Abstract
Context:
Adipocytes represent an important insulin-responsive tissue taking an active part in glucose metabolism.
Objective:
This study sought to assess adipose tissue insulin resistance (IR) across the spectrum of glucose tolerance and to test its relation with free fatty acid (FFA) suppression during an oral glucose tolerance test (OGTT).
Design and Setting:
A cross-sectional analysis of a pediatric clinic–derived cohort of obese adolescents.
Patients or Other Participants:
Participants age 7–20 y with a body mass index that exceeded the 95th percentile for their age and sex.
Intervention(s):
A standard oral glucose tolerance test.
Main Outcome Measures:
The adipose tissue insulin resistance index (calculated as the product of fasting insulin and FFA concentrations) (Adipose IR) and the area under curve of FFAs during the OGTT were compared between glucose tolerance categories.
Results:
A total of 962 obese children and adolescents participated in this study. Adipose IR significantly increased across glucose tolerance categories (P for trend < .001). Within the normal glucose tolerance participants, an increase in adipose IR was observed related to an increase in 2-hr glucose levels. In a subsample of participants who underwent abdominal imaging for determination of lipid partitioning (n = 115), a tight relation of visceral fat (r = 0.34; P < .001) and the visceral/sc fat ratio (r = 0.55; P < .001) with the Adipose IR index was evident. Greater area under the curve FFAs (lower FFA suppression) during the OGTT was evident with worsening glucose tolerance (P for trend < .001). Glucose tolerance category, degree of obesity (body mass index–z score), IL-6, and low adiponectin emerged as significant predictors of the Adipose IR.
Conclusions:
Adipose IR is associated with reduced suppression of FFAs during the OGTT and with an altered adipocytokine profile. The negative relation with insulin secretion deserves further longitudinal investigation in the context of deteriorating glucose tolerance.
“Adipose tissue insulin resistance is associated with reduced suppression of free fatty acids during the oral glucose tolerance test and with an altered adipocytokine profile. Adipose tissue insulin resistance is also linked to abdominal lipid partitioning and the circulating lipid profile.”
Insulin resistance is a reduction in the response to insulin in specific tissues and includes among others reduced hepatic sensitivity, reduced peripheral muscle glucose uptake and reduced adipose tissue sensitivity (1). Although insulin sensitivity may be reduced in most insulin-responsive tissues it may also spare tissues. A very common cause of whole-body reduced insulin sensitivity is seen in childhood obesity (2, 3). It has previously been shown that the pattern of lipid partitioning, ie, the deposition of lipid within insulin-responsive tissues (such as liver and muscle) as well as in the intra-abdominal compartment are specific determinants of whole-body insulin sensitivity with greater effect than the degree of obesity per se (4). Although hepatic and muscular insulin resistance (IR) have gained much attention and investigation, the resistance of adipose tissue to the effects of basal and postprandial insulin concentrations has been less investigated. The gold standard for measuring insulin sensitivity is the euglycemic-hyperinsulinemic clamp (5), yet this technique allows assessment of muscle and liver insulin sensitivity with less emphasis on adipose tissue (unless traced glycerol is used to assess lipolysis). An adipose tissue insulin index has been described as a product of fasting FFA and fasting insulin concentrations (22).
Adipose tissue release of free fatty acids (FFA) via unsuppressed lipolysis is the main manifestation of adipose insulin resistance (IR). Circulating FFAs play a major role in the development of reduced insulin sensitivity (7, 8) and β-cell dysfunction (9, 10), both via lipotoxicity. Infiltration of some adipose tissue depots by macrophages may be the cause of the typical subclinical inflammatory state commonly detected in obese children (11). Previous studies have shown that among obese adolescents, the 2-hour glucose measurement following an oral glucose tolerance test (OGTT) is negatively associated with insulin sensitivity and with β-cell function (12, 13). We postulated that the adipose IR would parallel this observation so that obese children and adolescents with altered glucose tolerance will have a greater adipose tissue IR manifested also as lower suppression of FFAs during the OGTT. The aim of this study was therefore to assess adipose IR across the spectrum of glucose tolerance and to test its relation with FFA suppression during the OGTT.
Materials and Methods
Subjects were recruited to the Yale Pathophysiology of Type 2 Diabetes in Youth Study, a long-term, multiethnic cohort aimed at studying early alternations in glucose metabolism in obese children and adolescents (NCT01967849). Subjects were eligible to participate if they were at the age of 7–20 years and had a body mass index (BMI) that exceeded the 95th percentile for their age and sex. In addition to subject assent and parental consent, complete medical histories and thorough physical examinations were obtained from each participant. All subjects tested negative for autoimmune markers for type 1 diabetes (insulin antibody, GAD65, and islet cell antibody 512). Glucose tolerance categories were defined according to the American Diabetes Association guidelines for diagnosis and classification of diabetes (14). The study was approved by the Human Investigations Committee of the Yale School of Medicine.
