Abstract
Context:
Mechanisms explaining documented associations of 25-hydroxyvitamin D [25(OH)D] deficiency with increased risks of cardiovascular disease (CVD) and venous thromboembolism may relate to adverse hemostatic and inflammatory responses.
Objective:
To evaluate whether 25(OH)D deficiency is associated with a prothrombotic and proinflammatory biological profile.
Design:
Cross-sectional analyses.
Setting:
The Multi-Ethnic Study of Atherosclerosis, a multicenter prospective cohort of American adults.
Participants:
Up to 6554 adults free of CVD.
Main Outcome Measures:
Ten hemostatic biomarkers (D-dimer, fibrinogen, factor VIII, plasmin-antiplasmin, and homocysteine [n = 6443]; von Willebrand factor, soluble tissue factor, plasminogen activator inhibitor-1 (PAI-1), total tissue factor pathway inhibitor (TFPI), and soluble thrombomodulin [n = 814]), and three inflammatory biomarkers (IL-6, C-reactive protein [n = 6443], and TNF-α soluble receptor [n = 3802]).
Results:
Among 6443 subjects (46.6% men; mean age, 62.1 years; mean body mass index, 28.3 kg/m2) of White (37.8%), Black (27.2%), Chinese (12.2%), and Hispanic (21.8%) race/ethnicity, mean 25(OH)D was 25.3 ng/mL. After multiple adjustment, 25(OH)D concentrations were associated with concentrations of IL-6 and homocysteine and also with concentrations of PAI-1 and TFPI: per 10 ng/mL decrement in 25(OH)D, 5.1% higher IL-6 (95% confidence interval [CI], 3.4–6.9; P < .001); 3.7% higher homocysteine (95% CI, 3.0–4.3; P < .001); 7.0% higher PAI-1 (95% CI, 0.9–13.6; P = .025); and 2.1% higher TFPI (95% CI, 0.0–4.2; P = .047), without racial/ethnic heterogeneity. No significant associations were observed for other hemostatic and inflammatory biomarkers.
Conclusions:
Increased inflammation as reflected by higher circulating IL-6 and increased homocysteine concentrations may represent mechanisms linking 25(OH)D deficiency to greater risks of CVD and perhaps venous thromboembolism. Low concentrations of 25(OH)D were also associated with PAI-1 and TFPI concentrations, but not with other hemostatic biomarkers.
“Among 13 hemostatic/inflammatory biomarkers, low levels of serum 25-hydroxyvitamin D were cross-sectionnaly associated with higher levels of IL-6, homocysteine, TFPI and PAI-1 but not with others.”
Up to three out of four adults in the United States have deficient (<20 ng/mL) or insufficient (<30 ng/mL) concentrations of 25-hydroxyvitamin D [25(OH)D] (1). Associations of vitamin D deficiency with both detrimental skeletal and extraskeletal conditions underlie its potential relevance from a clinical and public health perspective (2).
In particular, cardiovascular disease (CVD) has gathered substantial attention. In observational studies, individuals with low concentrations of 25(OH)D had higher risk of future coronary events and perhaps of future venous thromboembolism (VTE) events (3–5). Although the impact of vitamin D supplementation on these diseases remains to be determined through large ongoing clinical trials, mechanistic studies can bring insights in our understanding of possible pathways linking vitamin D insufficiency to cardiovascular risk. Potential influences of vitamin D on the cardiovascular system may include suppression of the renin-angiotensin-aldosterone system, modulation of the endothelial or platelet function, decrease of vascular smooth cell proliferation and immunogenic effects. Proinflammatory and prothrombotic influences of vitamin D deficiency may represent two other important plausible pathways. However, large observational studies investigating these associations remain scarce (6, 7).
Using a large contemporary cohort of multiethnic adults, we hypothesized that vitamin D deficiency, defined by low serum 25(OH)D concentration, would be associated with concentrations of hemostatic biomarkers suggestive of a more prothrombotic profile and with greater circulating concentrations of inflammatory biomarkers.
Subjects and Methods
This is an analysis of the Multi-Ethnic Study of Atherosclerosis (MESA), a large U.S. prospective cohort aimed at characterizing subclinical and clinical CVD and their risk factors in four racial/ethnic groups (8).
Population
The MESA enrolled 6814 men and women aged 45–84 years of four racial/ethnic groups (White, Black, Hispanic, and Chinese) between 2000 and 2002. Participants were free of clinical CVD at baseline and were recruited from the population near six field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; New York, New York; St. Paul, Minnesota; and Los Angeles, California) (8). They gave written informed consent, and local institutional review boards approved the study protocol.
From the full cohort at baseline, we excluded 341 participants without 25(OH)D measurements; six participants with 25(OH)D concentrations above 100 ng/mL, suggestive of supplementation with a high dose of vitamin D; 21 participants with current use of anticoagulant drugs; and three participants with missing hemostatic/inflammatory biomarkers. The analytic sample comprised 6443 subjects.
Measurements
All measurements were made using blood drawn at the first examination of the MESA cohort, processed in field centers, and shipped to a central laboratory (Laboratory for Clinical Biochemistry Research, University of Vermont, Burlington) for storage at −80°C.
Vitamin D
Serum 25(OH)D concentrations were measured by mass spectrometry, with 25(OH)D calibrated to NIST (National Institute of Standards and Technology) standards (interassay coefficient of variation [CV] <3.4%) (9). We used the actual measure of 25(OH)D, not an annualized estimate (10), because we hypothesized short-term influences of 25(OH)D on hemostatic/inflammatory biomarker concentrations measured concurrently.
