Abstract
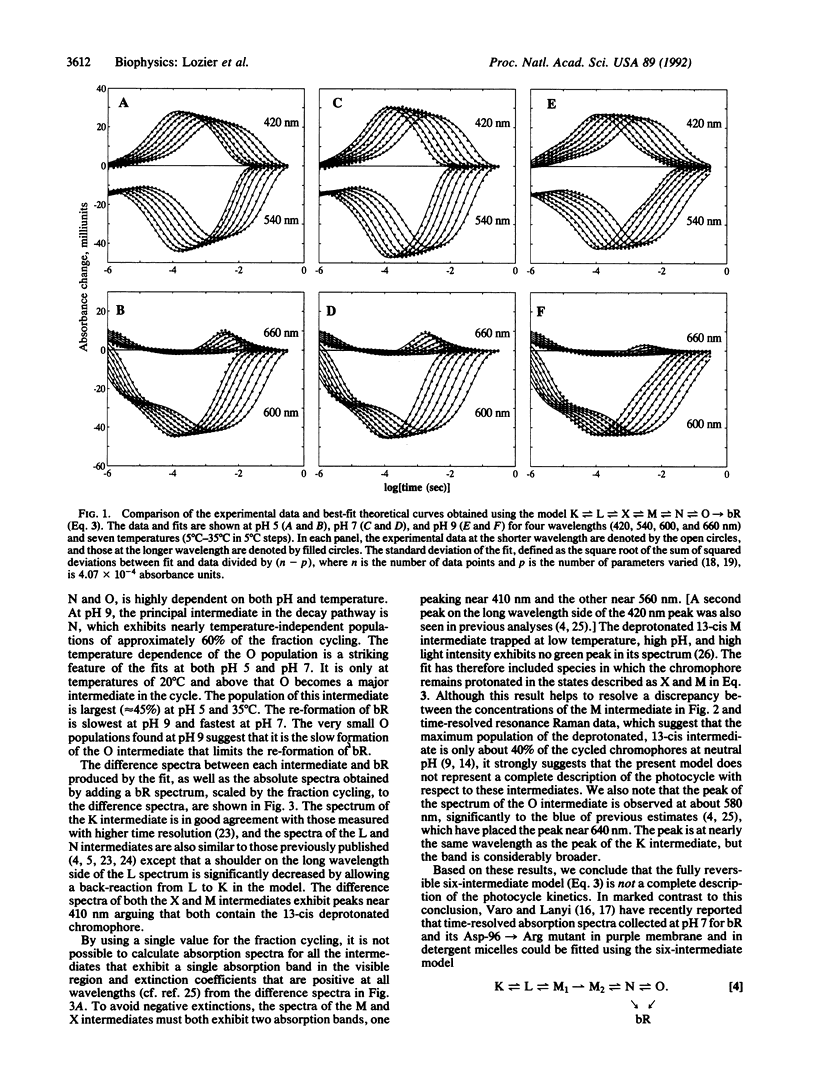
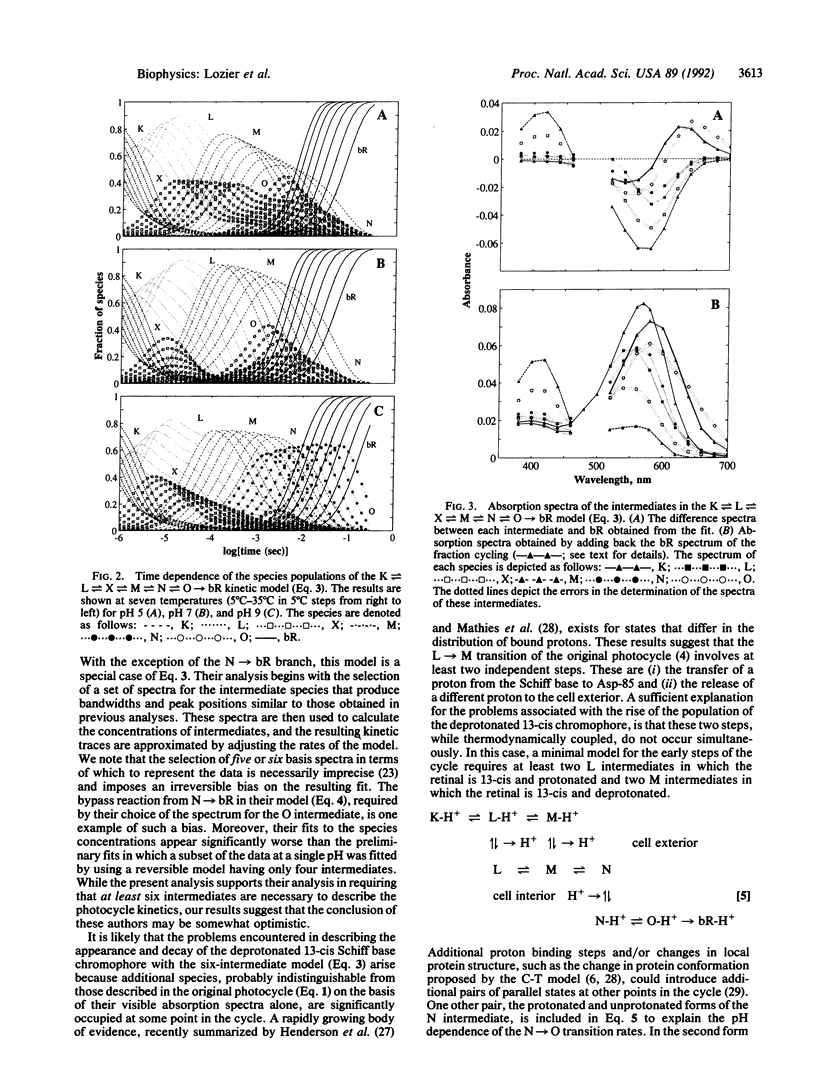
The absorbance changes that accompany the light-driven proton-pumping cycle of bacteriorhodopsin measured over a broad range of times, wavelengths, temperatures, and pH values have been globally fitted to the kinetic model K in equilibrium with L in equilibrium with X in equilibrium with M in equilibrium with N in equilibrium with O----bR. A remarkably good fit to the data was obtained by optimizing the rate constants at 20 degrees C and the corresponding activation energies at each pH value, together with the extinction coefficients for each intermediate, which were assumed to be independent of both pH and temperature. Back-reactions are included for all but the last step of the cycle and are found to be essential for fitting the data. The rates of these reactions are large, and the analogous irreversible model produced significantly worse fits to the data. Small systematic differences between the fit and the experimental data associated with the X, M, and O intermediates, together with the inability of the model to produce spectra for the X and M intermediates consistent with their assignment as molecular species, indicate that this model must be an incomplete description of the photocycle. We suggest that these problems arise from the presence of additional occupied states that are difficult to distinguish on the basis of their visible absorption spectra alone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Bousché O., Rothschild K. J. Protein dynamics in the bacteriorhodopsin photocycle: submillisecond Fourier transform infrared spectra of the L, M, and N photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2388–2392. doi: 10.1073/pnas.88.6.