Abstract
Context:
Neither uncorrected- nor albumin-corrected total calcium reliably predict ionized calcium in patients with end-stage renal disease. However, little is known about the consequences of inaccurate assessment of calcium concentration using total calcium.
Objective:
We hypothesized that hidden hypercalcemia (ie, elevated ionized calcium with normal total calcium) and apparent hypercalcemia (ie, elevated ionized calcium with elevated total calcium) are both associated with increased mortality risk.
Design, Setting, and Patients:
We identified 874 incident hemodialysis patients with measured serum ionized calcium, total calcium, albumin, phosphorus, and bicarbonate from October 2007 to December 2011, using data from a large dialysis organization in the United States.
Exposures:
Serum concentrations of ionized calcium and total calcium were measured.
Main Outcome Measure:
The primary outcome was all-cause mortality.
Results:
There was only fair interindex agreement with calcium status between ionized calcium and uncorrected or corrected total calcium (κ = 0.32 and 0.27, respectively). Among patients with high ionized calcium (>1.32 mmol/liter), 88% and 70% patients were incorrectly categorized as being normocalcemic using uncorrected and corrected total calcium, respectively, and were thus considered to have “hidden hypercalcemia.” Compared to patients with low-normal ionized calcium (1.16–1.24 mmol/liter), patients with high ionized calcium had a significantly higher mortality risk (adjusted hazard ratio, 1.77; 95% confidence interval, 1.13–2.75). Furthermore, compared to patients with normocalcemia (ionized calcium 1.16–1.32 mmol/liter), those with hidden hypercalcemia by uncorrected and corrected total calcium also had a higher risk for death (adjusted hazard ratio 1.75 [95% confidence interval 1.11–2.75] and 1.80 [95% confidence interval, 1.11–2.90], respectively).
Conclusion:
The majority of end-stage renal disease patients with elevated ionized calcium are incorrectly categorized as normocalcemic using conventional total calcium measurements; these patients have a higher death risk. Future research is needed to establish whether reducing ionized calcium concentrations in these patients improves clinical outcomes.
“The majority of end-stage renal disease patients with elevated ionized calcium are incorrectly categorized as normocalcemic using conventional total calcium measurements; these patients are at higher death risk. Further research is needed to establish whether reducing ionized calcium concentrations in these patients improves clinical outcomes.”
Hypercalcemia is an established risk factor for death among patients with end-stage renal disease (ESRD) (1–8). In the predialysis period, serum calcium concentrations decrease as kidney function declines because of decreased intestinal calcium absorption and diminished renal tubular reabsorption, which is attributed to blunted activation of vitamin D in the kidney resulting from elevated fibroblast growth factor-23 and reduced functioning renal mass (9–11). Serum calcium concentrations then rise following the initiation of hemodialysis (4), likely from decreased renal calcium excretion, active vitamin D treatment, calcium-based phosphate binders, and/or hyperparathyroidism. Elevated extracellular calcium levels, along with hyperphosphatemia, raise the risk of vascular calcification, which leads to the development of cardiovascular disease in this population (12–14). Given these observations, current clinical practice guidelines for patients with ESRD suggest maintaining total serum calcium concentrations within the normal range (15).
Approximately 45% of total serum calcium is in the form of physiologically active ionized calcium. However, in clinical practice, total calcium is typically measured in lieu of ionized calcium given its lower cost and less time and effort needed to process and test samples. Several correction equations using both total calcium and serum albumin have been developed to predict ionized calcium concentrations given the experimental findings that approximately 40% of total calcium is bound to albumin with a ratio of 0.8 mg of calcium per 1 g of albumin in normal subjects (16). However, the proportion of albumin-bound serum calcium varies according to acid-base balance. Additionally, serum calcium also binds to multiple organic and inorganic anions including sulfate, bicarbonate, and phosphate. Previous studies have demonstrated that neither uncorrected nor corrected total calcium adequately predict ionized calcium concentrations in patients with advanced kidney disease (17–20), in part because of frequent accompaniment of metabolic acidosis, hyperphosphatemia, and high plasma sulfate concentrations. As a result, clinical practice guidelines also support ionized calcium measurement as the preferred method to evaluate calcium status in patients with advanced kidney diseases (15).
Although the prevalence of high albumin-corrected total calcium (>10.2 mg/dl) has been decreased over time from 20% in the early 2000s to 4–5% in the 2010s (21, 22), there are currently few data regarding the implications of discrepant total and ionized calcium concentrations upon clinical outcomes in patients with ESRD. Because ionized calcium is infrequently measured in clinical practice, we examined a 5-year cohort of incident hemodialysis patients treated in facilities operated by a large dialysis organization in the United States, hypothesizing that hidden hypercalcemia (ie, elevated ionized calcium with normal total calcium) and apparent hypercalcemia (ie, elevated ionized calcium with elevated total calcium) are both associated with increased mortality risk.
Subjects and Methods
This study was approved by the Institutional Review Boards of the Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles, University of California Irvine Medical Center, and the University of Washington as exempt from informed consent.