Hemostatic/inflammatory biomarkers
We selected 10 hemostatic (including homocysteine) and three inflammatory biomarkers, which were assayed at the central laboratory. D-dimer, fibrinogen, factor VIII, plasmin-antiplasmin complex (PAP), high-sensitivity C-reactive protein (CRP), IL-6, and homocysteine were measured in the entire cohort. Plasminogen activator inhibitor-1 (PAI-1), von Willebrand factor (vWF), soluble tissue factor, tissue factor pathway inhibitor (TFPI), and soluble thrombomodulin were measured in a 1000-person random sample. TNF-α receptor 1 (TNFαR1) was measured in participants enrolled for an early ancillary study (subsample of 2880 participants, with equal representation from the four ethnic groups and matched for age and sex; n = 2641 after exclusions).
The following laboratory methods were used: immunoturbidometry for D-dimer and vWF (Liatest D-DI, Diagnostica Stago; on Sta-R analyzer, Diagnostica Stago; analytical CV = 8 and 4.5%, respectively); immunonephelometry using the BNII nephelometer for fibrinogen antigen and CRP (N Antiserum to Human Fibrinogen, N High Sensitivity CRP; Dade Behring Inc.; CV = 2.6 and 2.1–5.7%, respectively); clotting time of a sample in factor VIII-deficient plasma in the presence of activators for factor VIII (STA-Deficient VIII on Sta-R analyzer; CV = 10%); in-house immunoassay sensitive for PAI-1 (CV = 3.5%); ultrasensitive ELISA for IL-6 and TNFαR1 (R&D Systems; CV = 6.3 and 5.0%, respectively); fluorescence polarization immunoassay for homocysteine (IMx Homocysteine Assay, Axis Biochemicals ASA; using the IMx Analyzer, Abbott Diagnostics; CV = 3.8–5.1%); enzyme-linked sandwich ELISA for TFPI (Imubind Total TFPI ELISA Kit; American Diagnostica Inc.; CV = 6.2–7.1%); enzyme-linked immunoassay for soluble tissue factor (Imubind Tissue Factor ELISA Kit; American Diagnostica Inc.; CV = 14.6%); enzyme immunoassay for soluble thrombomodulin (Asserachrom Thrombomodulin; Diagnostica Stago; CV = 12%); and two-site ELISA for PAP (reagents provided by Drs. Désiré Collen and Paul Declerck; CV = 1.7%).
Covariates
At enrollment, demographic data, smoking status, medical conditions, physical activity, and the current use of medications were recorded in a questionnaire. Physical activity was estimated using reported weekly total intentional exercise. Waist circumference, body mass index (BMI), and resting blood pressure were measured. Blood analyses included fasting glucose, creatinine, and cystatin C. Diabetes was defined as the report of treated diabetes or a fasting glucose ≥126 mg/dL. We calculated the glomerular filtration rate (GFR) with the combined creatinine-cystatin C equation (11).
Statistical analysis
We evaluated cross-sectional associations of 25(OH)D with the 13 log-transformed hemostatic/inflammatory biomarkers using linear regression with robust standard errors. Results can be interpreted as a percentage difference in geometric means between categories of 25(OH)D or for a continuous difference in 25(OH)D. For categorical 25(OH)D, we compared participants with 25(OH)D concentrations below 20 ng/mL, and between 20 and 30 ng/mL with participants with 25(OH)D concentrations above 30 ng/mL. These categories correspond to concentrations of vitamin D deficiency, insufficiency, and sufficiency, respectively (12). We also explored associations for participants with very low 25(OH)D concentrations (<10 ng/mL). The presence of nonlinear trends was also excluded graphically. P values were obtained from Wald tests. A marginal level <.05 determined statistical significance. Corresponding Bonferroni-corrected P values accounting for the 13 biomarkers in the main analysis were <.0038.
We defined two sets of covariates for adjustment before analysis. The first model adjusted for demographic variables: age (continuous), sex, race, site of enrollment, education (less than high school, complete high school or equivalent, complete college), and annual income (<$20,000, $20,000 to $39,999, $40,000 to $74,499, and >$75,000). The second model further adjusted for confounders defined before analyses: physical activity (in quartiles), BMI (continuous), waist circumference (continuous), diabetes, current smoking, GFR (continuous), and current use of statin, hormone replacement therapy, and aspirin.
In secondary analyses, we evaluated the presence of heterogeneity of the associations by sex, smoking status (current vs not current), BMI (>30 vs ≤30 kg/m2), and race/ethnicity (White, Black, Chinese, Hispanic) by adding multiplicative interaction terms of those variables with 25(OH)D.
Sensitivity analyses were conducted on all hemostatic/inflammatory biomarkers by excluding values outside the normal range or very high values, specified in Supplemental Table 1. We also explored the potential for confounding by folate (vitamin B9) and vitamin B12 of the observed associations between 25(OH)D concentrations and hemostatic or inflammatory biomarkers by further adjusting the regression models with estimated daily intake of folate and B12 from food (based on a food frequency questionnaire) and supplements in quartiles. Finally, we conducted a sensitivity analysis with exclusion of participants using medications with possible influence on 25(OH)D concentrations (hormone replacement therapy, thiazide, calcium channel blocker, proton pump inhibitors, and bile acid sequestrants).
Missing covariate data were infrequent in covariates (≤5.3%) and were multiple-imputed with five imputed datasets using imputation by chained-equations. All analyses were conducted using Stata 11 (StataCorp LP).
Results
The sample comprised 3005 men and 3438 women, with White (37.8%), Chinese (12.2%), Black (27.2%), and Hispanic (21.8%) race/ethnicities; mean age of 62.1 (SD, 10.3) years; and mean BMI of 28.3 (SD, 5.4) kg/m2 (Table 1). The prevalence of diabetes and current smoking was 12.4 and 13.0%, respectively. Two percent of participants self-reported a previous deep vein thrombosis or pulmonary embolism.
Table 1.