Participants were instructed to consume a high-carbohydrate diet 3 days prior to the OGTT. A standard OGTT (1.75 g/kg body weight [up to 75 g]) was performed in all adolescents to establish glucose-tolerance status. Subjects were studied at the Yale Clinical Center Investigation at 0800 hours after a 10-hour overnight fast as previously reported (15). Blood samples were taken for FFA throughout the study at 0, 30, 60, and 120 minutes. All blood samples were drawn at the predetermined intervals, immediately put on ice, centrifuged for 30 minutes, and stored at −80○. Those sampled for FFAs were allowed to clot first and then serum was separated per instructions of the assay manufacturer. Body weight and percent body fat were measured using a body composition analyzer (Tanita Corp), and height was measured in triplicate with a wall-mounted stadiometer. Obese adolescents were later categorized as having normal glucose tolerance (NGT), prediabetes (impaired fasting glucose [IFG], impaired glucose tolerance [IGT], or both) or type 2 diabetes mellitus (T2DM) per American Diabetes Association criteria. For some analyses, the NGT category was further subdivided into ranges of 2-hour glucose. All participants were offered the opportunity to undergo a more extensive body composition evaluation, and a subsample of participants (n = 115) who consented underwent abdominal magnetic resonance imaging for quantification of visceral and sc abdominal fat as previously described (16).
A convenience sample of participants who had all the relevant samples was used for the present analysis. The sex and ethnicity distribution between those who had the complete data (n = 962) and those who did not (n = 2185) was similar (P χ2 = 0.70 and P χ2 = 0.54, respectively). Age was significantly lower (13.57 ± 2.95 vs 13.87 ± 3.15 y, respectively; P < .001) and BMI-z score was higher (2.33 ± 0.50 vs 2.27 ± 0.62, respectively; P = .001) among those who had complete data compared to those who did not.
Analytical methods
Plasma glucose was determined with a YSI 2700 Analyzer (Yellow Springs Instruments) and lipid levels (total cholesterol, triglycerides and high-density lipoprotein [HDL] cholesterol) were measured with the use of an AutoAnalyzer (Model 747–200, Roche-Hitachi). Low-density lipoprotein (LDL) cholesterol was derived from the lipids measured using the Friedewald calculation. Plasma insulin and total adiponectin levels were measured using double antibody RIAs from Millipore.(insulin intra- and interassay coefficients of variation are 6.8 and 11.6%, respectively; adiponectin intra- and interassay coefficients of variation are 7.1 and 9.5%, respectively). C-reactive protein (CRP) levels were measured using the ultrasensitive assay (Kamiya Biomedical). For CRP, the intra-assay coefficient of variation is no greater than 3.0%, and the interassay coefficient of variation is no greater than 11.6%.) IL-6 levels were measured using a highly sensitive solid-phase ELISA (R&D Systems) (lower limit of detection: 0.16 pg/mL; intra- and interassay coefficients of variation: 7.4 and 7.8%, respectively). Plasma FFAs were determined using an enzymatic colorimetric method assay for the quantitative determination of nonesterified fatty acids in serum (Wako-chem, Ind.).
Calculations
Insulin sensitivity was calculated using the OGTT-derived whole-body insulin sensitivity index (WBISI) (Matsuda index). The composite WBISI is based on values of insulin (microunits per milliliter) and glucose (milligrams per deciliter) obtained from the OGTT and the corresponding fasting values (17). The acute insulin response was derived from the insulinogenic index (18) as previously described (19). The insulinogenic index (IGI), a commonly used index of β-cell function, was calculated from the OGTT data: IGI = Δ insulin (0–30) in μU/mL divided by the Δ glucose (0–30) in mg/dL. The oral disposition index (DI) was calculated as the product of insulin sensitivity (WBISI) and the acute insulin response (IGI) (20). The hepatic insulin sensitivity index was calculated as the product of the glucose and insulin areas under the curve (AUCs) during the first 30 minutes during the OGTT (21). The Adipose Insulin Resistance index (Adipose IR) was calculated as the product of fasting insulin and FFA concentrations (22). An additional index of adipose insulin sensitivity to circulating FFA (ISI-FFA) (23) was calculated with the formula: 2/(insulin [mU/L] × FFA [mmol/L]) +1). AUC of FFAs during the OGTT was calculated using the trapezoidal rule.
Statistical analysis
Data are presented as the mean ± SD. Parameters not normally distributed were natural-log transformed for the analysis. Group comparisons (glucose tolerance categories) were performed using ANCOVA with post-hoc adjustment for multiple comparisons using the Bonferroni correction. Simple Pearson correlations were performed to test the associations among parameters. Linear regression models were used to assess the determinants of the Adipose IR and the AUC of FFAs during the OGTT as dependent variables and with sex, age, degree of obesity, pubertal status, ethnicity, glucose tolerance, and multiple biochemical parameters as independent variables in different models. No correction for the number of models was applied. Similarly, linear regressions were performed with plasma lipids as dependent variables. P < .05 was considered to be statistically significant. The analysis was performed using SPSS version 20.0 for Windows.