Patients
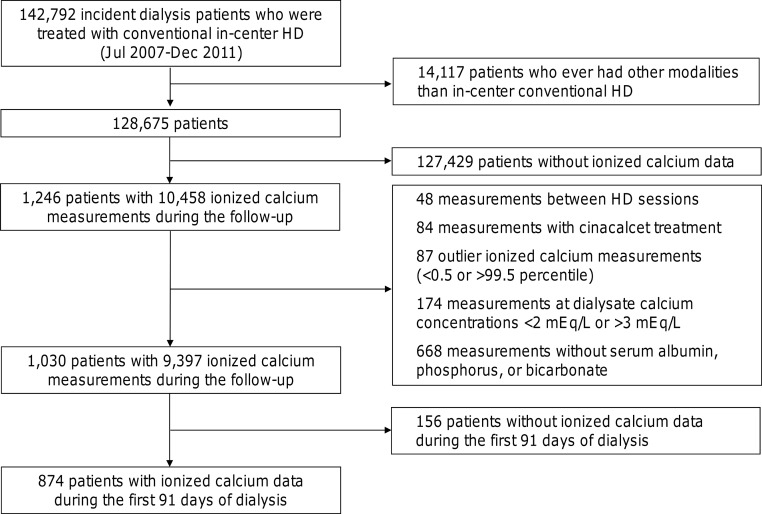
We extracted, refined, and examined electronic data from all incident dialysis patients who were age at least 18 years and received conventional hemodialysis treatment in facilities operated by a large dialysis organization in the United States from October 1, 2007, to December 31, 2011 (23). We excluded patients who ever been treated with peritoneal dialysis, home hemodialysis, or nocturnal in-center hemodialysis from this study. Of 128 675 patients who were treated with conventional hemodialysis only during follow-up, we identified 1246 patients with 10 458 ionized calcium measurements. We excluded 48 measurements performed in between hemodialysis sessions (ie, during the interdialytic period), 84 measurements done in patients receiving cinacalcet, 87 measurements with extreme values (<0.5 or >99.5th percentile), and 174 measurements in patients dialyzed against <2.0 mEq/liter or >3.0 mEq/liter calcium. Because the fraction of ionized calcium against total calcium is influenced by serum concentrations of albumin, phosphorus, and bicarbonate (17–20, 33, 34), we also excluded 668 measurements with missing simultaneous evaluation of these variables. We then restricted measurements to those obtained during the first 91 days of dialysis treatment. The final analytic cohort was comprised of 874 incident hemodialysis patients (Figure 1).
Figure 1.
Study flow diagram. HD, hemodialysis.
Demographic, clinical, and laboratory measures
Information on race/ethnicity, primary insurance, access type, and International Classification of Diseases-9 codes were obtained from the electronic database of the dialysis provider. International Classification of Diseases-9 codes were used to determine the following comorbidities: diabetes mellitus, hypertension, dyslipidemia, atherosclerotic heart disease, congestive heart failure, cerebrovascular disease, and other cardiovascular disease. Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the central laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured by automated and standardized methods. Specifically, serum ionized calcium and albumin was measured by using ion-selective electrode and bromcresol green. Most blood samples were collected predialysis with the exception of the postdialysis urea that was obtained to calculate urea kinetics. Single-pool Kt/V delivered by dialysis was calculated using urea kinetic modeling equations (24, 25). Albumin-corrected total calcium was calculated as follows (15):
Because serum concentrations of albumin, total calcium, phosphorus, and bicarbonate, and medication use (ie, oral/IV active vitamin D and calcium-based phosphorus binders) are closely related to calcium status, those data were extracted from the same days of ionized calcium measurements, whereas data for body mass index (BMI) and other laboratory variables including single-pool Kt/V, hemoglobin, creatinine, and ferritin, were extracted during the first 91 days of dialysis. Those repeated measures were then averaged and used in all analyses to minimize measurement variability.
Statistical analysis
Patient characteristics are expressed as mean ± SD, medians (interquartile range), or percentages, as appropriate. Differences between included and excluded patients were compared by standardized differences because of the large sample size of this study (26, 27).
Total and ionized calcium was categorized as low (<8.6 mg/dL and <1.16 mmol/liter, respectively), low-normal (8.6 to 9.4 mg/dl and 1.16 to 1.24 mmol/liter, respectively), high-normal (>9.4 to 10.2 mg/dl and > 1.24 to 1.32 mmol/liter, respectively), and high (>10.2 mg/dl and >1.32 mmol/liter, respectively). The κ-statistic measure was used to evaluate the interindex agreement for categories of calcium status.
Cox proportional hazards models were used to analyze the association between calcium status and all-cause mortality. For comparison between calcium indices, uncorrected total calcium, albumin-corrected total calcium, and ionized calcium values were also normalized by conversion to a Z score based on the normal range in the central laboratory (not a data-derived Z score) as follows (17–20): the lower and upper limits of the normal ranges for ionized calcium (1.16 and 1.32 mmol/liter) and total calcium (8.6 and 10.2 mg/dl) were treated as the 95% confidence intervals (CIs) and used to calculate the mean and SD by the formula “” where the mean and SD were 1.24 and 0.08 mmol/liter and 9.4 and 0.8 mg/dl for ionized and total calcium, respectively. The association of each Z score with mortality was then modeled using restricted cubic splines with knots placed at the fifth, 35th, 65th, and 95th percentiles of exposure. Median values of each calcium index were used as a reference.
