Abstract
Context:
The association of hypoalbuminemia with osteoporosis in human studies is controversial.
Objective:
We tested the independent association between hypoalbuminemia and osteoporosis in a national dataset.
Design:
This is a cross-sectional observation.
Setting and Participants:
Participants are individuals selected from the National Health and Nutrition Examination Survey (NHANES) with available clinical, laboratory, and densitometry data from the 2005–2010 and 2013–2014 cycles.
Exposure:
Exposure is hypoalbuminemia defined as serum albumin <3.5 g/dL.
Main Outcome Measure:
Osteoporosis is defined as bone mineral density of ≤2.5 SD below the mean peak bone mass of young, healthy adults. Analysis took into account the hidden variance and the weighting methodology pertinent to analysis of NHANES.
Results:
Overall, 15 539 individuals were included. The mean age was 48.6 years (SE = 0.27). Fifty percent of the individuals were male, and 10.2% were of the black race. There was a graded decrease in the rate of osteoporosis from 15.2% at albumin <3.5 g/dL to 2.6% at albumin >4 g/dL (P = .007) at the femoral neck and from 11.0 to 1.4% at total femur (P = .009). In a fully adjusted model, the odds of osteoporosis with hypoalbuminemia were 5.37-fold (95% confidence interval [CI], 1.43 to 20.20; P = .014) at the femoral neck, 12.46-fold (95% CI, 3.24 to 48.01; P < .001) at total femur, and 4.59-fold (95% CI, 1.49 to 14.16; P = .025) at the lumbar spine higher as compared to albumin >4 mg/dL.
Conclusion:
In the NHANES dataset, we report an independent association of osteoporosis with hypoalbuminemia at different anatomical sites.
“In the NHANES dataset we report an independent association of osteoporosis with hypoalbuminemia at different anatomical sites.”
It is estimated that there are over 53 million adults with low bone mass at the femoral neck and lumbar spine in the United States (1). This is associated with higher morbidity and mortality with significant public health burden (2–5). In 2005, the number of osteoporosis (OP)-related fractures exceeded 2 million, with close to a $17 billion societal burden (6). Albumin is the most abundant protein in the blood (7), with various physiological roles including maintenance of oncotic pressure, metabolite transportation, plasma buffer, and antioxidant properties, besides several other functions (8–10). Hypoalbuminemia is linked with several systemic disorders such as malignancy, hepatopathy, nephrotic syndrome, and inflammation-malnutrition complex (10, 11). Despite well-established links between hypoalbuminemia with various systemic disorders, the association of hypoalbuminemia with OP in humans has been a matter of controversy with conflicting results (13–17). In a recent study, we suggested that the controversy in human studies is due to methodological limitations including the lack of diversity of selected participants, low sample size and power of studies, and limited range in which serum albumin has been studied, besides other limitations (18). Indeed, when some of these limitations were addressed, we were able to demonstrate the independent association between hypoalbuminemia and OP in a large diverse cohort in our health care system (18). In this study, our aim is to replicate the association of hypoalbuminemia with OP at various anatomical sites in independent samples randomly selected from the U.S. general population using the National Health and Nutrition Examination Survey (NHANES). We hypothesize that hypoalbuminemia is independently associated with OP at various anatomical sites in participants of the NHANES.
Subjects and Methods
This is a cross-sectional observational study. The study population is the general population of the United States. Study participants were selected from the publically available datasets of the NHANES from the periods 2005–2010 and 2013–2014. Because this is the analysis of publically available deidentified data, no local institutional review board approval or participant consent was obtained. Inclusion criteria were: availability of bone densitometry, age ≥20 years, and estimated glomerular filtration rate (eGFR) ≥30 mL/min. Individuals younger than 20 years and those with eGFR <30 mL/min were excluded from the analysis. The rationale for excluding advanced kidney disease is enriching the study population with OP as opposed to renal osteodystrophy. From the dataset demographics (age, race, gender), body mass index (BMI), history of smoking at least 100 cigarettes in a lifetime, awareness from comorbid conditions, laboratory values (serum albumin, sodium, glucose, calcium, phosphorous, creatinine), 24-hour dietary information, and densitometry indices were retrieved (Supplement 1). Hologic QDR 4500A fan-beam dual-energy x-ray absorptiometry densitometers (Hologic, Inc) were used to assess the bone mineral density (BMD) of the anterior-posterior lumbar spine and proximal femur in specially equipped mobile examination centers. The details of the NHANES BMD examination protocols and the corresponding quality controls have been published elsewhere (19–23). The coefficient of variation of BMD at the hip or spine was 0.6% or less according to a daily assessment at the spine and a weekly assessment at the hip. If at the time of daily or weekly calibration, a higher coefficient of variation (>0.6%) was achieved on three attempts, the chief technologist and the mobile examination center manager were contacted, and the information had to be reported to Hologic for corrective actions (21–23). BMD cutoff values to define OP at the hip and femur were based on 2.5 SD or less below the mean peak bone mass of young, healthy adults and were obtained from the previously published reference ranges of the NHANES III report (24). The corresponding cutoff values to define OP at the lumbar spine were obtained from the Vital and Health Statistics from Centers for Disease Control and Prevention (25). Hypoalbuminemia was defined as serum albumin <3.5 g/dL (35 g/L) (26, 27). The Chronic Kidney Disease-Epidemiology Collaboration formula was used to calculate eGFR (28).
