Abstract
Sleep deprivation produces deficits in hippocampal synaptic plasticity and hippocampal-dependent memory storage. Recent evidence suggests that sleep deprivation disrupts memory consolidation through multiple mechanisms, including the down-regulation of the cAMP-response element-binding protein (CREB) and of mammalian target of rapamycin (mTOR) signaling. In this study, we tested the effects of a Bioactive Dietary Polyphenol Preparation (BDPP), comprised of grape seed polyphenol extract, Concord grape juice, and resveratrol, on the attenuation of sleep deprivation-induced cognitive impairment. We found that BDPP significantly improves sleep deprivation-induced contextual memory deficits, possibly through the activation of CREB and mTOR signaling pathways. We also identified brain-available polyphenol metabolites from BDPP, among which quercetin-3-O-glucuronide activates CREB signaling and malvidin-3-O-glucoside activates mTOR signaling. In combination, quercetin and malvidin-glucoside significantly attenuated sleep deprivation-induced cognitive impairment in -a mouse model of acute sleep deprivation. Our data suggests the feasibility of using select brain-targeting polyphenol compounds derived from BDPP as potential therapeutic agents in promoting resilience against sleep deprivation-induced cognitive dysfunction.
Keywords: Sleep deprivation, Polyphenols, Cognitive dysfunction, Resilience, Memory consolidation
1. Introduction
Chronic sleep loss is a common problem in our society; an estimated 50–70 million adults in the United States have sleep or wakefulness disorder (Institute of Medicine, 2006). Insufficient sleep is co-morbid with chronic problems such as heart disease, kidney disease, high blood pressure, diabetes, obesity, and mental illness (Ford and Kamerow, 1989; Gillin, 1998; Knutson and Van, 2008; Hirotsu et al., 2010; Vijayan, 2012; Palagini et al., 2013; Najafian et al., 2013). Sleep loss can also contribute to irritability, aggression, inattentiveness, and diminished psychomotor vigilance (Rajaratnam, 2001; Van Dongen et al., 2003; Kamphuis et al., 2012). The negative impacts of sleep loss on physical and mental health place a strain on our healthcare system (Kapur et al., 2002) and a large financial burden on our economy (Goel et al., 2009). Unfortunately, many people are unable to obtain sufficient sleep on a daily basis. Therefore, it is important to explore the molecular and cellular impacts of sleep loss in an effort to identify novel therapeutic approaches to counteract these effects.
A number of studies indicate that sleep deprivation (SD) disrupts the consolidation period and impairs memory (Fishbein, 1971; Graves et al., 2003). It has been reported that SD inhibits the induction of long-term potentiation (LTP) in the hippocampus of rodents (Campbell et al., 2002) and that the LTP deficit induced by SD may be rescued by increasing cAMP/PKA signaling (Vecsey et al., 2009), which is known to play an important role in memory consolidation. Recent studies suggest multiple molecular mechanisms by which SD disrupts memory consolidation, including down-regulation of the cAMP-response element-binding protein (CREB) and down-regulation of mammalian target of rapamycin (mTOR) signaling (Vecsey et al., 2012).
Synaptic efficacy mediating memory storage requires the activation of specific gene expression programs. For example, CREB signaling is essential for long-lasting changes in synaptic plasticity that mediate the conversion of short-term memory to long-term memory. The crucial role of CREB signaling as a cellular mechanism underlying synaptic plasticity and memory has led to the hypothesis that targeting CREB signaling could be a therapeutic approach for memory disorders (Tully et al., 2003). mTOR is a protein kinase involved in translation control and long-lasting synaptic plasticity. While the importance of mTOR signaling in synaptic plasticity has mostly been derived from studies using rapamycin (Beaumont et al., 2001; Tang et al., 2002), more specific genetic targeting of the mTOR signaling cascade has also suggested that mTOR couples receptors to the translation machinery to establish long-lasting synaptic changes (Shima et al., 1998; Pende et al., 2004; Goorden et al., 2007). Disruption of mTOR signaling appears to be a common physiological feature of many neurological disorders, including Alzheimer's disease (AD), autism spectrum disorders, and mental retardation syndromes (Hoeffer and Klann, 2010; Ma et al., 2010).