Characteristics of Participants, Stratified by 25(OH)D Concentrations (n = 6443)
| 25(OH)D, ng/mL |
|||
|---|---|---|---|
| <20 | 20.0–29.9 | ≥30.0 | |
| n | 2205 | 2136 | 2102 |
| Age, y | 60.8 (10.1) | 62.4 (10.4) | 63.3 (10.1) |
| Male gender | 985 (44.7%) | 1074 (50.3%) | 946 (45%) |
| Race | |||
| White | 467 (21.2%) | 800 (37.5%) | 1230 (58.5%) |
| Chinese | 200 (9.1%) | 331 (15.5%) | 257 (12.2%) |
| Black | 1046 (47.4%) | 482 (22.6%) | 225 (10.7%) |
| Hispanic | 492 (22.3%) | 523 (24.5%) | 390 (18.6%) |
| Site | |||
| Wake Forest | 347 (15.7%) | 289 (13.5%) | 330 (15.7%) |
| Columbia | 413 (18.7%) | 334 (15.6%) | 263 (12.5%) |
| John Hopkins | 439 (19.9%) | 309 (14.5%) | 282 (13.4%) |
| University of Minnesota | 326 (14.8%) | 318 (14.9%) | 381 (18.1%) |
| Northwestern | 390 (17.7%) | 382 (17.9%) | 369 (17.6%) |
| UCLA | 290 (13.2%) | 504 (23.6%) | 477 (22.7%) |
| Educational achievement | |||
| <High school | 413 (18.8%) | 422 (19.8%) | 319 (15.2%) |
| High school | 406 (18.5%) | 369 (17.3%) | 375 (17.9%) |
| >High school | 1375 (62.7%) | 1340 (62.9%) | 1403 (66.9%) |
| Annual income | |||
| <$20 000 | 542 (26.1%) | 527 (25.5%) | 414 (20.2%) |
| $20 000 to $39 999 | 599 (28.8%) | 517 (25.0%) | 526 (25.7%) |
| $40 000 to $74 999 | 563 (27.1%) | 552 (26.7%) | 548 (26.8%) |
| ≥$75 000 | 377 (18.1%) | 471 (22.8%) | 560 (27.3%) |
| Treated or untreated diabetes | 320 (14.5%) | 319 (14.9%) | 160 (7.6%) |
| Hypertension | 1041 (47.2%) | 959 (44.9%) | 864 (41.1%) |
| SBP, mm Hg | 128.3 (22.1) | 126.3 (21.2) | 124.7 (21.1) |
| BMI, kg/m2 | 29.9 (6.1) | 28.1 (4.9) | 26.8 (4.7) |
| Waist circumference, cm | 101.2 (15.4) | 98 (13.5) | 94.5 (13.2) |
| Total intentional exercise, Mets-min/wk | 1299.4 (2090.1) | 1520.1 (2249.9) | 1887.1 (2674.2) |
| Total cholesterol, mg/dL | 193.1 (37.4) | 194.1 (35.8) | 195.3 (33.8) |
| Current smoking | 372 (17.0%) | 255 (12%) | 208 (9.9%) |
| Current use of statin | 293 (13.3%) | 325 (15.3%) | 339 (16.2%) |
| Current use of aspirin | 329 (14.9%) | 398 (18.7%) | 515 (24.5%) |
| Current use of HRT (among women) | 246 (22.9%) | 305 (32%) | 443 (41.4%) |
| Estimated GFR | 86.1 (17.8) | 83.9 (17.1) | 80.5 (16.4) |
| Self-reported leg/lung blood clot | 45 (2%) | 37 (1.7%) | 48 (2.3%) |
Abbreviations: SBP, systolic blood pressure; HRT, hormone replacement therapy. Data are expressed as number (percentage) or means (SD).
The mean concentration of 25(OH)D was 25.3 ng/mL (SD, 10.9), with a prevalence of vitamin D deficiency (<20 ng/mL) of 34.2% and of vitamin insufficiency (20–30 ng/mL) of 33.2%. Participants with low 25(OH)D concentrations were more likely to be of Black race; to have diabetes, greater BMI, and greater waist circumference; and to be current smokers. They were less likely to be current users of statin, aspirin, and, for women, hormone replacement therapy. Very low concentrations of 25(OH)D (<10ng/mL) were found in 414 participants (6.4%). Most of them were of the Black race (64.7%).
Hemostatic biomarkers
In regression models adjusted for demographic factors, we observed significant associations of 25(OH)D concentrations with fibrinogen, PAP, PAI-1, TFPI, and homocysteine (Table 2, model 1). However, adjustment for confounders (model 2) attenuated most of these associations, and only inverse associations of continuous 25(OH)D with homocysteine (P < .001), TFPI (P = .047), and PAI-1 (P = .025) persisted, with marginal significance for TFPI and PAI-1. Compared to participants with 25(OH)D concentrations >30 ng/mL, participants with 25(OH)D concentrations <20 ng/mL had 19.9% greater adjusted PAI-1 concentrations (95% confidence interval [CI], 2.2 to 39.3), 6.0% greater adjusted TFPI concentrations (95% CI, 0.2–12.1), and 10.1% greater adjusted homocysteine concentrations (95% CI, 8.3–12.0).
Table 2.