Statistical analysis
We used the 2003–2004 guidelines set forth by the National Center for Health Statistics, Centers for Disease Control and Prevention, for analysis of NHANES complex datasets accounting for the hidden variance and using the proposed weighting methodology (29). Linear regression analysis was used to explore the linear trend of covariates across categories of serum albumin. We applied logistic regression to explore the association of hypoalbuminemia with OP at different anatomical sites having serum albumin >4 g/dL (40 g/L) as the reference category. To do so, we applied three sets of models with an increasing degree of adjustment. Model 1 was an unadjusted case-mixed model. Model 2 was adjusted for age, gender, race, BMI categories, and history of smoking. Model 3 carried the components of model 2, plus the dietary components of the diet in the past 24 hours, awareness of comorbid conditions, and laboratory values including serum sodium, glucose, calcium, phosphorous, and eGFR, as shown in Table 1. The study has over 97% power to detect the significance of the minimum odds ratio (OR) observed at the hypoalbuminemic range of femoral neck or total femur anatomical sites compared with serum albumin >4 g/dL (40 g/L, reference) at the α level of 0.01 using a two-sided test.
Table 1.
Comparison of Characteristics of the Selected Individuals by Categories of Serum Albumin
| Albumin |
|||
|---|---|---|---|
| <3.5 g/dL | 3.5–4 g/dL | >4 g/dL | |
| Unweighted, n | 132 | 3892 | 11 515 |
| Weighted estimate per cycle | 1 036 062 | 34 672 687 | 122 856 079 |
| Age (range), yc | 55 (51 to 59) | 52 (51 to 53) | 48 (47 to 48) |
| Female, %c | 63.4 | 68.8 | 44.5 |
| Black race, %c | 22.5 | 15.5 | 7.9 |
| Smoked at least 100 cigarettes, % | 55.4 | 48.0 | 47.0 |
| 24-hour diet | |||
| Energy, kcalc | 1938 (1709 to 2166) | 1921 (1879 to 1963) | 2104 (2071 to 2137) |
| Protein, gc | 76 (65 to 87) | 76 (74 to 78) | 84 (83 to 86) |
| Carbohydrate, gc | 232 (213 to 251) | 241 (235 to 246) | 255 (252 to 260) |
| Total sugars, g | 104 (89 to 120) | 111 (107 to 115) | 114 (112 to 116) |
| Dietary fiber, gc | 16.3 (13.6 to 19.0) | 15.8 (15.3 to 16.3) | 17.2 (16.8 to 18.0) |
| Total fat, gc | 77 (61 to 93) | 72 (70 to 74) | 80 (78 to 82) |
| Total SFA, gc | 26 (20 to 32) | 24 (23 to 25) | 26.2 (25.7 to 26.8) |
| Total MUFA, gc | 28 (22 to 36) | 26 (25 to 27) | 29 (28 to 30) |
| Total PUFA, gc | 16.7 (13.5 to 19.9) | 15.8 (15.1 to 16.4) | 17.4 (17 to 17.8) |
| Cholesterol, mgc | 265 (196 to 333) | 255 (244 to 267) | 281 (274 to 288) |
| Calcium, mgc | 975 (809 to 1141) | 896 (862 to 931) | 976 (951 to 994) |
| Phosphorus, mgc | 1269 (1114 to 1424) | 1245 (1213 to 1281) | 1381 (1358 to 1403) |
| Comorbidities, % | |||
| Arthritisc | 50.6 | 33.4 | 24.0 |
| Heart failurec | 7.7 | 3.5 | 1.4 |
| Thyroid diseasec | 4.7 | 0.7 | 0.4 |
| Liver diseaseb | 4.9 | 0.4 | 0.2 |
| Malignancyb | 2.7 | 0.9 | 0.4 |