For each model, three levels of adjustments were used as follows: 1) minimally adjusted models that included age, sex, race, and ethnicity (non-Hispanic white, non-Hispanic Black, and other race/ethnicity), central venous catheter use as vascular access, and diabetes; 2) case-mix adjusted models that included the above plus primary insurance (Medicare, Medicaid, and other), natural log-transformed BMI, and history of hypertension and cardiovascular disease); and 3) fully adjusted models that included all covariates in the case-mix model plus laboratory variables (ie, single-pool Kt/V; hemoglobin; serum concentrations of albumin, creatinine, phosphorus, and bicarbonate; and natural log-transformed intact PTH and ferritin) and medication use (active vitamin D [either oral or IV] and oral calcium salts [either calcium acetate or calcium carbonate]). Because of the limited number of outcomes, we used the minimally adjusted models as the primary analyses. When estimating the mortality risk of hidden hypercalcemia, we also used backwards Akaike's information criterion (AIC)-based Cox regression to the fully adjusted models, which included age, race and ethnicity, central venous catheter use, history of hypertension, hemoglobin, serum albumin, serum phosphorus, serum bicarbonate, the use of active vitamin D, and the use of oral calcium salts, to balance the tradeoff between precision and overfitting. Proportional hazards assumptions were tested using Schoenfeld residuals and log-log plots against survival.
The frequency of missing covariate data was low (1.0%, 0.1%, 0.6%, 0.6%, and 0.9% for single-pool Kt/V, hemoglobin, creatinine, and natural log-transformed intact PTH and ferritin, respectively), and multiple imputation method with five data sets was used in Cox regression analyses. Available data for oral medication use were limited to clopidogrel or medications related to lipid and bone mineral metabolism. Fourteen percent of patients had no available oral medication data, and they were considered nonusers of active vitamin D compounds or calcium salts. We conducted all analyses using STATA MP, version 13.1 (StataCorp).
Results
Baseline demographic, clinical, and laboratory characteristics
There were 874 patients who met eligibility criteria, among whom the mean ± SD age was 63 ± 15 years old, 57% were male, 55% were non-Hispanic white, 35% were non-Hispanic Black, 56% were diabetic, and 77% used central venous catheters as their vascular access during the first 91 days of dialysis treatment (Table 1). The mean ± SD concentrations of ionized calcium, uncorrected total calcium, and albumin-corrected total calcium were 1.19 ± 0.10 mmol/liter, 8.6 ± 0.7 mg/dl, and 9.1 ± 0.6 mg/dl, respectively. Compared to excluded incident hemodialysis patients in whom ionized calcium, serum albumin, total calcium, phosphorus, and bicarbonate were not simultaneously measured during the first 91 days of dialysis treatment, included patients were less likely to be Hispanics or minorities; were more likely to be treated with lower dialysate calcium baths; and were more likely to have higher bicarbonate concentrations (standard differential >20%, Supplemental Table 1). In the overall cohort, 77 patients (9%) had hypercalcemia defined by ionized calcium (>1.32 mmol/liter) (Table 1).
Table 1.
Characteristics of 874 Incident HD Patients According to Ionized Calcium Concentrations
| Total n = 874 | Low <1.16 mmol/liter n = 284 (32%) | Low-Normal 1.16–1.24 mmol/liter n = 335 (38%) | High-Normal >1.24–1.32 mmol/liter n = 178 (20%) | High >1.32 mmol/liter n = 77 (9%) | P for Trend | |
|---|---|---|---|---|---|---|
| Age | 63 ± 15 | 62 ± 15 | 63 ± 15 | 64 ± 15 | 67 ± 15 | 0.01 |
| Male | 57% | 62% | 58% | 51% | 47% | .003 |
| Race | ||||||
| Non-Hispanic white | 55% | 44% | 58% | 60% | 69% | <.001 |
| Non-Hispanic Black | 35% | 42% | 33% | 32% | 26% | .003 |
| Others | 10% | 14% | 10% | 8% | 5% | .01 |
| Insurance | ||||||
| Medicare | 46% | 44% | 50% | 44% | 47% | .78 |
| Medicaid | 6% | 6% | 7% | 4% | 5% | .56 |
| Others | 48% | 50% | 44% | 52% | 48% | >.99 |
| Comorbidities | ||||||
| Diabetes | 56% | 62% | 54% | 51% | 55% | .04 |
| Hypertension | 46% | 47% | 44% | 49% | 42% | .78 |
| Cardiovascular disease | 46% | 46% | 50% | 40% | 39% | .15 |
| CV catheter | 77% | 85% | 77% | 67% | 70% | <.001 |