Associations of Serum 25(OH)D Concentrations With Hemostatic Biomarkers
| 25(OH)D | n | Unadjusted Means | Adjusted Percentage Difference |
|
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| D-dimer, μg/mL | ||||
| ≥30.0 | 2097 | 0.36 (0.83) | Ref. | Ref. |
| 20.0–29.9 | 2135 | 0.36 (0.88) | 2.1% (−3.1 to 7.6) | 0.5% (−4.6 to 5.9) |
| <20 | 2201 | 0.40 (0.82) | 6.0% (0.1 to 12.2) | 1.6% (−4.1 to 7.7) |
| Per 10 ng/mL decrement | 1.2% (−1.1 to 3.5) | −0.7% (−2.9 to 1.6) | ||
| P value | .31 | .56 | ||
| Fibrinogen, mg/dL | ||||
| ≥30.0 | 2097 | 339 (70) | Ref. | Ref. |
| 20.0–29.9 | 2132 | 342 (72) | 0.2% (−1.0 to 1.4) | −1.2% (2.4 to 0.0) |
| <20 | 2202 | 356 (76) | 2.6% (1.3 to 3.9) | −0.2% (−1.4 to 0.1) |
| Per 10 ng/mL decrement | 1.2% (0.6 to 1.7) | 0.0% (−0.5 to 0.5) | ||
| P value | <.001 | 1.00 | ||
| Factor VIII, % | ||||
| ≥30.0 | 2097 | 97 (35) | Ref. | Ref. |
| 20.0–29.9 | 2134 | 98 (37) | 0.6% (−1.6 to 2.8) | 0.4% (−1.8 to 2.6) |
| <20 | 2198 | 101 (40) | 0.1% (−2.3 to 2.6) | 0.1% (−2.3 to 2.6) |
| Per 10 ng/mL decrement | −0.1% (−1.1 to 0.8) | −0.1% (−1.1 to 0.8) | ||
| P value | .77 | .78 | ||
| vWF, % | ||||
| ≥30.0 | 288 | 133 (52) | Ref. | Ref. |
| 20.0–29.9 | 251 | 139 (59) | 2.4% (−4.5 to 9.9) | 1.8% (−5.0 to 9.1) |
| <20 | 273 | 139 (55) | 2.4% (−4.7 to 10.1) | 0.5% (−6.6 to 8.1) |
| Per 10 ng/mL decrement | 0.4% (−2.5 to 3.3) | −0.3% (−3.2 to 2.6) | ||
| P value | .8 | .81 | ||
| Plasmin-antiplasmin, nmol/L | ||||
| ≥30.0 | 2040 | 4.9 (2.1) | Ref. | Ref. |
| 20.0–29.9 | 2084 | 4.6 (1.9) | −5.6% (−7.6 to −3.5) | −3.6% (−5.6 to −1.5) |
| <20 | 2173 | 4.8 (2.5) | −4.0% (−6.3 to −1.7) | −0.7% (−3.1 to 1.7) |
| Per 10 ng/mL decrement | −2.0% (−2.8 to −1.1) | −0.5% (−1.5 to 0.4) | ||
| P value | <.001 | .28 | ||
| Soluble tissue factor, pg/mL | ||||
| ≥30.0 | 289 | 122 (79) | Ref. | Ref. |
| 20.0–29.9 | 251 | 118 (84) | −8.0% (−19.7 to 5.4) | −5.7% (−18.2 to 8.7) |
| <20 | 274 | 122 (96) | −10.2% (−21.8 to 0.3) | −5.8% (−18.9 to 9.5) |
| Per 10 ng/mL decrement | −3.6% (−8.9 to 1.9) | −2.0% (−8.0 to 4.4) | ||
| P value | .20 | .54 | ||
| PAI-1, ng/mL | ||||
| ≥30.0 | 289 | 22.7 (22.9) | Ref. | Ref. |
| 20.0–29.9 | 251 | 27.1 (33.5) | 13.7% (−4.6 to 35.6) | 9.8% (−5.6 to 27.6) |
| <20 | 274 | 29.4 (27.7) | 49.9% (26.2 to 78.0) | 19.3% (2.2 to 39.3) |
| Per 10 ng/mL decrement | 15.7% (8.3 to 23.7) | 7.0% (0.9 to 13.6) | ||
| P value | <.001 | .025 | ||
| TFPI, ng/mL | ||||
| ≥30.0 | 289 | 47.0 (14.3) | Ref. | Ref. |
| 20.0–29.9 | 251 | 45.9 (13.8) | −2.3% (−7.2 to 2.8) | −2.0% (−6.7 to 3.0) |
| <20 | 274 | 50.7 (14.8) | 9.3% (3.5 to 15.4) | 6.0% (0.2 to 12.1) |
| Per 10 ng/mL decrement | 3.1% (1.1 to 5.2) | 2.1% (0.0 to 4.2) | ||
| P value | .003 | .047 | ||
| Soluble thrombomodulin, ng/mL | ||||
| ≥30.0 | 289 | 37.4 (17.4) | Ref. | Ref. |
| 20.0–29.9 | 251 | 37.4 (22.2) | 0.6% (−7.1 to 8.9) | 1.6% (−5.7 to 9.5) |
| <20 | 274 | 34.0 (18.2) | −4.0% (−11.6 to 4.3) | −3.4% (−11.2 to 5.0) |
| Per 10 ng/mL decrement | −1.3% (−4.6 to 2.3) | −1.0% (−4.4 to 2.6) | ||
| P value | .48 | .59 | ||
| Homocysteine, μmol/L | ||||
| ≥30.0 ng/mL | 812 | 9.0 (3.0) | Ref. | Ref. |
| 20.0–29.9 ng/mL | 916 | 9.2 (4.0) | 2.2% (0.5 to 3.9) | 3.6% (2.0 to 5.2) |
| <20 ng/mL | 913 | 9.7 (4.0) | 8.5% (6.6 to 10.5) | 10.1% (8.3 to 12.0) |
| Per 10 ng/mL decrement | 3.0% (2.3 to 3.7) | 3.7% (3.0 to 4.3) | ||
| P value | <.001 | <.001 | ||
Estimates from regression model can be interpreted as a percentage difference in geometric means. Model 1, adjusted for age, sex, race/ethnicity, site of enrollment, education and income. Model 2, further adjusted for physical activity, BMI, waist circumference, diabetes, current smoking, GFR, and use of statin, aspirin, and hormone replacement therapy.