| Diabetesc | 21.9 | 11.7 | 6.8 |
| BMI, kg/m2c | 30 (29 to 32) | 30 (30 to 31) | 27 (27 to 28) |
| Sodium, mmol/Lc | 138 (136 to 139) | 139 (139 to 139) | 139 (139 to 140) |
| Glucose, mg/dLc | 121 (107 to 135) | 104 (103 to 106) | 97 (97 to 98) |
| Calcium, mg/dLc | 8.9 (8.8 to 9.1) | 9.3 (9.2 to 9.3) | 9.5 (9.5 to 9.55) |
| Phosphorous, mg/dLc | 3.7 (3.5 to 3.8) | 3.7 (3.7 to 3.8) | 3.8 (3.8 to 3.8) |
| eGFR, mL/minc | 89 (82 to 96) | 90 (89 to 91) | 92 (92 to 93) |
| Femoral neck BMD, g/cm2a | 0.79 (0.75 to 0.83) | 0.82 (0.81 to 0.83) | 0.83 (0.82 to 0.83) |
| Total femur BMD, g/cm2b | 0.92 (0.88 to 0.96) | 0.96 (0.95 to 0.97) | 0.97 (0.97 to 0.98) |
| L1–L4 BMD, g/cm2b | 1.03 (0.98 to 1.08) | 1.05 (1.04 to 1.06) | 1.03 (1.0 to 1.04) |
Abbreviations: SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. Data are expressed as percentage or mean (95% CI) in the weighted samples accounting for hidden variance. To convert values for glucose to millimoles per liter, multiply by 0.05551. To convert values of calcium to millimoles per liter, multiply by 0.25. To convert values of phosphorous to millimoles per liter, multiply by 0.32. To convert values of sodium to millimoles per liter, multiply by 1. Unweighted sample sizes for lumbar BMD from low to high categories of serum albumin are 105, 3082, and 9358, respectively.
P < .05;
P < .01;
P < .001.
Results
Baseline characteristics
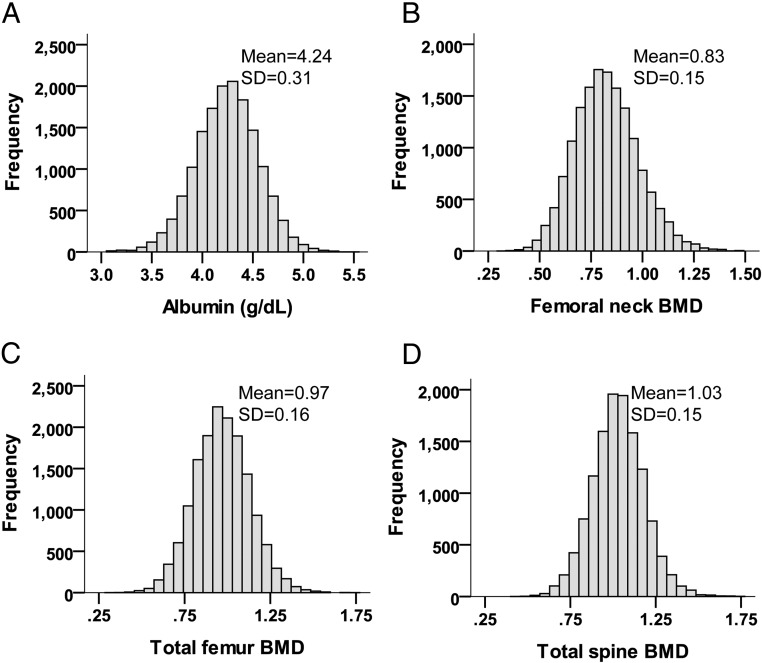
Overall, 15 539 eligible individuals selected from the publically available NHANES were included in the final analysis. Mean age was 48.6 years (SE = 0.27). Fifty percent of the individuals were male, and 10.2% were of the black race. The mean of weighted serum albumin was 4.28 g/dL (42.8 g/L), with a 95% confidence interval (CI) of 4.26 to 4.29 g/dL (42.6 to 42.9 g/L) and a range of 2.5 to 5.5 g/dL (25 to 55 g/L). Figure 1 shows the distribution of unweighted serum albumin and BMD at different anatomical sites. Table 1 shows the distribution of baseline characteristics of the participants by categories of serum albumin. The rates and estimates are calculated accounting for hidden variance and using the weighting methodology mentioned above. There is a significant decline in linear trend of mean age, BMI, serum glucose, and L1–L4 BMD, as well as in the frequency of female gender, black race, and awareness of other comorbidities by increasing level of albumin category (P < .01). On the other hand, the linear trend of mean sodium, calcium, phosphorous, eGFR, and BMD of the femoral neck and total femur increased significantly by the serum albumin categories (P < .05). Similarly, there was an increase in caloric intake, protein, carbohydrate, dietary fiber, total fat, fatty acids, cholesterol, calcium, and phosphorous consumption from a low to high category of serum albumin (P < .001). The history of smoking at least 100 cigarettes in the past was not significantly different by the categories.