Results
Study participants
A total of 962 obese children and adolescents were eligible to participate in the study, having all data for FFAs as well as other metabolic parameters (Table 1). Participants were classified by glucose tolerance. The prediabetes group included 104 children with IGT, 36 children with IFG, and 30 children with both IFG and IGT. Upon their classification into glucose-tolerance categories, there were no significant differences among the groups (Table 1) with regard to age, sex, height, BMI, and BMI z score, yet weight was higher in those with worse glucose tolerance (P = .04). Of note, there was a significant difference in the racial distribution of participants in which Caucasians were slightly overrepresented in the NGT category.
Table 1.
Study Participants by Glucose Tolerance Category and Metabolic Indices
| NGT (n = 770) | Prediabetes (n = 170) | Diabetes (n = 22) | P ANOVA/χ2 | |
|---|---|---|---|---|
| Age, y | 13.7 ± 3 | 13.9 ± 2.9 | 14.6 ± 2.2 | .24 |
| Height, cm | 160.5 ± 12.7 | 160.9 ± 11.1 | 166.7 ± 11.1 | .07 |
| Weight, kg | 93.2 ± 27.4 | 97.1 ± 28.1 | 104.9 ± 24.4 | .04 |
| BMI, kg/m2 | 35.6 ± 7.7 | 37.2 ± 8.0 | 37.7 ± 8.1 | .06 |
| BMI-z score | 2.34 ± 0.52 | 2.40 ± 0.42 | 2.40 ± 0.50 | .27 |
| Body fat, % | 44.66 ± 8.68 | 45.47 ± 7.65 | 43.85 ± 8.96 | .50 |
| Sex (m/f), % | 305/465 (39/61) | 62/108 (36/64) | 8/14 (36/64) | .72 |
| Race (C/AA/HIS), No. (%) | 367/242/161 (48/31/21) | 70/50/50 (42/29/29) | 7/12/3 (32/54/14) | .02 |
| Fasting glucose, mg/dL | 91.0 ± 6.75 | 97.5 ± 10.1 | 114.6 ± 18.9 | <.001a |
| Fasting insulin, μU/mL | 32.7 ± 18.5 | 44.6 ± 23.1 | 76.5 ± 89.1 | <.001a |
| Fasting FFA, mmol/L | 0.51 ± 0.16 | 0.55 ± 0.15 | 0.56 ± 0.18 | .007b |
| IGI | 5.1 ± 4.3 | 4.6 ± 6.4 | 2.7 ± 2.7 | .04 |
| WBISI | 2.04 ± 1.2 | 1.1 ± 0.6 | 1.1 ± 1.7 | <.001c |
| Oral DI | 8.6 ± 7.6 | 4.08 ± 4.3 | 1.4 ± 0.8 | <.001d |
| Adipose IR (mmol/L × μU/mL) | 16.4 ± 10.1 | 24.6 ± 15.3 | 33 ± 25.1 | <.001e |
| ISI-FFA index | 0.15 ± 0.09 | 0.10 ± 0.05 | 0.11 ± 0.12 | <.001f |
Abbreviation: AA, African American; HIS, Hispanic; ISI-FFA, insulin sensitivity index of circulating FFAs.
P < .001 for NGT vs prediabetes and T2DM, P < .001 for prediabetes vs T2DM.
P = .006 for NGT vs prediabetes.
P < .001 for NGT vs prediabetes, P = .001 for NGT vs T2DM.
P < .001 for NGT vs prediabetes and T2DM.
P < .001 for NGT vs prediabetes and T2DM, P = .005 for prediabetes vs T2DM.
P < .001 for NGT vs prediabetes.
Fasting glucose and insulin were greater in those with worse glucose tolerance, as was fasting FFA concentration (P < .001, P < .001, and P = .006, respectively). The hepatic insulin sensitivity index was significantly higher in NGT vs prediabetes (0.18 ± 0.1 vs 0.1 ± 0.06; P < .001). The oral DI significantly decreased across glucose tolerance categories (P < .001 NGT vs prediabetes and NGT vs T2DM).