Null results between 25(OH)D concentrations and D-dimer, fibrinogen, and factor VIII were precise and excluded an average percentage difference >0.5–2.9% for a decrement of 10 ng/mL in 25(OHD) concentrations.
Exploratory analyses of very low 25(OH)D concentrations (<10 ng/mL) yielded similar results, and no further association was observed.
Inflammatory biomarkers
There was strong evidence for an inverse association between 25(OH)D and IL-6, with 13.4% greater concentrations of IL-6 (95% CI, 8.9 to 18.0; P < .001) for participants with vitamin D deficiency compared with vitamin D sufficiency after full adjustment (Table 3). No associations were observed for CRP and TNFαR1 with confident exclusions of percentage difference >1.5–3.1% for a 10 ng/mL decrement in 25(OH)D concentrations. For participants with very low 25(OH)D concentrations (<10 ng/mL), results were similar, with null associations for CRP and TNFαR1.
Table 3.
Associations of 25(OH)D Concentrations With Inflammatory Biomarkers
| 25(OH)D, ng/mL | n | Unadjusted Means | Adjusted Percentage Difference |
|
|---|---|---|---|---|
| Regression Model 1 | Regression Model 2 | |||
| CRP, mg/L | ||||
| ≥30.0 ng/mL | 2095 | 3.4 (5.4) | Ref. | Ref. |
| 20.0–29.9 ng/mL | 2127 | 3.4 (5.5) | 4.7% (−2. to 12.0) | −4.4% (−10.1 to 1.8) |
| <20 ng/mL | 2202 | 4.3 (6.2) | 17.3% (0.9 to 26.1) | −0.8% (−7.2 to 6.2) |
| Per 10 ng/mL decrement | 6.7% (3.7 to 9.8) | −0.4% (−3.1 to 2.3) | ||
| P value | <.001 | .76 | ||
| IL-6, pg/mL | ||||
| ≥30.0 ng/mL | 2067 | 1.42 (1.30) | Ref. | Ref. |
| 20.0–29.9 ng/mL | 2093 | 1.52 (1.27) | 10.0% (5.7 to 14.5) | 4.1% (0.2 to 8.0) |
| <20 ng/mL | 2150 | 1.78 (1.43) | 26.1% (20.9 to 31.6) | 13.4% (8.9 to 18.0) |
| Per 10 ng/mL decrement | 9.9% (8.1 to 11.8) | 5.1% (3.4 to 6.9) | ||
| P value | <.001 | <.001 | ||
| TNFαR1, pg/mL | ||||
| ≥30.0 ng/mL | 812 | 1405 (433) | Ref. | Ref. |
| 20.0–29.9 ng/mL | 916 | 1379 (478) | −0.7 (−3.0 to 1.7) | −0.2% (−1.9 to 1.6) |
| <20 ng/mL | 913 | 1357 (411) | −0.4 (−2.8 to 2.1) | −1.0% (−2.8 to 0.8) |
| Per 10 ng/mL decrement | −0.3 (−1.3 to 0.7) | −0.7% (−1.5 to 0.1) | ||
| P value | .55 | .09 | ||
Model 1 adjusted for age, race/ethnicity, site of enrollment, education, and income. Model 2 further adjusted for physical activity, BMI, waist circumference, diabetes, current smoking, GFR, and use of statin, aspirin, and hormone replacement therapy.
Sensitivity and subgroup analyses
Overall results were not modified in sensitivity analyses excluding possible outliers or values outside the normal range (Supplemental Table 1). The associations with IL-6, TFPI, and homocysteine persisted, and the association with PAI-1 was somewhat less strong. In the sensitivity analysis with further adjustment for daily intake of folate and vitamin B12, we did not observe any material changes in the regression estimates. Per 10-ng/mL decrement in 25(OH)D concentrations, IL-6, PAI-1, TFPI, and homocysteine concentrations were 5.2% (95% CI, 3.4 to 6.9), 6.7% (95% CI, 0.5 to 13.4), 1.9% (95% CI, −0.2 to 4.1), and 3.5% (95% CI, 2.8 to 4.2) greater, respectively.
When excluding 2258 current users of medications with possible influence on 25(OH)D levels, findings were not different from the primary results (data not shown).