Figure 1.
Distribution of serum albumin and BMD at different anatomical sites according to unweighted estimates. Weighted mean albumin was estimated to be 4.28 g/dL. Unweighted N is 15 539 for panels A to C and 12 545 for panel D. To convert values of serum albumin to grams per liter, multiply by 10.
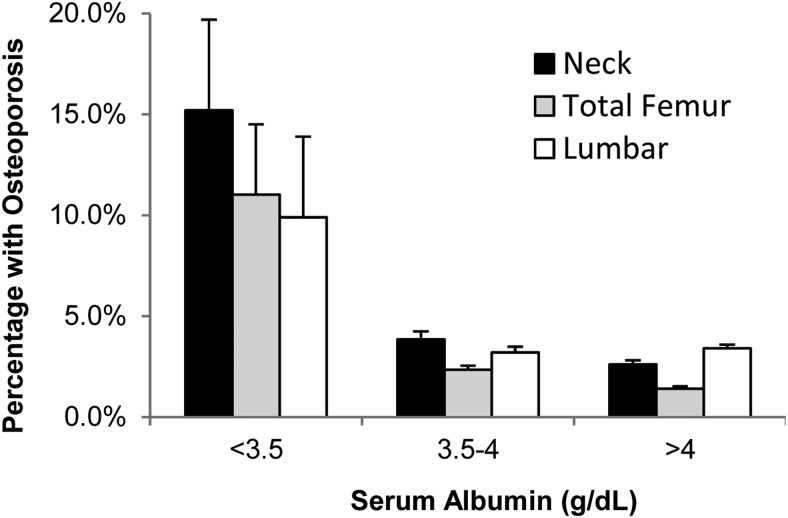
Rate of OP by categories of albumin
Figure 2 illustrates a significant declining trend of OP at the femoral neck, total femur, and lumbar spine by increasing categories of serum albumin (P ≤ .001). There was a graded decrease in the rate of OP from 15.2% at albumin <3.5 g/dL (35 g/L) to 2.6% at albumin >4 g/dL (40 g/L; P = .007) at the femoral neck; from 11.0 to 1.4% at the total femur (P = .009); and from 9.9 to 3.4% at the lumbar spine (P = .022).
Figure 2.
Comparison of the prevalence of OP at different levels of serum albumin at various anatomical sites shows a significantly higher rate of OP at all anatomical sites compared with albumin >4 g/dL (P ≤ .025). Bars represent percentage plus standard error in the weighted samples accounting for hidden variance. To convert values of serum albumin to grams per liter, multiply by 10.
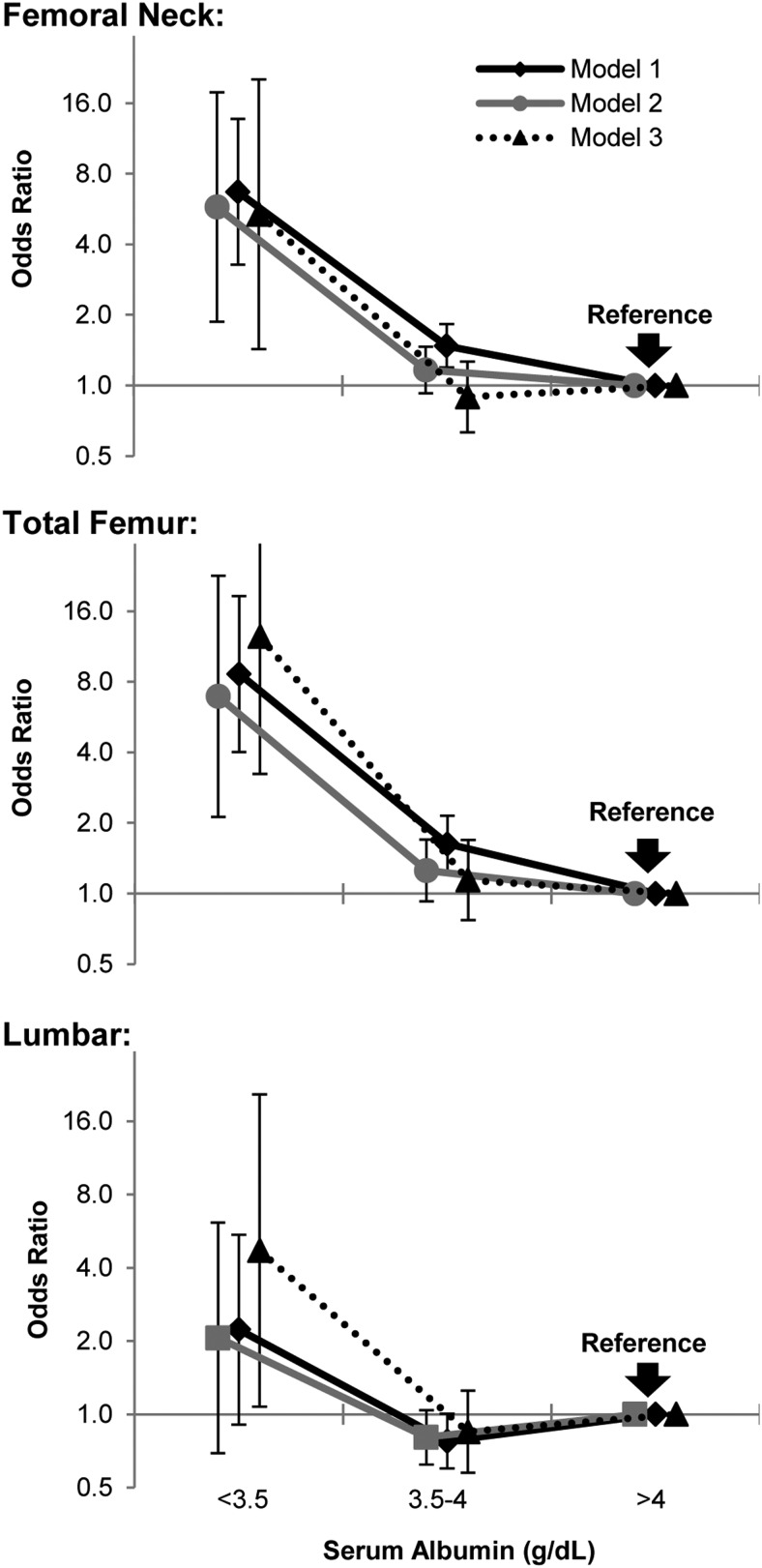
Risk of OP by categories of albumin