| Single-pool Kt/V | 1.47 ± 0.33 | 1.45 ± 0.34 | 1.47 ± 0.32 | 1.49 ± 0.31 | 1.48 ± 0.33 | .22 |
| BMI | 26.8 (IQR, 23.0–31.7) | 26.4 (IQR, 23.0–31.5) | 26.9 (IQR, 22.8–31.4) | 27.1 (IQR, 23.4–32.2) | 26.8 (IQR, 24.5–32.8) | .34 |
| Dialysate calcium (mEq/liter) | 2.25 (IQR, 2.0–2.5) | 2.3 (IQR, 2.0–2.5) | 2.33 (IQR, 2.0–2.5) | 2.50 (IQR, 2.0–2.5) | 2.25 (IQR, 2.0–2.5) | .93 |
| Active vitamin D (%) | 32% | 31% | 35% | 32% | 25% | .42 |
| Calcium salts (%) | 12% | 13% | 10% | 16% | 10% | .92 |
| Laboratories | ||||||
| Hemoglobin (g/dl) | 10.9 ± 1.3 | 10.6 ± 1.3 | 10.9 ± 1.4 | 11.0 ± 1.3 | 11.1 ± 1.4 | <.001 |
| Albumin (g/dl) | 3.4 ± 0.6 | 3.3 ± 0.6 | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.6 | <.001 |
| Creatinine (mg/dl) | 5.7 ± 2.3 | 6.2 ± 2.4 | 5.7 ± 2.4 | 5.2 ± 2.1 | 5.0 ± 1.9 | <.001 |
| Uncorrected calcium (mg/dl) | 8.6 ± 0.7 | 8.0 (IQR, 7.7–8.5) | 8.7 (IQR, 8.4–8.9) | 9.0 (IQR, 8.8–9.4) | 9.6 (IQR, 9.2–9.9) | <.000 |
| Corrected calcium (mg/dl) | 9.1 ± 0.6 | 8.7 (IQR, 8.4–9.0) | 9.1 (IQR, 8.9–9.3) | 9.4 (IQR, 9.3–9.6) | 10.0 (IQR, 9.7–10.3) | <.001 |
| Phosphorus (mg/dl) | 4.7 ± 1.3 | 5.1 ± 1.5 | 4.7 ± 1.3 | 4.4 ± 1.0 | 4.3 ± 1.3 | <.001 |
| Intact PTH (pg/ml) | 315 (IQR, 190–490) | 413 (IQR, 263–627) | 330 (IQR, 198–467) | 249 (IQR, 142–351) | 193 (IQR, 57–313) | <.001 |
| Ferritin (ng/ml) | 295 (IQR, 173–494) | 292 (IQR, 170–476) | 304 (IQR, 181–512) | 269 (IQR, 161–447) | 362 (IQR, 200–542) | .66 |
| Bicarbonate (mEq/liter) | 24 ± 3 | 24 ± 3 | 24 ± 3 | 24 ± 3 | 25 ± 3 | .30 |
Values are expressed as mean (sd), median (IQR), or percentage, appropriately. SI conversion factors: to convert hemoglobin to g/liter, multiply by 10; albumin to g/liter, multiply by 10; creatinine to μmol/liter multiply by 88.4; calcium to mmol/liter, multiply by 0.25; phosphorus to mmol/liter, multiply by 0.323; PTH to ng/liter, multiply by 1.0; ferritin to pmol/liter, multiply by 2.247; bicarbonate to mmol/liter, multiply by 1.0.
Abbreviations: CV, central venous; IQR, interquartile range.
We categorized patients into a low, low-normal, high-normal, or high calcium group based on each index, and then compared the concordance of calcium status between ionized calcium and uncorrected/corrected total calcium (Table 2). Among 77 patients with high ionized calcium (>1.32 mmol/liter), 68 (88%) and 55 (71%) were incorrectly categorized as normocalcemic using uncorrected and corrected total calcium (>10.2 mg/dl), respectively. Likewise, among 284 patients with low ionized calcium (<1.16 mmol/liter), 55 (19%) and 164 (58%) were incorrectly categorized as normocalcemic using uncorrected and corrected total calcium (>10.2 mg/dl), respectively. Overall, the kappa statistics against ionized calcium status were 0.32 and 0.27 for uncorrected and corrected total calcium, respectively, indicating only a fair agreement with each index (28).
Table 2.
Concordance and Discordance of Calcium Status Between Ionized Calcium and Uncorrected and Corrected Calcium
| Uncorrected Calcium |
Total | ||||
|---|---|---|---|---|---|
| Low (<8.6 mg/dl) | Low-Normal (8.6–9.4 mg/dl) | High-Normal (>9.4–10.2 mg/dl) | High (>10.2 mg/dl) | ||
| Ionized calcium | |||||
| Low (<1.16 mmol/liter) | 229 (26%) | 55 (6.3%) | 0 (0%) | 0 (0%) | 284 (33%) |
| Low-normal (1.16–1.24 mmol/liter) | 133 (15%) | 191 (22%) | 11 (1.3%) | 0 (0%) | 335 (39%) |
| High-normal (>1.24–1.32 mmol/liter) | 22 (2.5%) | 123 (14%) | 32 (3.7%) | 1 (0.1%) | 178 (20%) |
| High (>1.32 mmol/liter) | 2 (0.2%) | 31 (3.6%) | 35 (4.0%) | 9 (1.0%) | 77 (8.9%) |
| Total | 386 (44%) | 400 (46%) | 78 (9.0%) | 10 (1.2%) | 874 |
| Corrected Calcium |
Total | ||||
|---|---|---|---|---|---|
| Low (<8.6 mg/dL) | Low-Normal (8.6–9.4 mg/dl) | High-Normal (>9.4–10.2 mg/dl) | High (>10.2 mg/dl) | ||
| Ionized calcium | |||||
| Low (<1.16 mmol/liter) | 120 (14%) | 152 (17%) | 12 (1.4%) | 0 (0%) | 284 (33%) |
| Low-normal (1.16–1.24 mmol/liter) | 18 (2.1%) | 261 (30%) | 55 (6.3%) | 1 (0.1%) | 335 (39%) |
| High-normal (>1.24–1.32 mmol/liter) | 0 (0%) | 87 (10%) | 86 (10%) | 5 (0.6%) | 178 (20%) |
| High (>1.32 mmol/liter) | 0 (0%) | 5 (0.6%) | 50 (5.8%) | 22 (2.5%) | 77 (8.9%) |
| Total | 138 (16%) | 505 (58%) | 203 (23%) | 28 (3.2%) | 874 |
Boldface, normal, and italic fonts indicate underestimation, correct classification, and overestimation of ionized calcium status by uncorrected/corrected total calcium, respectively.