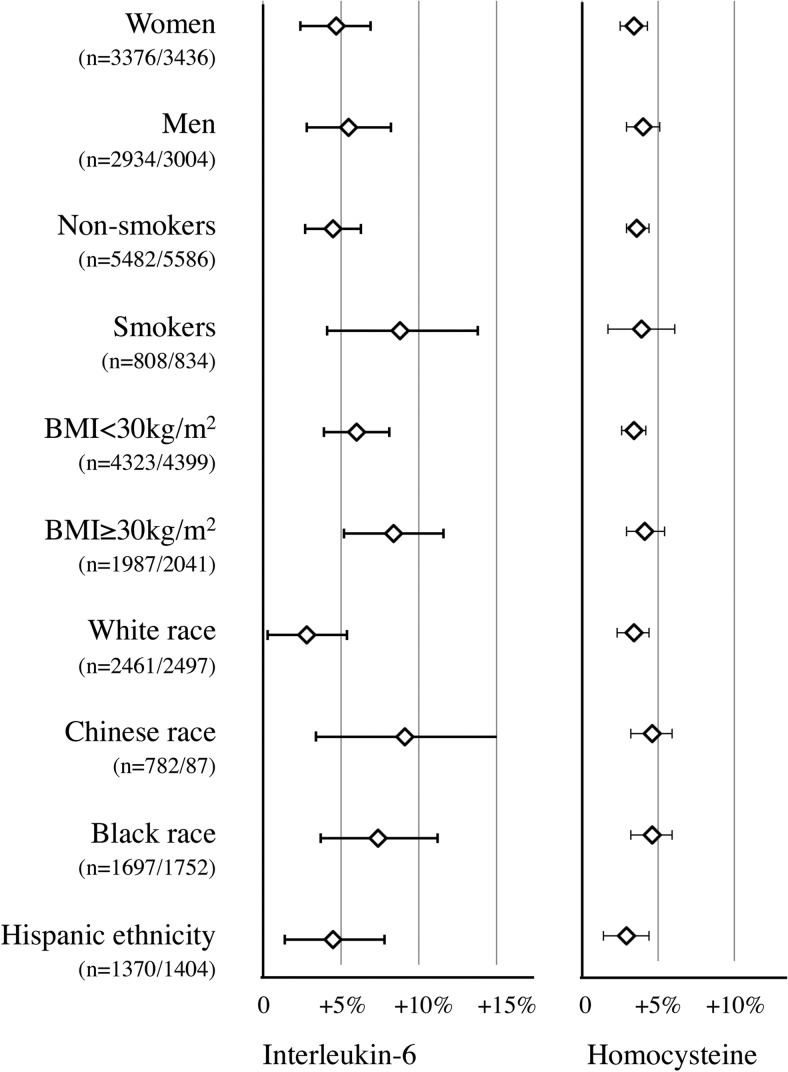
Subgroup analyses did not suggest heterogeneity by gender, smoking status, BMI, and race/ethnicity for any of the 13 biomarkers. Null results for D-dimer, fibrinogen, factor VIII, or CRP persisted in all subgroups. We found consistently statistically significant associations of 25(OH)D concentrations with IL-6 and homocysteine concentrations in all subgroups (Figure 1).
Figure 1.
Subgroup-adjusted associations of continuous 25(OH)D concentrations (per 10 ng/mL decrement) with IL-6 and homocysteine concentrations.
Discussion
In this study of racially and ethnically diverse American adults, we found strong evidence for inverse associations of 25(OH)D concentrations with IL-6 and homocysteine concentrations and weak evidence for inverse associations of 25(OH)D concentrations with TFPI and PAI-1 concentrations. Results for IL-6 and homocysteine were consistent across all races/ethnicities.
Hemostatic biomarkers
Vitamin D status may be associated with fibrinolytic activity through PAI-1, its main inhibitor. Our data suggest that plasma from vitamin D-deficient participants may show a hypofibrinolytic tendency, with 20% greater concentrations of PAI-1 than vitamin D-sufficient participants, after adjustment. In two small samples from the Tromsø study, 25(OH)D concentrations were not associated with PAI-1 concentrations in models adjusting for BMI, but the power of both studies was limited, and participants had been selected based on previous abnormal mineral metabolism measurements and obesity, therefore limiting the interpretation of these results (13, 14). Four small randomized trials estimated the direct influence of vitamin D supplementation on PAI-1 concentrations. PAI-1 concentrations did not decrease after 1 year of weekly treatment with 40 000 IU of cholecalciferol per week, compared with placebo, in the Tromsø substudy of 158 obese patients with mean baseline 25(OH)D concentrations of 24.8 ng/mL (14). Two studies found lower PAI-1 concentrations, although this was not statistically significant, in 52 obese Danish adults and 62 healthy Swiss adults randomized to oral vitamin D supplementation (15, 16). Finally, a significant decrease in PAI-1 over 4 weeks was observed in healthy Chinese women living in the United Kingdom who were randomized to a single dose of 100 000 IU of oral vitamin D, compared with placebo (17). Such a positive result may emanate from the sampling of vitamin D-deficient women with baseline mean 25(OH)D concentrations of 10.8 ng/mL, in whom effects from vitamin D supplementation may be easier to observe, or it may be a chance finding. However, an association between vitamin D and PAI-1 concentrations is also supported by in vitro studies, showing decreased concentrations of PAI-1 production by aortic smooth muscles treated with vitamin D3 analogs (18).
Little is known about the relationship between vitamin D and homocysteine. Three cross-sectional studies of healthy representative Canadians (Canadian Health Measures Survey), healthy US adults (National Health and Nutrition Examination Survey), and healthy older Dutch adults yielded different results, showing null, inverse, and U-shaped associations, respectively (19–21). In our sample, we found strong evidence for an inverse association, with 10% greater concentrations in participants with low concentrations of 25(OH)D than in participants with normal concentrations. In vitro studies suggest a potential influence of treatment with 1,25-dihydroxyvitamin D on the metabolism of homocysteine through the regulation of expression of the cystathionine β-synthase (21). We could therefore hypothesize that vitamin D deficiency may increase homocysteine concentrations.
The evaluation of associations of 25(OH)D concentrations with factor VIII, PAP, soluble thrombomodulin, soluble tissue factor, and TFPI is novel. We observed borderline evidence for an inverse association between 25(OH)D and total TFPI concentrations, which was in the opposite direction to our hypothesis of greater thrombogenicity of 25(OH)D deficiency. TFPI is a major inhibitor of the tissue factor VIIa complex, thought to represent the trigger of blood coagulation in vivo (22). The relationship of total TFPI with thrombogenicity remains unclear, however, because most TFPI is found in vivo in the endothelium, and most of the plasma TFPI is complexed with lipoproteins, with less anticoagulant activity than free plasma TFPI. Paradoxically, low total TFPI concentrations have been associated with greater risk of VTE (23), and high concentrations have been associated with atherosclerosis (increased intimal medial thickness and coronary artery calcium) (24). Therefore, the significance of high total plasma TFPI remains unclear, but perhaps relates to endothelial dysfunction.