Figure 3 shows significantly higher odds of OP at albumin <3.5 g/dL (35 g/L) at the femoral neck and total femur as compared to albumin >4 g/dL (40 g/L) using an unadjusted case-mixed model (P < .001). The OR remained highly statistically significant after adjusting for components of model 2 (P ≤ .003) as well as with the full adjustment in model 3 in the femoral neck and total femur (P ≤ .014). At the lumbar spine, the OR was not significant in unadjusted and with partial adjustment; however, full adjustment revealed significantly higher odds of OP at the hypoalbuminemic range as compared to albumin >4 g/dL (40 g/L). In the fully adjusted models, the odds of OP with hypoalbuminemia were 5.37-fold (95% CI, 1.43 to 20.20; P = .014) at the femoral neck, 12.46-fold (95% CI, 3.24 to 48.01; P < .001) at the total femur, and 4.59-fold (95% CI, 1.49 to 14.16; P = .025) at the lumbar spine higher than albumin >4 g/dL (40 g/L).
Figure 3.
Comparing the OR of OP by categories of serum albumin shows significantly higher odds of OP with hypoalbuminemia at the femoral neck and total femur compared with albumin >4 g/dL in all three models (P ≤ .003) using logistic regression on the weighted samples accounting for hidden variance. At the lumbar spine only, full adjustment has disclosed the association of hypoalbuminemia with OP. Model 1, case-mixed unadjusted model. Model 2, adjusted for age, sex, race, BMI categories, and history of smoking. Model 3, Model 2 plus additionally adjusted for other laboratory values and comorbidities, and components of 24-hour diet recall. Values represent OR ± 95% CI. To convert values of serum albumin to grams per liter, multiply by 10.