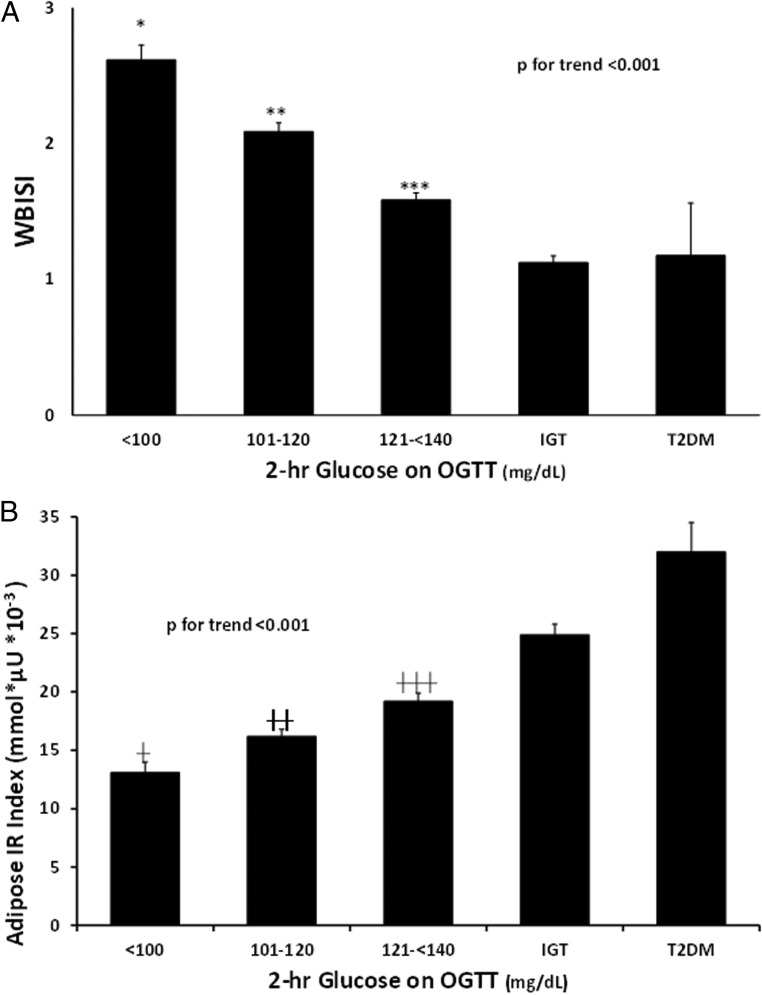
We divided the NGT category into subgroups based on ranges of 2-hour glucose. Whole-body insulin sensitivity index (WBISI) was significantly higher in the first two NGT categories compared with that of the prediabetes and T2DM groups (P for trend < .001; P < .001 for category 1 vs all groups; P < .001 for category 2 vs NGT and prediabetes; P = .03 category 2 vs T2DM) (Figure 1A). Subjects with high-normal 2-hour glucose among the NGT participants (121–139 mg/dL) had lower insulin sensitivity than other NGT groups and from prediabetes (P < .05).
Figure 1.
Whole-body insulin sensitivity (WBISI) and the adipose IR index across glucose tolerance categories. Participants with NGT were divided into subcategories based on their 2-hour glucose (2-hour glucose < 100 mg/dL, 101 mg/dL > 2-hour glucose > 120 mg/dL and 121 mg/dL > 2-hour glucose < 140 mg/dL). Whole body insulin sensitivity (A, WBISI) and the adipose IR index (B) were compared across categories. *, P < .001 vs all other categories; **, P < .001 vs other NGT categories and prediabetes, P = .003 vs T2DM; ***, P < .001 vs other NGT categories. †, P = .04 vs NGT 101–120 mg/dL, P < .001 vs NGT 121–140 mg/dL, prediabetes and T2DM; ††, P = .01 vs NGT 121–140 mg/dL, P < .001 vs prediabetes and T2DM; †††, P < .001 vs prediabetes and T2DM.
Adipose insulin resistance
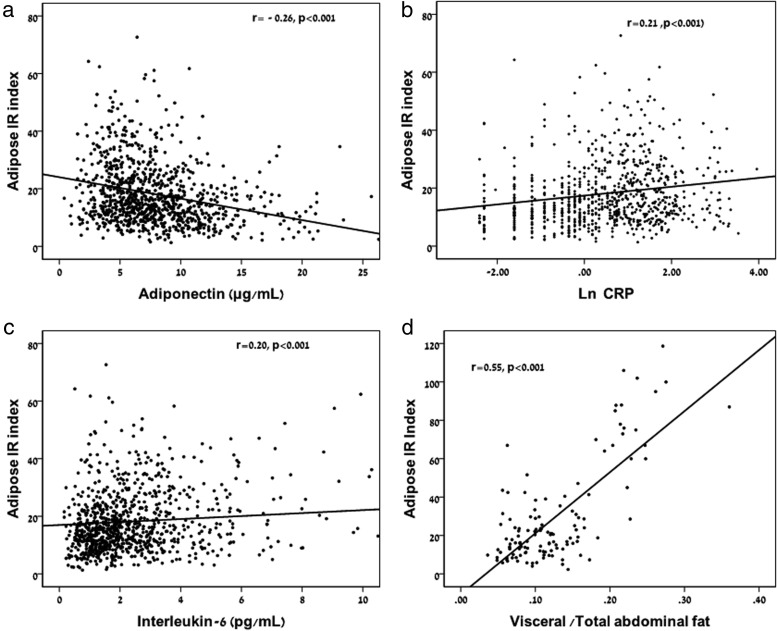
Adipose IR significantly increased across glucose tolerance categories (P for trend < .001; Figure 1B). Within the NGT participants, an increase in Adipose IR was observed related to an increase in 2-hour glucose levels. Although fasting FFAs were significantly higher in prepubertal compared with pubertal participants (0.57 ± 0.17 vs 0.51 ± 0.16 mmol/L; P = .001), fasting insulin was lower (30 ± 20 vs 36 ± 23 μU/mL; P = .004) and the Adipose IR index was lower in prepubertal children (16.04 ± 10.15 vs 18.46 ± 11.46 mmol/L × μU/mL; P = .03). The Adipose IR index was correlated with BMI z score (0.353; P < .001), fat mass (r = 0.32; P < .001), and percent body fat (r = 0.31; P < .001). Adipose IR was negatively associated with adiponectin (Figure 2A; r = −0.26; P < .001) as well as with the oral DI (r = −0.33; P < .001). Adipose IR was associated with C-reactive protein (CRP) (Figure 2B; r = 0.21; P < .001) and with IL-6 concentrations (Figure 2C; r = 0.20; P < .001).