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of fully reduced membrane-bound cytochrome oxidase with oxygen at 176K. Biochem J. 1978 Sep 1;173(3):799–810. doi: 10.1042/bj1730799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Groma G. I., Dancshazy Z. How Many M Forms are there in the Bacteriorhodopsin Photocycle? Biophys J. 1986 Aug;50(2):357–366. doi: 10.1016/S0006-3495(86)83469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Lindau M., Heyn M. P. Distributed kinetics of the charge movements in bacteriorhodopsin: evidence for conformational substates. Biophys J. 1988 Apr;53(4):623–633. doi: 10.1016/S0006-3495(88)83141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Korenstein R., Hess B., Kuschmitz D. Branching reactions in the photocycle of bacteriorhodopsin. FEBS Lett. 1978 Sep 15;93(2):266–270. doi: 10.1016/0014-5793(78)81118-1. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Resonance Raman spectroscopy of the retinylidene chromophore in bacteriorhodopsin (bR570), bR560, M421, and other intermediates: structural conclusions based on kinetics, analogues, models, and isotopically labeled membranes. Biochemistry. 1978 Oct 31;17(22):4722–4735. doi: 10.1021/bi00615a019. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi L. A., Lozier R. H., Bhattacharjee S. M., Nagle J. F. Testing kinetic models for the bacteriorhodopsin photocycle--II. Inclusion of an O to M backreaction. Photochem Photobiol. 1984 Oct;40(4):501–506. doi: 10.1111/j.1751-1097.1984.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Váró G., Duschl A., Lanyi J. K. Interconversions of the M, N, and O intermediates in the bacteriorhodopsin photocycle. Biochemistry. 1990 Apr 17;29(15):3798–3804. doi: 10.1021/bi00467a029. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