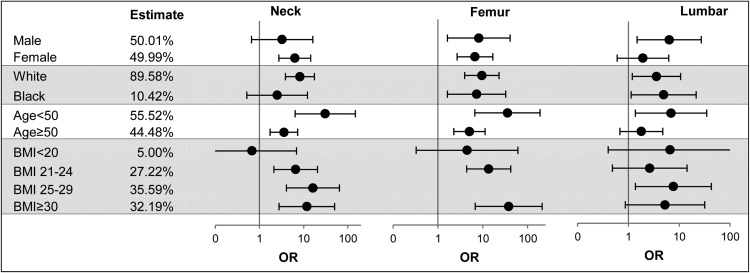
Subgroup analysis
Figure 4 shows that at the femoral neck there are significantly higher odds of association of hypoalbuminemia with OP at all subgroups (P ≤ .002), except in males, black race, and lean body mass. Similarly, at total femoral anatomical sites, all subgroups had higher odds of association of hypoalbuminemia with OP (P < .011), except in lean body mass. At the lumbar spine, the association was less consistent and did not reach statistical significance in females, age ≥50 years, and BMI <25 or ≥30 kg/m2.
Figure 4.
OR of association of hypoalbuminemia with OP compared with albumin >4 g/dL by subgroups of age, sex, race, and BMI at different anatomical sites using logistic regression models on the weighted samples accounting for hidden variance. Note that values for the subgroup BMI of 25–29 kg/m2 at the femur are not shown due to inaccurate estimates owing to the differentially low prevalence of OP and low rate of hypoalbuminemia in that subgroup, with the net effect of inaccuracy of the estimate in that subgroup.
Discussion
In this study, we report significantly higher odds of OP in individuals with hypoalbuminemia as compared to those with an albumin level >4 g/dL (40 g/L) at the femoral neck, total femur, and lumbar spine after full multivariable adjustment in the NHANES dataset.
The first report of the link between hypoalbuminemia and OP was proposed by Albright and Reifenstein (13) in the late 1940s. In 1997, Morii et al reported a lower BMD in rats that were fed a low-albumin diet (30). Aligned with these results, two human observations by Rico et al (16) and Saito et al (17) similarly reported lower BMD associated with hypoalbuminemia. Contrary to these findings, D'Erasmo et al (14) failed to show the association in a group of healthy postmenopausal women, and the weak association between albumin and BMD disappeared after adjusting for age in the Rancho Bernardo study (15). We believe that the discrepancy in the results of these studies is rooted in methodological limitations including relatively low sample size (16, 17, 31); lack of multivariable adjustment (14, 16, 17); lack of age, gender, or race diversity (14–17); and narrow range of study variables (15). For example, the Rancho Bernardo study had only four patients out of 1593 values in the hypoalbuminemic range, and therefore it did not have enough patients at the hypoalbuminemic range necessary to test the impact of hypoalbuminemia on OP. Similarly, the study of the healthy postmenopausal women defined the inclusion criteria narrowly by age, so that it eliminated the variability necessary to capture the association (14). To that end, we recently examined the association in a large cohort of patients in our health care system with sufficient gender and race diversity with an age range of 20 to over 100 years, along with adequate multivariable adjusting for potential confounders, and we found significantly higher odds of association between hypoalbuminemia and OP at the femoral neck, total hip, and lumbar spine (18). However, a limitation of our previous work (18) was that the results could not be extrapolated to the general population or other patient populations with different characteristics. Therefore, a relevant question becomes whether or not what we reported as an independent association between hypoalbuminemia and OP could be replicated in independent cohorts. In this study, we have used the publically available datasets from NHANES and have replicated the independent association of hypoalbuminemia with OP at the femoral neck, total femur, and lumbar spine after full multivariable adjustments. The OR at the lumbar spine was relatively smaller than what was observed at hip areas, and therefore in the subgroup analysis fewer subgroups reached statistical significance due to loss of power. A larger sample size at the hypoalbuminemic range is required to show the statistical significance at the lumbar area as was achieved in our previous observation (18). The smaller OR at the lumbar spine as compared to hip areas suggests that trabecular and compact bones may differentially be associated with hypoalbuminemia. In the subgroup analysis, we also noticed that significant associations remain in a majority of subgroups in hip areas. The exceptions were male gender and black race in the femoral neck and lean body mass in all areas. This is likely related to a lower prevalence of OP in males as compared to females and a smaller proportion of individuals with black race or lean body mass, altogether translating to a loss of power and widening of CI in those subgroups. Otherwise, the absolute value of OR (in males and black race at the femoral neck and lean body mass at the total femur and lumbar spine) is comparable with the rest of the corresponding subgroups.