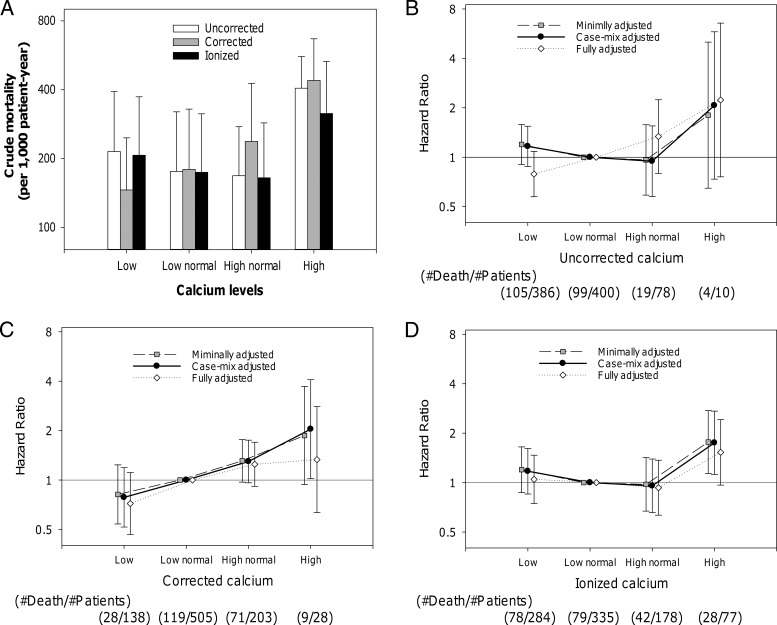
Association of calcium indices with mortality
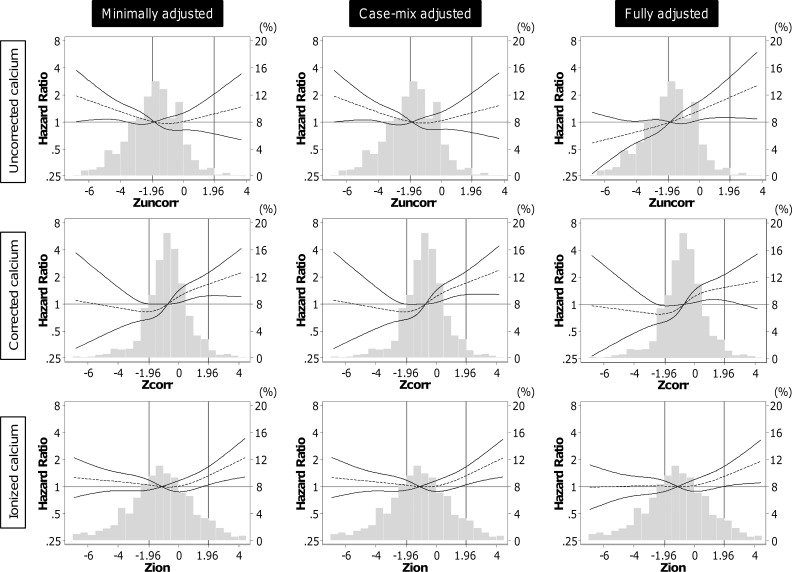
A total of 227 deaths were observed during a total follow-up period of 1174 patient-years, with an overall crude rate of death of 193 per 1000 patient-years. Figure 2 depicts the crude mortality and the adjusted hazard ratios (HRs) for all-cause death according to calcium status by uncorrected, corrected, and ionized calcium. The highest mortality was observed in patients with hypercalcemia regardless of calcium indices. In the minimally adjusted models (the primary analysis), the estimated HRs of hypercalcemia were high in uncorrected and corrected total calcium, but the number of events in these categories were limited (n = 4 and n = 9, respectively), and these associations were not statistically significant (Figure 2, B and C). Meanwhile, 28 of 77 patients with hypercalcemia (>1.32 mmol/liter) defined by ionized calcium died during the follow-up, and ionized hypercalcemia showed a significantly higher mortality risk with an HR of 1.76 (95% confidence interval [CI], 1.13–2.75); reference: low-normal ionized calcium [1.16–1.24 mmol/liter]) (Figure 2D). High-normal ionized calcium (>1.24–1.32 mmol/liter) was not associated with mortality (HR, 0.98 [95%CI, 0.67–1.42], P = .90). Consistent findings were observed in the restricted cubic spline models by using Z scores of three calcium indices (Figure 3). High ionized calcium concentrations were significantly associated with death in the range beyond the upper normal limit of the central laboratory (>1.96 of Z score or >1.32 mmol/liter, the right vertical line; reference, 1.20 mmol/liter).
Figure 2.
The associations of uncorrected calcium, corrected calcium, and ionized calcium with mortality in 874 incident hemodialysis patients (2008–2011). Low, low normal, high normal, and high calcium status is defined as less than 8.6 mg/dl, 8.6–9.4 mg/dl, higher than 9.4–10.2 mg/dl, and higher than 10.2 mg/dl in total calcium, or less than 1.16 mmol/liter, 1.16–1.24 mmol/liter, higher than 1.24–1.32 mmol/liter, and higher than 1.32 mmol/liter in ionized calcium, respectively. Points and lines represent point estimates and 95% CIs, respectively.