Figure 2.
Relation of adiponectin, markers of inflammation and visceral fat to total abdominal fat ratio with the Adipose IR index. Adiponectin (A), CRP (B), and IL-6 (C) were all significantly associated with the Adipose IR index. The visceral to total abdominal fat ratio (D) was significantly associated with the Adipose IR index (Of note, the analysis of abdominal fat was performed in 115 participants).
The adipose insulin sensitivity index (ISI-FFA) was significantly higher in the NGT compared with prediabetes participants. Within the NGT participants, a decrease in ISI-FFA was observed with increasing 2-hour glucose levels. As expected, the ISI-FFA showed very similar correlations (of opposite direction as it is a measure of sensitivity and not of resistance) to those of the Adipose IR index with BMI z score, percent body fat, adiponectin, CRP, and IL-6 (data not shown).
In a subsample of participants who underwent and abdominal magnetic resonance imaging for determination of lipid partitioning (n = 115; 81 NGT/28 prediabetes/6 T2DM; 76 female/39 male; 49 Caucasian/31 Hispanic/35 African American), a tight relation of visceral fat (r = 0.34; P < .001) and the visceral/total abdominal fat ratio (Figure 2D; r = 0.55; P < .001) with the Adipose IR index was evident whereas the relation with abdominal sc fat was nonsignificant.
Determinants of the Adipose IR index
We evaluated the determinants of the Adipose IR index by a series of regression models (Table 2). Regression Models 1–4 were restricted to NGT subjects only to eliminate the effects of glucose intolerance. In model 1, sex and race were not significantly associated with Adipose IR whereas BMI z score was positively associated with Adipose IR (B = 6.68; P < .001) and pubertal status was also a significant determinant (B= −0.61; P < .001 for prepubertal vs pubertal). Adding triglyceride to HDL-cholesterol ratio (TG/HDL; as a surrogate of whole-body insulin resistance [IR] to the model, Model 2) showed a significant association of TG/HDL and the Adipose IR index without altering the effects seen in Model 1. When adiponectin, CRP, and IL-6 were added to the model (Model 3), adiponectin was negatively associated with Adipose IR (B = −0.43; P < .001) whereas IL-6 was positively associated with it (B = 0.57; P = .007). Adding the oral DI (which includes a measure of secretion and sensitivity in its calculation) showed that whereas variables in Model 3 remained significant predictors of the Adipose IR index, the oral DI was also significantly negatively associated with it (B = −0.24; P < .001). Worse glucose tolerance categories (Model 5, including all participants), were negatively associated with Adipose IR (B = −18.66, P < .001 for NGT vs T2DM; and B = −12.88, P < .001 for prediabetes vs T2DM). Importantly, even when participants from all glucose tolerance categories were included, overall obesity (BMI z score), pubertal status, adiponectin, and IL-6 remained significant predictors of the Adipose IR index.
Table 2.
Determinants of the Adipose IR index
| Characteristic | Model 1-NGT |
Model 2: NGT |
Model 3: NGT |
Model 4: NGT |
Model 5: All |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | P | B | P | B | P | B | P | B | P | |
| Sex | ||||||||||
| Male | −0.69 | .33 | −0.99 | .16 | −0.70 | .38 | −0.80 | .31 | −1.61 | .07 |
| Female | — | — | — | — | — | |||||
| Race | ||||||||||
| Caucasian | −1.16 | .20 | −1.26 | .16 | −1.66 | .10 | −1.50 | .14 | −0.18 | .86 |
| Black | −0.71 | .46 | 0.06 | .94 | −0.45 | .69 | 0.35 | .75 | 0.43 | .72 |
| Hispanic | — | — | — | — | — | |||||
| Age | 0.42 | .006 | 0.46 | .004 | 0.36 | .04 | 0.36 | .04 | 0.41 | < .001 |
| BMIZ | 6.68 | < .001 | 6.77 | < .001 | 6.44 | < .001 | 6.41 | < .001 | 7.03 | < .001 |
| Pubertal status (pre vs pubertal) | −6.15 | < .001 | −6.21 | < .001 | −4.95 | < .001 | −5.65 | < .001 | −7.32 | < .001 |
| TG/HDL | 0.69 | < .001 | 0.61 | .001 | 0.61 | .001 | 0.36 | .05 | ||
| Adiponectin | −0.43 | < .001 | −0.39 | < .001 | −0.54 | < .001 | ||||
| CRP | 0.13 | .11 | 0.12 | .12 | 0.14 | .09 | ||||
| IL-6 | 0.57 | .007 | 0.61 | .004 | 0.57 | .01 | ||||
| Oral DI | −0.24 | < .001 | −0.22 | < .001 | ||||||
| Glucose tolerance | ||||||||||
| NGT | −18.66 | < .001 | ||||||||
| IGT | −12.88 | < .001 | ||||||||
| T2DM | — | — | ||||||||
| R2 | 0.13 | 0.16 | 0.21 | 0.24 | 0.27 | |||||
Linear regression models were used to identify predictors of the Adipose IR index. Models 1–4 include only participants with NGT (to exclude the effect of glucose tolerance on the index) and Model 5 includes all participants. Presented are the unstandardized β.