The underpinning mechanism(s) of the association between low albumin and low BMD is unknown. Hypoalbuminemia may directly activate osteoclasts and suppress osteogenesis via its link with the nuclear factor-κB; or by virtue of being an acute phase reactant, it may indirectly be linked with nuclear factor-κB and other inflammatory cytokines driving the osteoclastic activities (32–34). Other plausible mechanisms may include association with prolonged immobility (35), alteration flux of albumin and minerals to and from the bone with the net effect of declining formation of calcium phosphate apatite crystals, change in the metabolism of PTH and vitamin D binding protein, and decreased Gla-protein, besides other unknown mechanisms still to be identified (30, 31, 36–38). Variation in coding regions of the human albumin gene revealed the genetic basis of the congenital analbuminemia, a rare condition with a flurry of metabolic defects (12). Studying the bone density of patients with congenital analbuminemia can also provide mechanistic insight and further assess a potential causal effect.
This study has several strengths. This is the analysis of randomly selected samples from national datasets through validated and standardized methods of data collection; the results are representative of the general population and therefore can be extrapolated to the entire nation. The randomness of the sample selection and the uniform data collection irrespective of albuminemia status have also eliminated the possibility for selection or ascertainment bias, allowing robust comparison of the hypoalbuminemic subgroup with the rest of the samples. This is an observation of a large dataset with sufficient age, gender, and race diversity. The large sample size has provided very high power for multivariable analysis of the associations at hip areas, and the availability of covariates (to the extent available through NHANES) provided the opportunity for multivariable adjustments to control for potential confounders. This study also has important limitations. It is an observation of cross-sectional data that does not provide any mechanistic explanation for the association. Fracture is a relevant outcome, but because the prevalence of fracture was a small fraction of OP, there were too few individuals with fracture at the hypoalbuminemic range, which precluded any meaningful analysis. The small proportion of participants with hypoalbuminemia may lead to a source bias from differentially distributed characteristics and residual confounders, and the corresponding individuals might have specific characteristics and unique features that provide a mechanistic explanation for the association. Our current analysis might not have fully captured the entire residual confounders, and therefore the potential for bias from differentially distributed characteristics and residual confounders should be addressed with further studies. Although there is the potential for residual confounders such as medications, because the results from the unadjusted to fully adjusted models were almost identical and aligned with the our previous observation (18), we suggest that the potential for any further residual confounder capable of significantly changing the associations is low.
In a previous report, we showed that not only the severity of hypoalbuminemia in a dose-dependent manner but also its longer duration was associated with OP (18). The findings in this study reinforce our previous findings and signify novel aspects of mineral metabolism above and beyond traditional markers of bone metabolism. Further mechanistic studies are needed to better understand the mechanism of the link between hypoalbuminemia and OP, which may enhance the opportunities for novel therapeutic targets. We conclude that hypoalbuminemia is strongly and independently associated with OP at the femoral neck, total femur, and lumbar spine in the general population of the United States. The association at the lumbar spine is weaker.
Acknowledgments
F.A. is funded by National Institute of Diabetes, Digestive, and Kidney Diseases Grant DK106523.
Disclosure Summary: F.A. and S.P. declare that they have no conflict of interest.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- OP
- osteoporosis
- OR
- odds ratio.
References
- 1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. [DOI] [PubMed] [Google Scholar]
- 3. Johansson C, Black D, Johnell O, Odén A, Mellström D. Bone mineral density is a predictor of survival. Calcif Tissue Int. 1998;63:190–196. [DOI] [PubMed] [Google Scholar]
- 4. Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974–1980. [DOI] [PubMed] [Google Scholar]
- 5. Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10:592–602. [DOI] [PubMed] [Google Scholar]
- 6. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. [DOI] [PubMed] [Google Scholar]
- 7. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20:271–273. [DOI] [PubMed] [Google Scholar]
- 8. Evans TW. Review article: albumin as a drug–biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(suppl 5):6–11. [DOI] [PubMed] [Google Scholar]