Figure 3.
A panel of the associations of between all-cause mortality and each Z score of ionized (Zion), uncorrected (Zuncorr) total calcium, and corrected (Zcorr) total calcium with three-level adjustments. Vertical lines at –1.96 and 1.96 of Z score indicate the upper and lower limits of the normal range for each calcium index in the central laboratory, respectively.
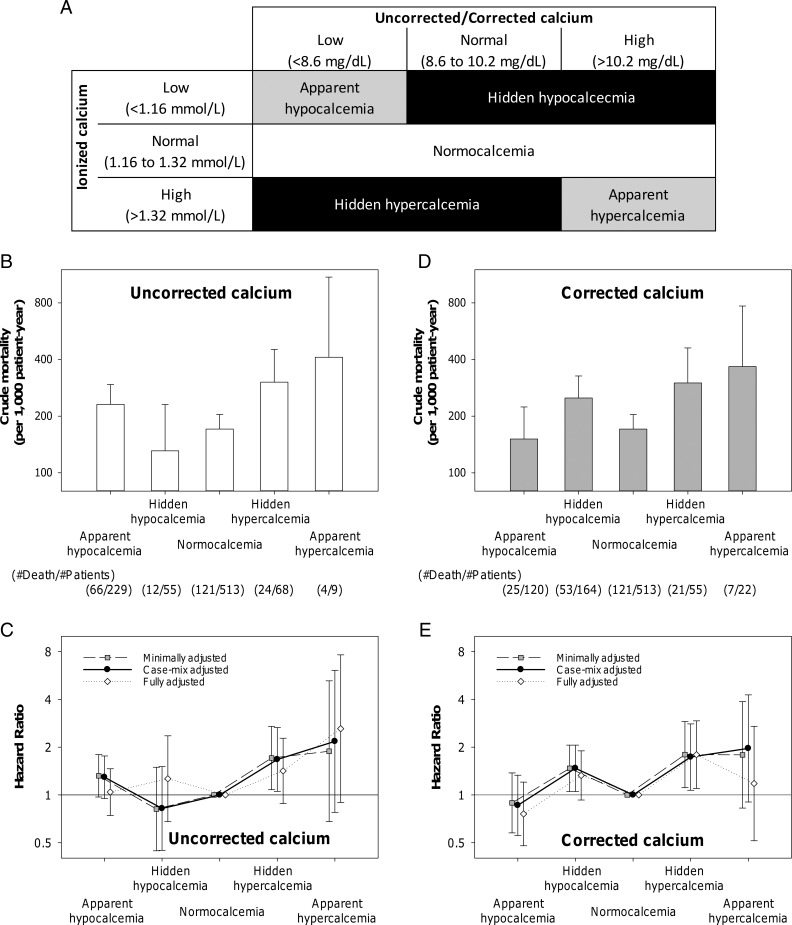
Hidden calcium abnormality and mortality
To examine the mortality risk associated with hidden calcium abnormality (ie, misclassification of calcium status using uncorrected and corrected calcium concentrations, in which ionized calcium served the “gold standard”), we conducted Cox regression analyses after categorizing patients into five groups, namely, “apparent hypocalcemia,” “hidden hypocalcemia,” “normocalcemia,” “hidden hypercalcemia,” and “apparent hypercalcemia” (Figure 4A).
Figure 4.
Calcium status by the combination of ionized calcium and uncorrected/corrected calcium and their associations with mortality. (A) Definition of calcium status by the combination of ionized calcium and uncorrected/corrected calcium. (B) Crude mortality and (C) adjusted HR according to calcium status by the combination of ionized calcium and uncorrected total calcium. (D) Crude mortality and (E) adjusted HR according to calcium status by the combination of ionized calcium and corrected total calcium. The number of patients and all-cause death in each group is shown in the bottom of panels B and D. Points and lines represent point estimates and 95% CIs, respectively.
For uncorrected total calcium, the highest crude mortality was observed in patients with apparent hypercalcemia (Figure 4B). Patients with hidden hypercalcemia showed the second highest crude mortality rates next to apparent hypercalcemia. In the minimally adjusted models (primary analysis), the mortality risk was significant in patients with hidden hypercalcemia (HR, 1.75 [95% CI, 1.11–2.75], P = .02) but not in those with hidden hypocalcemia (HR, 0.82 [95% CI, 0.45–1.50], P = .52) (Figure 4C). In the sensitivity analysis using backwards AIC-based Cox regression, there was a trend toward a higher mortality risk of hidden hypercalcemia (HR 1.49 [95% CI, 0.94–2.35], P = .09).
For corrected total calcium, the highest crude mortality rate was observed in patients with apparent hypercalcemia (Figure 4D). Patients with hidden hypercalcemia and hidden hypocalcemia showed the second and third highest crude mortality rates. In the minimally adjusted models (primary analysis), the mortality risk was significant both in patients with hidden hypercalcemia (HR, 1.80 [95% CI, 1.11–2.90], P = .02) and in those with hidden hypocalcemia (HR, 1.47 [95% CI, 1.05–2.06], P = .02) (Figure 4E). Further adjustments for case-mix variables did not change these associations. The mortality risk of hidden hypocalcemia was attenuated and lost its statistical significance in the fully adjusted model, but the risk of hidden hypercalcemia remained significant. The mortality risk of hidden hypercalcemia was still significant in the sensitivity analysis using backwards AIC-based Cox regression (HR, 1.71 [95% CI, 1.05–2.78], P = .03).