FFAs during the OGTT
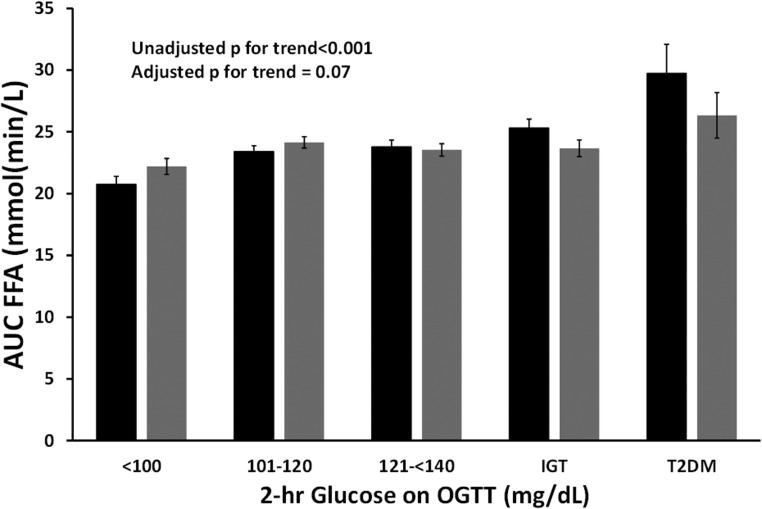
As expected, FFAs were suppressed during the OGTT (Figure 3). The AUC for FFAs was significantly lower (indicating greater suppression) across glucose tolerance categories (P for trend < .001) with those with NGT having greater FFA suppression. AUC FFAs was significantly correlated with the Adipose IR index (r = 0.42; P < .001). We tested AUC FFAs as the dependent variable in a liner regression and adjusted for age, sex, race, BMI z score, pubertal status, the oral DI, adiponectin, CRP, IL-6, glucose tolerance category, and the Adipose IR index as independent variables. Importantly, the Adipose IR index emerged as a significant determinant of AUC FFAs (B = 0.20; P < .001) along with BMI z score (B = 1.50; P = .03) and sex (with males having greater AUC FFAs than females B = 1.13; P = .05). IL-6 was significantly associated with AUC-FFA (B = 0.53, P < .001) yet adiponectin and CRP were not. In this model, the adjusted AUC-FFA was not associated with glucose tolerance categories (P = .07).
Figure 3.
AUC of FFAs during the OGTT. Unadjusted AUC FFA increased with deteriorating glucose tolerance categories (black; P for trend < .001). Adjusted AUC FFA (adjusted for age, sex, ethnicity, BMI-z score, the Adipose IR index and glucose tolerance categories) similarly increased across glucose tolerance categories yet failed to reach statistical significance (gray; P = .07).
Relation of the adipose IR and circulating plasma lipids
In linear regression models, we used plasma lipids as dependent variables and age, BMI z score, ethnicity, sex, glucose tolerance, and the Adipose IR indices as independent variables. The Adipose IR index emerged as a significant predictor of plasma triglycerides in these models (standardized β = 0.89; P < .001) as did the ISI-FFA index (standardized β = −0.21; P < .001). Both were not significant predictors of LDL-cholesterol. The ISI-FFA emerged as a significant predictor of total cholesterol (standardized β = −0.12; P = .002) as did the Adipose IR index (standardized β = 0.19; P = .05).
Discussion
Adipocytes represent an important insulin-responsive tissue, taking an active part in whole-body glucose metabolism. In this analysis we show that the Adipose IR index is greater in those with altered glucose metabolism, even within the NGT range. This index is associated with overall obesity as reflected by BMI z score yet is also tightly linked to abdominal lipid partitioning. Moreover, this index is a strong determinant of FFA suppression during the OGTT indicating its major involvement in glucose metabolism. A significant association of Adipose IR with adiponectin and IL-6 is in line with previous observations of the adverse adipocytokine profile secreted by insulin-resistant adipose tissue. The negative association with the oral DI is consistent with a negative effect of FFAs on insulin secretion.