All other hemostatic biomarkers were not related to 25(OH)D concentrations. We did not observe any independent association of vitamin D status with global hemostatic measures such as D-dimer and with specific coagulation factors such as fibrinogen and vWF. Given the large sample, these null findings appear precise enough to exclude associations of clinical or public health importance. Such null results have also been suggested by two other studies: in 6538 participants from the 1958 British Birth Cohort [mean 25(OH)D concentrations of 21.1 ng/mL] for D-dimer, fibrinogen, or vWF (6), and in 1381 participants from the Framingham Offspring Study [mean 25(OH)D concentrations of 19.8 ng/mL] for fibrinogen. Reasonably, these associations should not be further evaluated in the general population.
Inflammatory biomarkers
Vitamin D-deficient individuals appear to have a proinflammatory state as evidenced by greater IL-6 concentrations. Our cross-sectional results are consistent with prior smaller studies (7, 25–28). The plausibility of this association is also supported by in vitro models (29). However, in placebo-controlled randomized trials, the influence of vitamin D supplementation on IL-6 concentrations has been conflicting. These trials differ in their interventional dose of vitamin D (700 IU/d to single dose of 250 000 IU) and their duration of treatment (single dose to 3 years). They often included selected populations of small or modest sample sizes (Chinese women [17], Black subjects [30], obese subjects [16, 31, 32], and subjects with diabetes [33], lupus [34], and cystic fibrosis [35]), who in the majority were vitamin D deficient. IL-6 concentrations decreased 1 week after a single dose of vitamin D3 of 250 000 IU in patients with exacerbated cystic fibrosis (35) and at 12 weeks in Iranian diabetic patients after the daily intake of yogurts fortified with 1000 IU of vitamin D3 (33). In the largest study, including 332 obese Norwegian subjects, supplementation with 20 000 to 40 000 IU of vitamin D3 per week resulted in a trend for decreased IL-6 concentrations (P = .08) (31). All other studies found null results. Larger-scale studies in more general populations are warranted to better evaluate the association of vitamin D supplementation on IL-6 concentrations.
Early studies reported associations of 25(OH)D concentrations with CRP concentrations (25), although not consistently (28, 36). In MESA, we observed that such an association disappeared after adjustment for confounding variables, in particular BMI and abdominal circumference. The lack of clinical CVD in MESA participants further reinforces the validity of our results by reducing the risk of confounding by CVD. A direct influence of vitamin D status on CRP concentrations therefore appears unlikely.
Significance of the findings
We believe that the demonstrated proinflammatory and perhaps prohemostatic states in individuals with vitamin D deficiency may represent possible mechanisms explaining their greater risk of arterial cardiovascular and venous thromboembolic disease. Vitamin D receptors are ubiquitous. Specific modulations of the function of endothelial cells (producing most of circulating PAI-1 and TFPI) and of immunological cells have been demonstrated (37, 38), adding biological plausibility for these mechanisms.
Elevated PAI-1 concentrations have long been recognized as a risk factor for myocardial infarctions and their complications (39) and may perhaps also be associated with the risk of incident VTE (40). Furthermore, inflammation is an inherent mechanism of the initiation and progression of atherosclerosis. The evidence linking IL-6 with coronary heart disease is strong, in particular through Mendelian randomization studies, suggesting a causal role (41, 42). Hemostasis is also influenced by the inflammatory balance, and chronic inflammatory diseases are recognized risk factors for VTE. However, epidemiological data on IL-6 concentrations and risk of VTE remain scarce and mainly emanate from case-control studies with blood draw taken after the VTE diagnosis (43).
Observational studies have almost consistently detected greater risks of CVD and VTE with greater homocysteine concentrations (44). However, also consistently, clinical trials with folic acid have yielded null results: folic acid reduced homocysteine concentrations but not CVD or VTE risks (44, 45). Furthermore, Mendelian randomization approaches using common MTHFR gene polymorphisms do not suggest a causative role of homocysteine in the risk of arterial CVD (46). Whether an influence of vitamin D on homocysteine concentrations translates into increased clinical risks therefore remains very speculative.
The lack of racial heterogeneity of our results contrasts with documented differences in the inverse association between 25(OH)D concentrations and CVD risk. In MESA, this association was observed among White and Chinese participants, but not among Black and Hispanic participants (47). The postulated mechanisms that are explored in our study cannot account for these differences.
Limitations and strengths
Our results should be interpreted in view of their limitations. First, causality and its direction cannot be inferred from observational and cross-sectional analyses, which are also prone to survivorship bias. We cannot exclude residual confounding by unmeasured variables or reverse causality, ie, that IL-6, homocysteine, TFPI, or PAI-1 concentrations may influence 25(OH)D concentrations. In particular, we did not have blood measurements of folate or vitamin B12 concentrations, but adjustment for daily intake of these vitamins did not suggest a strong confounding potential. Second, whereas 25(OH)D concentrations estimate vitamin D status, they may not reflect the actual biological effect of vitamin D. Third, the statistical significance of our findings should be viewed in light of the smaller sample size for some biomarkers. The somewhat weaker associations found for PAI-1 and TFPI may therefore arise from the lower statistical power than that for IL-6 and homocysteine. Some biomarkers were measured in a restricted sample, and we cannot exclude the possibility of a type 2 error. Finally, the exploration of hemostasis through coagulation biomarker measurements is very partial, given that in vivo hemostasis is a very complex process including coagulation, platelets, endothelial cells, and other factors (microparticles, neutrophil extracellular traps). Strengths of our results include the precise measure of 25(OH)D by mass spectrometry, high power given the large sample size for most biomarkers, and external validity derived from the diverse community-based population.