Discussion
To our knowledge, this is the largest study examining ionized calcium in patients with chronic kidney disease conducted to date. In this 5-year cohort of incident hemodialysis patients from a large dialysis organization in the United States, high ionized calcium was associated with higher mortality risk. However, we observed that there was only fair interindex agreement with calcium status between ionized and total calcium. Approximately 90% and 70% of patients with ionized hypercalcemia were incorrectly categorized as normocalcemic by uncorrected and corrected total calcium concentrations, respectively, and were considered to have “hidden hypercalcemia.” When compared to normocalcemic patients, those with hidden hypercalcemia using either uncorrected or corrected total calcium had significantly higher mortality risk, as did those with apparent hypercalcemia.
Our study is the first to demonstrate that hemodialysis patients with hidden hypercalcemia have higher mortality risk. The low correlation between ionized vs uncorrected or corrected total calcium has been shown in multiple previous studies (17–20). However, these findings have not been acknowledged well in clinical practice guidelines because of the lack of data showing that misclassification of calcium status is clinically relevant (15). A recent cohort study of 160 hemodialysis patients did not find such an association between ionized calcium and all-cause death (29). However, there were only three patients with ionized hypercalcemia (>1.32 mmol/liter), and the impact of the discrepancy between ionized and total calcium on survival was not examined. Our study found that the mortality risk appeared to increase with ionized calcium concentrations above the normal range irrespective of total calcium concentrations, suggesting that many hypercalcemic patients may not come to clinical attention when calcium status is evaluated by uncorrected or corrected total calcium concentrations alone.
Common criticisms of measuring ionized calcium include lower reproducibility, a more time-consuming process, and higher costs than total calcium measurements (30). Clinical application of this technique requires periodic maintenance, electrode replacement with associated downtime, and redundancy of instrumentation and personnel (31). However, the previously used colorimetric and biological assays have been replaced by much more reliable ion-sensitive electrodes that are available both in blood-gas instruments and automated chemistry analyzers with equivalent analytical coefficients of variation to total calcium (32). Additionally, it is important when evaluating cost-effectiveness of a diagnostic test to weigh its measurement cost against potential medical costs induced by false-negative or false-positive diagnosis, especially when such misdiagnosis is associated with severe adverse events including cardiovascular disease and death.
An important limitation of this study is that the large size of the dialysis organization may have limited optimal processing needed for measurements of ionized calcium. To optimally measure ionized calcium, samples should be collected and managed anaerobically with complete filling of the blood sampling tube to avoid change in pH resulting from a loss of carbon dioxide (33, 34). A closed-tube automation system and analyzers would make serum ionized calcium measurement more reliable in clinical practice. Additionally, time to centrifugation and temperature during transfer also influences ionized calcium concentrations through pH change. However, those samples used in this study were collected for serum bicarbonate measurement as well. Several studies also demonstrated that serum ionized calcium can be measured without a significant error up to 24 hours from blood draw if samples are anaerobically collected and kept at below 4 C (35–37). Additionally, although ionized calcium concentrations in serum samples from dialysis patients show large positive and negative changes after 6-hour storage at 4 C, the mean difference across the patient population is low (37). This finding suggests that the mean ionized calcium concentrations from an adequate number of subjects can be used to estimate the population-level associations with clinical outcomes, albeit vulnerable to bias toward the null.
Several other limitations of our study should be noted when interpreting our results. First, available ionized calcium measures may not be representative of the entire source population. Potential selection bias may exist, such that physicians may be likely to measure ionized calcium in patients with low dialysate calcium concentration or unstable patients with suspected disease conditions that may induce calcium abnormality. Second, the risk of hidden hypocalcemia, but not hidden hypercalcemia, might have been overestimated if there remained residual confounding by central venous catheter use as vascular access. Indeed, heparin used for catheter lock lowers the fraction of ionized calcium by binding (33), and catheter use is a risk factor for mortality in hemodialysis patients (38). Third, the small sample size of patients with hidden or apparent calcium abnormality resulted in wide CIs for the estimated associations and might have inflated the likelihood of type II error in our analyses.
In conclusion, hidden hypercalcemia is common in incident hemodialysis patients and associated with higher mortality risk in this population. Prospective studies are needed to identify which subpopulations of chronic kidney disease patients who would most benefit from ionized calcium measurement vs routinely used uncorrected and corrected total calcium assays and to establish whether reducing ionized calcium concentrations in these patients improves clinical outcomes.
Acknowledgments
The authors thank DaVita Clinical Research for providing the clinical data for this research.
The work in this manuscript has been performed with the support of the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) of the National Institutes of Health research grants R01-DK095668 (to R.M. and K.K.-Z.), K24-DK091419 (to K.K.-Z.), and R01-DK078106 (to K.K.-Z.); philanthropic grants from Harold Simmons, Louis Chang, Joseph Lee, and AVEO (to K.K.-Z.); NIDDK grants R01-DK096920 and U01-DK102163 (to C.P.K.); NIDDK grant K23-DK102903 (to C.M.R.); and the Shinya Foundation for International Exchange of Osaka University Graduate School of Medicine Grant (to Y.O.).