The effects of insulin on adipose tissue are to promote glucose uptake and lipogenesis while suppressing lipolysis. In conditions of adipose IR, glucose uptake, as well as lipogenesis are reduced and lipolysis is suppressed to a lesser extent. The result is a higher concentration of circulating FFAs. The adverse effect of high concentrations of FFAs in plasma on muscle and liver insulin responsiveness as well as on β-cell insulin secretion is well known (24). Specifically, elevated FFAs have been shown to acutely reduce whole-body insulin sensitivity during euglycemic-hypreinsulinemic clamps (7), to increase postabsorptive hepatic glucose production (25) and to reduce insulin secretion (26). Taken together, it is thus not surprising that greater adipose IR (manifested as a higher Adipose IR index) resulted in lower suppression of FFAs during the OGTT and both were tightly associated with worse glucose tolerance. Importantly, prepubertal participants had slightly higher fasting FFAs yet in the context of lower fasting insulin concentrations and thus overall lower adipose IR. Given that whole-body insulin sensitivity is reduced in midpubertal adolescents (27), and given that circulating androgens are associated with adipose tissue metabolism leading to greater IR (28), it seems reasonable that a reduction in adipose insulin sensitivity will develop in mid puberty. Adipose IR was negatively associated with the oral DI indicating the potential deleterious effects of higher circulating FFAs on insulin secretion (29).
The causes of significant variability of adipose sensitivity to insulin among a large sample of obese adolescents seem to be a key question in deciphering the determinants of its role in glucose metabolism in this context. The observation shown in a subsample of the participants, that a higher visceral-to-sc abdominal fat ratio is associated with greater adipose IR may provide clues to answering this question. Although intra-abdominal fat has been shown not to contribute a greater proportion of systemic FFAs in relation to its share of total fat (30), it may be the source of other mediators having a mechanistic role in the development of systemic IR (16, 31). Specifically, macrophage infiltration into adipose depots has been shown to instigate whole-body low-grade inflammation, which in turn may have adverse effects in insulin-responsive tissues such as muscle and liver (32). In addition, infiltration of macrophages and up-regulation of the NLRP3-inflammasome together with high ceramide content in sc abdominal fat of obese adolescents with a high visceral-to-sc fat ratio may contribute to the limited expansion of the sc abdominal adipose depot and the development of IR (33). Moreover, the negative association of adipose IR and adiponectin and the positive one with CRP and IL-6 suggests that the insulin-resistant adipocyte secretes an adverse adipocytokine profile characterized by increased inflammatory cytokines in the context of IR (34). As weight loss in this age group has been shown to be associated with a reduction of inflammatory adipocyte–derived cytokines (35), it is reasonable to assume that adipose IR is responsive to such interventions. Adipose insulin sensitivity/resistance was a significant predictor of circulating plasma lipids in this analysis. Specifically, both indices were associated with circulating triglycerides and total cholesterol but not with LDL cholesterol. These findings are in partial agreement (regarding triglycerides) with Reinehr et al (36) who described an analysis with a broader spectrum of obesity. The lack of agreement with regard to relation to LDL cholesterol may be due to slight differences in the variables used in the regression models.
Greater systemic FFAs in the context of the postabsorptive state, as observed with worsening glucose tolerance in this study, may alter fuel utilization patterns in the muscle and liver in a manner promoting hyperglycemia by inducing alterations in the insulin signal transduction pathway leading to muscle and liver IR (37). Our findings regarding FFA suppression patterns during the OGTT are in agreement with those of Toledo-Corral et al (38) who showed differences in fasting but not in postprandial or nocturnal FFA concentrations between Hispanic children with normal vs impaired glucose tolerance. Our unadjusted comparison indeed showed significant differences between glucose tolerance categories, yet the adjusted one failed to reach statistical significance. Subtle differences between these studies may stem from the different analytical approach (adjustments for different covariates) used by Toledo-Corral as well as from the differences of the populations studied. The finding of less FFA suppression in males vs females may be explained by the greater FFA flux described previously in obese adolescent females (39). We did not detect race/ethnic differences in Adipose IR among our participants, in agreement with previous observations on the similar responses to an acute lipid load in obese children of varying ethnic backgrounds (8). Given that the FFA profile is affected by weight loss as previously shown in children (40) but also by dietary constituents (6), additional investigation into potential mechanisms that modify FFA metabolism in the postabsorptive state is warranted.
The strengths of this analysis are the large sample of obese adolescents with a spectrum of glucose tolerance levels that allowed us to isolate discrete determinants of the Adipose IR index. Despite this, the group with type 2 diabetes is relatively small, which may potentially have prevented us from detecting significant characteristics of this group. Despite the fact that statistically significant differences were detected between the sample used and the entire cohort, we can carefully state that a mean difference of 0.3 years and 0.05 in the BMI z score is not of major clinical significance in this context. The limitations include the cross-sectional nature of the analysis, which prevents us from delineating mechanistic explanations of the findings. Another significant limitation is the lack of a nonobese age-matched reference group that would put our findings in a broader perspective. This currently prevents us from quantifying accurate “normal” thresholds for adipose IR in this age group. Investigating protein expression in adipocytes derived from individuals with varying degrees of adipose insulin sensitivity would provide insights into the molecular mechanisms leading to adipocyte resistance to insulin. A longitudinal assessment of the ability of Adipose IR to predict β-cell decompensation in comparison with a standard OGTT (and its insulin-derived indices) is warranted.
Acknowledgments
We thank the adolescents who participated in this trial as well as their parents.
This study was registered in ClinicalTrials.gov as trial number NCT01967849.