In conclusion, we found that 25(OH)D concentrations are not associated with most hemostatic biomarkers, but the inverse association between 25(OH)D and PAI-1 may suggest fibrinolytic differences in vitamin D deficiency. Furthermore, insufficient 25(OH)D concentrations may contribute to inflammation, as evidenced by higher circulating concentrations of IL-6, and to greater concentrations of homocysteine. Whether these possible mechanisms may increase risks of arterial and/or venous cardiovascular risk remains to be elucidated.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts R01HL096875 and P30DK035816, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by Grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- CRP
- C-reactive protein
- CV
- coefficient of variation
- CVD
- cardiovascular disease
- GFR
- glomerular filtration rate
- 25(OH)D
- 25-hydroxyvitamin D
- PAI-1
- plasminogen activator inhibitor-1
- PAP
- plasmin-antiplasmin complex
- TFPI
- tissue factor pathway inhibitor
- TNFαR1
- TNFα receptor 1
- VTE
- venous thromboembolism
- vWF
- von Willebrand factor.
References
- 1. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. [DOI] [PubMed] [Google Scholar]
- 3. Kestenbaum B, Katz R, de Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brøndum-Jacobsen P, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. J Thromb Haemost. 2013;11:423–431. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hyppönen E, Berry D, Cortina-Borja M, Power C. 25-Hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: a cross sectional analysis in the 1958 British Birth Cohort. PLoS One. 2010;5:e10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 9. Phinney KW, Bedner M, Tai SS, et al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jorde R, Haug E, Figenschau Y, Hansen JB. Serum levels of vitamin D and haemostatic factors in healthy subjects: the Tromsø study. Acta Haematol. 2007;117:91–97. [DOI] [PubMed] [Google Scholar]
- 14. Jorde R, Sneve M, Torjesen P, Figenschau Y, Hansen JB. Parameters of the thrombogram are associated with serum 25-hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thromb Res. 2010;125:e210–e213. [DOI] [PubMed] [Google Scholar]
- 15. Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg. 2012;44:307–312. [DOI] [PubMed] [Google Scholar]
- 16. Wamberg L, Kampmann U, Stødkilde-Jørgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24:644–649. [DOI] [PubMed] [Google Scholar]
- 17. Witham MD, Adams F, Kabir G, Kennedy G, Belch JJ, Khan F. Effect of short-term vitamin D supplementation on markers of vascular health in South Asian women living in the UK–a randomised controlled trial. Atherosclerosis. 2013;230:293–299. [DOI] [PubMed] [Google Scholar]
- 18. Wu-Wong JR, Nakane M, Ma J. Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. J Vasc Res. 2007;44:11–18. [DOI] [PubMed] [Google Scholar]
- 19. Amer M, Qayyum R. The relationship between 25-hydroxyvitamin D and homocysteine in asymptomatic adults. J Clin Endocrinol Metab. 2014;99:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. García-Bailo B, Da Costa LA, Arora P, Karmali M, El-Sohemy A, Badawi A. Plasma vitamin D and biomarkers of cardiometabolic disease risk in adult Canadians, 2007–2009. Prev Chronic Dis. 2013;10:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kriebitzsch C, Verlinden L, Eelen G, et al. 1,25-Dihydroxyvitamin D3 influences cellular homocysteine levels in murine preosteoblastic MC3T3–E1 cells by direct regulation of cystathionine β-synthase. J Bone Miner Res. 2011;26:2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams M. Tissue factor pathway inhibitor: new insights into an old inhibitor. Semin Thromb Hemost. 2012;38:129–134. [DOI] [PubMed] [Google Scholar]
- 23. Zakai NA, Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Total tissue factor pathway inhibitor and venous thrombosis. The Longitudinal Investigation of Thromboembolism Etiology. Thromb Haemost. 2010;104:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell CT, Kamineni A, Palmas W, Cushman M. Tissue factor pathway inhibitor, vascular risk factors and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;207:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. [DOI] [PubMed] [Google Scholar]
- 26. De Vita F, Lauretani F, Bauer J, et al. Relationship between vitamin D and inflammatory markers in older individuals. Age (Dordr). 2014;36:9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laird E, McNulty H, Ward M, et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J Clin Endocrinol Metab. 2014;99:1807–1815. [DOI] [PubMed] [Google Scholar]
- 28. Srikanth P, Chun RF, Hewison M, et al. Associations of total and free 25OHD and 1,25(OH)2D with serum markers of inflammation in older men [published online February 23, 2016]. Osteoporos Int. doi: 10.1007/s00198-016-3537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J, Kong J, Duan Y, et al. Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–E322. [DOI] [PubMed] [Google Scholar]
- 30. Chandler PD, Scott JB, Drake BF, et al. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila). 2014;7:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-α and insulin resistance in overweight and obese subjects. Cytokine. 2012;60:870–874. [DOI] [PubMed] [Google Scholar]
- 32. Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. [DOI] [PubMed] [Google Scholar]
- 33. Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Kalayi A, Shariatzadeh N, Khalaji N, Gharavi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev. 2012;28:424–430. [DOI] [PubMed] [Google Scholar]
- 34. Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol. 2013;40:265–272. [DOI] [PubMed] [Google Scholar]
- 35. Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michos ED, Streeten EA, Ryan KA, et al. Serum 25-hydroxyvitamin D levels are not associated with subclinical vascular disease or C-reactive protein in the old order Amish. Calcif Tissue Int. 2009;84:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uberti F, Lattuada D, Morsanuto V, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99:1367–1374. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ploplis VA. Effects of altered plasminogen activator inhibitor-1 expression on cardiovascular disease. Curr Drug Targets. 2011;12:1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meltzer ME, Lisman T, de Groot PG, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–121. [DOI] [PubMed] [Google Scholar]
- 41. IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD, Jr, Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. 2012;33:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di Minno MN, Tremoli E, Coppola A, Lupoli R, Di Minno G. Homocysteine and arterial thrombosis: challenge and opportunity. Thromb Haemost. 2010;103:942–961. [DOI] [PubMed] [Google Scholar]
- 45. den Heijer M, Willems HP, Blom HJ, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: a randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–144. [DOI] [PubMed] [Google Scholar]
- 46. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]