Y.O., R.M., M.B.R., and K.K.-Z. Study conduct: Y.O., R.M., M.B.R., and E.S. Data collection: R.M. and K.K.-Z. Data analysis: Y.O. and E.S. Data interpretation: Y.O., R.M., M.B.R., E.S., C.M.R., W.L., C.P.K., and K.K.-Z. Drafting manuscript: Y.O. Revising manuscript content: R.M., M.B.R., E.S., C.M.R., W.L., C.P.K., and K.K.-Z. Approving final version of manuscript: Y.O., R.M., M.B.R., E.S., C.M.R., W.L., C.P.K., and K.K.-Z. K.K.-Z. takes responsibility for the integrity of the data analysis.
Preliminary results of this study have been partly presented as a poster at the National Kidney Foundation 2016 Spring Clinical Meetings, Boston, Massachusetts.
Disclosure Summary: K.K.-Z. has received honoraria from Abbott, Abbvie, Alexion, Amgen, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi-Aventis, Shire, Vifor, UpToDate, and ZS Pharma. C.P.K. has received honoraria from Abbott, Relypsa, Sanofi-Aventis, and ZS Pharma.
Footnotes
- AIC
- Akaike's information criterion
- BMI
- body mass index
- CI
- confidence interval
- ESRD
- end-stage renal disease
- HR
- hazard ratio.
References
- 1. Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. [DOI] [PubMed] [Google Scholar]
- 2. Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. [DOI] [PubMed] [Google Scholar]
- 3. Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. [DOI] [PubMed] [Google Scholar]
- 4. Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. [DOI] [PubMed] [Google Scholar]
- 5. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52:519–530. [DOI] [PubMed] [Google Scholar]
- 6. Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. J Am Soc Nephrol 2015;26:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danese MD, Belozeroff V, Smirnakis K, et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. [DOI] [PubMed] [Google Scholar]
- 10. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakano C, Hamano T, Fujii N, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7:810–819. [DOI] [PubMed] [Google Scholar]
- 12. Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. [DOI] [PubMed] [Google Scholar]
- 13. Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. [DOI] [PubMed] [Google Scholar]
- 14. Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496. [DOI] [PubMed] [Google Scholar]
- 15. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–S130. [DOI] [PubMed] [Google Scholar]
- 16. Moore EW. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J Clin Invest. 1970;49:318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clase CM, Norman GL, Beecroft ML, et al. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant. 2000;15:1841–1846. [DOI] [PubMed] [Google Scholar]
- 18. Jain A, Bhayana S, Vlasschaert M, et al. A formula to predict corrected calcium in haemodialysis patients. Nephrol Dial Transplant. 2008;23:2884–2888. [DOI] [PubMed] [Google Scholar]
- 19. Gauci C, Moranne O, Fouqueray B, et al. Pitfalls of measuring total blood calcium in patients with CKD. J Am Soc Nephrol. 2008;19:1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrari P, Singer R, Agarwal A, et al. Serum phosphate is an important determinant of corrected serum calcium in end-stage kidney disease. Nephrology (Carlton). 2009;14:383–388. [DOI] [PubMed] [Google Scholar]
- 21. 2012: Annual Report of the Dialysis Outcomes and Practice Patterns Study: Hemodialysis Data 1997–2011. Arbor Research Collaborative for Health, Ann Arbor, MI. [Google Scholar]
- 22. U.S. Renal Data System. USRDS 2015 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015. [Google Scholar]
- 23. Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. [DOI] [PubMed] [Google Scholar]
- 25. Miller JE, Kovesdy CP, Nissenson AR, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schacht A, Bogaerts K, Bluhmki E, et al. A new nonparametric approach for baseline covariate adjustment for two-group comparative studies. Biometrics. 2008;64:1110–1116. [DOI] [PubMed] [Google Scholar]
- 28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 29. Jean G, Granjon S, Zaoui E, et al. Usefulness and feasibility of measuring ionized calcium in haemodialysis patients. Clin Kidney J. 2015;8:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 31. Calvi LM, Bushinsky DA. When is it appropriate to order an ionized calcium? J Am Soc Nephrol. 2008;19:1257–1260. [DOI] [PubMed] [Google Scholar]
- 32. Larsson L, Magnusson P. Ionized calcium or corrected total calcium? J Bone Miner Res. 2003;18:1554–1556. [DOI] [PubMed] [Google Scholar]
- 33. Baird GS. Ionized calcium. Clin Chim Acta. 2011;412:696–701. [DOI] [PubMed] [Google Scholar]
- 34. Siyam FF, Klachko DM. What is hypercalcemia? The importance of fasting samples. Cardiorenal Med. 2013;3:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toffaletti J, Blosser N, Kirvan K. Effects of storage temperature and time before centrifugation on ionized calcium in blood collected in plain vacutainer tubes and silicone-separator (SST) tubes. Clin Chem. 1984;30:553–556. [PubMed] [Google Scholar]
- 36. Conceicao SC, Ward MK, Alvarez-Ude F, et al. Determination of serum ionised calcium by ion-exchange electrode in normal subjects. Clin Chim Acta. 1978;86:143–151. [DOI] [PubMed] [Google Scholar]
- 37. Nikolakakis NI, De Francisco AM, Rodger RS, et al. Effect of storage on measurement of ionized calcium in serum of uremic patients. Clin Chem. 1985;31:287–289. [PubMed] [Google Scholar]
- 38. Xue JL, Dahl D, Ebben JP, et al. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. [DOI] [PubMed] [Google Scholar]