- 9. Doweiko JP, Nompleggi DJ. Role of albumin in human physiology and pathophysiology. JPEN J Parenter Enteral Nutr. 1991;15:207–211. [DOI] [PubMed] [Google Scholar]
- 10. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–290. [DOI] [PubMed] [Google Scholar]
- 11. Ha CE, Bhagavan NV. Novel insights into the pleiotropic effects of human serum albumin in health and disease. Biochim Biophys Acta. 2013;1830:5486–5493. [DOI] [PubMed] [Google Scholar]
- 12. Minchiotti L, Galliano M, Caridi G, Kragh-Hansen U, Peters T., Jr Congenital analbuminaemia: molecular defects and biochemical and clinical aspects. Biochim Biophys Acta. 2013;1830:5494–5502. [DOI] [PubMed] [Google Scholar]
- 13. Albright F, Reifenstein EC. The parathyroid glands and metabolic bone disease: selected studies. Baltimore, MD: Williams, Wilkins; 1948. [Google Scholar]
- 14. D'Erasmo E, Pisani D, Ragno A, Raejntroph N, Letizia C, Acca M. Relationship between serum albumin and bone mineral density in postmenopausal women and in patients with hypoalbuminemia. Horm Metab Res. 1999;31:385–388. [DOI] [PubMed] [Google Scholar]
- 15. Lunde AV, Barrett-Connor E, Morton DJ. Serum albumin and bone mineral density in healthy older men and women: the Rancho Bernardo study. Osteoporos Int. 1998;8:547–551. [DOI] [PubMed] [Google Scholar]
- 16. Rico H, Revilla M, Villa LF, Hernandez ER, Fernandez JP. Crush fracture syndrome in senile osteoporosis: a nutritional consequence? J Bone Miner Res. 1992;7:317–319. [DOI] [PubMed] [Google Scholar]
- 17. Saito N, Tabata N, Saito S, et al. Bone mineral density, serum albumin and serum magnesium. J Am Coll Nutr. 2004;23:701S–703S. [DOI] [PubMed] [Google Scholar]
- 18. Afshinnia F, Wong KK, Sundaram B, Ackermann RJ, Pennathur S. Hypoalbuminemia and osteoporosis: reappraisal of a controversy. J Clin Endocrinol Metab. 2016;101:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cogswell ME, Looker AC, Pfeiffer CM, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89:1334–1342. [DOI] [PubMed] [Google Scholar]
- 20. Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. J Bone Miner Res. 1994;9:951–960. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Body Composition Procedures Manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Body_Composition_DXA.pdf April 2013.
- 22. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Dual Energy X-ray Absorptiometry (DXA) Procedures Manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dexa.pdf January 2007.
- 23. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Body Composition Procedures Manual. http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/BC.pdf January 2006.
- 24. Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. [DOI] [PubMed] [Google Scholar]
- 25. Looker AC, Borrud LG, Hughes JP, Fan B, Shepherd JA, Melton LJ., 3rd Lumbar spine and proximal femur bone mineral density, bone mineral content, and bone area: United States, 2005–2008. Vital Health Stat 11. 2012;251:1–132. [PubMed] [Google Scholar]
- 26. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(suppl 3):S193–S199. [DOI] [PubMed] [Google Scholar]
- 27. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Center for Health Statistics, Centers for Disease Control and Prevention. Analytic and reporting guidelines. The National Health and Nutrition Examination Survey (NHANES). http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Updated December 2005. Corrected September 2006.
- 30. Morii H, Shioi A, Inaba M, et al. Significance of albumin in the pathogenesis of osteoporosis: bone changes in genetically analbuminemic rats and rats fed a low albumin diet. Osteoporos Int. 1997;7(suppl 3):S30–S35. [DOI] [PubMed] [Google Scholar]
- 31. Diamond TH, Stiel D, Lunzer M, McDowall D, Eckstein RP, Posen S. Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla-protein in 80 patients with chronic liver disease. Gastroenterology. 1989;96:213–221. [PubMed] [Google Scholar]
- 32. Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao X, Lin W, Liang C, et al. Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-κB signaling pathway. Immunol Res. 2015;62:357–367. [DOI] [PubMed] [Google Scholar]
- 34. Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. [DOI] [PubMed] [Google Scholar]
- 35. Sinaki M. Effect of physical activity on bone mass. Curr Opin Rheumatol. 1996;8:376–383. [DOI] [PubMed] [Google Scholar]
- 36. Paiva AO, Costa N, Cachinho SC, Fernandes MH. Evaluation of the influence of albumin on the mineralization of a glass by Atomic Force Microscopy. J Mater Sci Mater Med. 2007;18:599–604. [DOI] [PubMed] [Google Scholar]
- 37. Johnston CC, Jr, Hui SL, Witt RM, Appledorn R, Baker RS, Longcope C. Early menopausal changes in bone mass and sex steroids. J Clin Endocrinol Metab. 1985;61:905–911. [DOI] [PubMed] [Google Scholar]
- 38. Fibbi B, Benvenuti S, Giuliani C, et al. Low extracellular sodium promotes adipogenic commitment of human mesenchymal stromal cells: a novel mechanism for chronic hyponatremia-induced bone loss. Endocrine. 2016;52:73–85. [DOI] [PubMed] [Google Scholar]