- 52. McCoard SA, Wise TH, Fahrenkrug SC, Ford JJ. Temporal and spatial localization patterns of Gata4 during porcine gonadogenesis. Biol Reprod. 2001;65:366–374. [DOI] [PubMed] [Google Scholar]
- 53. Oréal E, Mazaud S, Picard JY, Magre S, Carré-Eusèbe D. Different patterns of anti-Müllerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn. 2002;225:221–232. [DOI] [PubMed] [Google Scholar]
- 54. Kilcoyne KR, Smith LB, Atanassova N, et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA. 2014;111:E1924–E1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Landreh L, Spinnler K, Schubert K, et al. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99:E1227–E1235. [DOI] [PubMed] [Google Scholar]
- 56. Imai T, Kawai Y, Tadokoro Y, Yamamoto M, Nishimune Y, Yomogida K. In vivo and in vitro constant expression of GATA-4 in mouse postnatal Sertoli cells. Mol Cell Endocrinol. 2004;214:107–115. [DOI] [PubMed] [Google Scholar]
- 57. Lei N, Heckert LL. Gata4 regulates testis expression of Dmrt1. Mol Cell Biol. 2004;24:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. [DOI] [PubMed] [Google Scholar]
- 59. Bouma GJ, Washburn LL, Albrecht KH, Eicher EM. Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci USA. 2007;104:14994–14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. The regulation of Sox9 gene expression by the GATA4/FOG2 transcriptional complex in dominant XX sex reversal mouse models. Dev Biol. 2007;307:356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hermann BP, Heckert LL. Silencing of Fshr occurs through a conserved, hypersensitive site in the first intron. Mol Endocrinol. 2005;19:2112–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rahman NA, Kiiveri S, Rivero-Müller A, et al. Adrenocortical tumorigenesis in transgenic mice expressing the inhibin α-subunit promoter/simian virus 40 T-antigen transgene: relationship between ectopic expression of luteinizing hormone receptor and transcription factor GATA-4. Mol Endocrinol. 2004;18:2553–2569. [DOI] [PubMed] [Google Scholar]
- 63. Lui WY, Wong EW, Guan Y, Lee WM. Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A. J Cell Physiol. 2007;211:638–648. [DOI] [PubMed] [Google Scholar]
- 64. Feng ZM, Wu AZ, Zhang Z, Chen CL. GATA-1 and GATA-4 transactivate inhibin/activin β-B-subunit gene transcription in testicular cells. Mol Endocrinol. 2000;14:1820–1835. [DOI] [PubMed] [Google Scholar]
- 65. Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9:e1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu YC, Nicholls PK, Soh YQ, et al. Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 2015;11:e1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Buganim Y, Itskovich E, Hu YC, et al. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell. 2012;11:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Byers S, Graham R, Dai HN, Hoxter B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am J Anat. 1991;191:35–47. [DOI] [PubMed] [Google Scholar]
- 69. Hwang I, Yang H, Kang HS, et al. Spatial expression of claudin family members in various organs of mice. Mol Med Rep. 2014;9:1806–1812. [DOI] [PubMed] [Google Scholar]
- 70. Guillemot L, Spadaro D, Citi S. The junctional proteins cingulin and paracingulin modulate the expression of tight junction protein genes through GATA-4. PLoS One. 2013;8:e55873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 2003;540:151–156. [DOI] [PubMed] [Google Scholar]
- 72. Munshi NV, McAnally J, Bezprozvannaya S, et al. Cx30.2 enhancer analysis identifies Gata4 as a novel regulator of atrioventricular delay. Development. 2009;136:2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu J, He X, Corbett SA, et al. Integrins are required for the differentiation of visceral endoderm. J Cell Sci. 2009;122:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lustig L, Denduchis B, Ponzio R, Lauzon M, Pelletier RM. Passive immunization with anti-laminin immunoglobulin G modifies the integrity of the seminiferous epithelium and induces arrest of spermatogenesis in the guinea pig. Biol Reprod. 2000;62:1505–1514. [DOI] [PubMed] [Google Scholar]
- 75. Bennett J, Baumgarten SC, Stocco C. GATA4 and GATA6 silencing in ovarian granulosa cells affects levels of mRNAs involved in steroidogenesis, extracellular structure organization, IGF-I activity, and apoptosis. Endocrinology. 2013;154:4845–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. [DOI] [PubMed] [Google Scholar]
- 77. Nehar D, Mauduit C, Boussouar F, Benahmed M. Tumor necrosis factor-α-stimulated lactate production is linked to lactate dehydrogenase A expression and activity increase in porcine cultured Sertoli cells. Endocrinology. 1997;138:1964–1971. [DOI] [PubMed] [Google Scholar]
- 78. Li SS, O'Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989;40:173–180. [DOI] [PubMed] [Google Scholar]
- 79. Thomas K, Del Mazo J, Eversole P, et al. Developmental regulation of expression of the lactate dehydrogenase (LDH) multigene family during mouse spermatogenesis. Development. 1990;109:483–493. [DOI] [PubMed] [Google Scholar]
- 80. Jutte NH, Grootegoed JA, Rommerts FF, van der Molen HJ. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil. 1981;62:399–405. [DOI] [PubMed] [Google Scholar]
- 81. Courtens JL, Plöen L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol Reprod. 1999;61:154–161. [DOI] [PubMed] [Google Scholar]
- 82. Erkkilä K, Aito H, Aalto K, Pentikäinen V, Dunkel L. Lactate inhibits germ cell apoptosis in the human testis. Mol Hum Reprod. 2002;8:109–117. [DOI] [PubMed] [Google Scholar]
- 83. Bergeron F, Nadeau G, Viger RS. GATA4 knockdown in MA-10 Leydig cells identifies multiple target genes in the steroidogenic pathway. Reproduction. 2015;149:245–257. [DOI] [PubMed] [Google Scholar]
- 84. George RM, Hahn KL, Rawls A, Viger RS, Wilson-Rawls J. Notch signaling represses GATA4-induced expression of genes involved in steroid biosynthesis. Reproduction. 2015;150:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang C, Xu Q, Martin TD, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]