This work was supported by by the American Heart Association (13SDG14640038) (N.S.), the National Institutes of Health (NIH) (Grants R01-HD-40787, R01-HD-28016) and ADA (Distinguished Clinical Scientist Awards from the American Diabetes Association (DK-49230) (S.C.). This work was also made possible by the CTSA Grant No. UL1 RR024139 from the National Center for Advancing Translational Science and by DK045735 to the Yale Diabetes Endocrinology Research Center, a component of the NIH, and NIH roadmap for Medical Research.
The contents of this scientific contribution are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Adipose IR
- Adipose Insulin Resistance
- AUC
- area under the curve
- BMI
- body mass index
- CRP
- C-reactive protein
- DI
- disposition index
- FFA
- free fatty acid
- HDL
- high-density lipoprotein
- IFG
- impaired fasting glucose
- IGI
- insulinogenic index
- IGT
- impaired glucose tolerance
- IR
- insulin resistance
- ISI
- index of adipose insulin sensitivity
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- T2DM
- type 2 diabetes mellitus
- TG/HDL
- triglyceride to HDL cholesterol ratio
- WBISI
- whole body insulin sensitivity index.
References
- 1. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. [DOI] [PubMed] [Google Scholar]
- 2. Weiss R, Kaufman FR. Metabolic complications of childhood obesity: Identifying and mitigating the risk. Diabetes Care. 2008;31 Suppl 2:S310–S316. [DOI] [PubMed] [Google Scholar]
- 3. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. [DOI] [PubMed] [Google Scholar]
- 4. Weiss R. Fat distribution and storage: How much, where, and how? Eur J Endocrinol. 2007;157 Suppl 1:S39–S45. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 6. Varady KA, Dam VT, Klempel MC, et al. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci Rep. 2015;5:7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid–induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burns SF, Kelsey SF, Arslanian SA. Effects of an intravenous lipid challenge and free fatty acid elevation on in vivo insulin sensitivity in African American versus Caucasian adolescents. Diabetes Care. 2009;32:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaliszyn SF, Bonadonna RC, Sjaarda LA, Lee S, Farchoukh L, Arslanian SA. β-Cell lipotoxicity in response to free fatty acid elevation in prepubertal youth: African American versus Caucasian contrast. Diabetes. 2013;62:2917–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes. 2000;49:399–408. [DOI] [PubMed] [Google Scholar]
- 11. Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- 12. Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: A longitudinal study. Diabetes. 2012;61:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- 15. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–810. [DOI] [PubMed] [Google Scholar]
- 16. Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: A determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. [DOI] [PubMed] [Google Scholar]
- 17. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 18. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. [DOI] [PubMed] [Google Scholar]
- 19. Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. [DOI] [PubMed] [Google Scholar]
- 20. Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care. 2007;30:1845–1850. [DOI] [PubMed] [Google Scholar]
- 21. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. [DOI] [PubMed] [Google Scholar]
- 22. Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belfiore F, Iannello S, Volpicelli G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol Genet Metab. 1998;63:134–141. [DOI] [PubMed] [Google Scholar]
- 24. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roden M, Stingl H, Chandramouli V, et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes. 2000;49:701–707. [DOI] [PubMed] [Google Scholar]
- 26. Boden G. Effects of free fatty acids (FFA) on glucose metabolism: Significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:121–124. [DOI] [PubMed] [Google Scholar]
- 27. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. [DOI] [PubMed] [Google Scholar]
- 28. Corbould A. Effects of androgens on insulin action in women: Is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–532. [DOI] [PubMed] [Google Scholar]
- 29. Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab. 2012;97:3302–3309. [DOI] [PubMed] [Google Scholar]
- 30. Votruba SB, Jensen MD. Regional fat deposition as a factor in FFA metabolism. Annu Rev Nutr. 2007;27:149–163. [DOI] [PubMed] [Google Scholar]
- 31. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. [DOI] [PubMed] [Google Scholar]
- 32. Tchernof A, Després JP. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- 33. Kursawe R, Dixit VD, Scherer PE, et al. A role of the Inflammasome in the low storage capacity of the abdominal subcutaneous adipose tissue in obese adolescents. Diabetes. 2016;65:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodríguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab. 2015;309:E691–E714. [DOI] [PubMed] [Google Scholar]
- 35. Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60:445–452. [DOI] [PubMed] [Google Scholar]
- 36. Reinehr T, Kiess W, Andler W. Insulin sensitivity indices of glucose and free fatty acid metabolism in obese children and adolescents in relation to serum lipids. Metabolism. 2005;54:397–402. [DOI] [PubMed] [Google Scholar]
- 37. Dubé JJ, Coen PM, DiStefano G, et al. Effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility, and mitochondrial performance. Am J Physiol Endocrinol Metab. 2014;307:E1117–E1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ. Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol. 2015;52:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adler-Wailes DC, Periwal V, Ali AH, et al. Sex-associated differences in free fatty acid flux of obese adolescents. J Clin Endocrinol Metab. 2013;98:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143–2150. [DOI] [PubMed] [Google Scholar]