We previously reported that dietary supplementation with a Bioactive Dietary Polyphenol Preparation (BDPP), a combination of 3 bioactive and commercially available polyphenol products (Concord grape juice, grape seed extract (GSE), and resveratrol), is effective in protecting against diverse mechanisms associated with cognitive function and general brain health (Wang et al., 2008, 2010, 2013; Ho et al., 2013), including oxidative stress and inflammation, and of promoting neuroplasticity. We found that the grape-derived polyphenolic preparation significantly improved cognitive function through the activation of the CREB signaling pathway in a mouse model of AD (Wang et al., 2012). The current study was designed to test the hypothesis that BDPP can attenuate SD-induced cognitive impairments through activation of the CREB and mTOR signaling pathways, and to identify bioactive components of the BDPP that promote resilience against SD-mediated cognitive decline (Fig. 1).
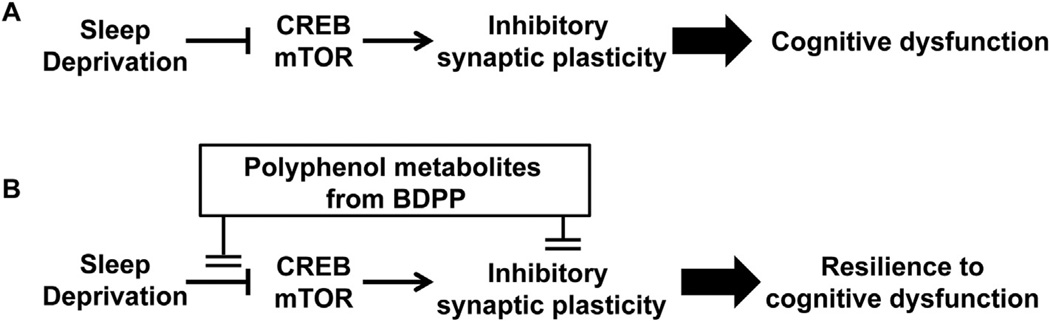
Fig. 1.
Scheme of the working hypothesis. (A) Sleep deprivation inhibits CREB signaling and mTOR signaling, thus leading to cognitive impairment. (B) Polyphenol metabolites activate the CREB and mTOR signaling pathways, thus promoting resilience to sleep deprivation-induced cognitive dysfunction.
2. Material and methods
2.1. Materials
Food grade resveratrol was purchased from ChromaDex (Irvine, CA). GSE was purchased from Supplement Warehouse (UPC: 603573579173). Only one lot of the resveratrol and one lot of the GSE were used for this particular study. Both resveratrol and GSE have been shown to be very stable when stored at 4 °C in the dark. Welch Concord purple grape juice was purchased at a local market. Quercetin-3-O-glucuronide was purchased from Sigma (St. Louis, MO). Malvidin-3-O-glucoside chloride, delphinidin-3-O-glucoside, and cyanidin-3-O-glucoside chloride were purchased from ChromaDex (Irvine, CA). 3′-O-methyl-epicatechin-5′-O-glucuronide was synthesized as previously described (Blount et al., 2012; Wang et al., 2012). All extraction and LC solvents were HPLC certified and were obtained from J.T. Baker (Phillipsburg, NJ).
2.2. Animals and treatment
C57BL6/J mice were purchased from Jackson's laboratory and housed in the centralized animal care facility of the Center for Comparative Medicine and Surgery at the Icahn School of Medicine at Mount Sinai. For the BDPP treatment, the calculated daily intake of GSE was 200 mg/kg body weight (BW) (Wang et al., 2012; Blount et al., 2012), resveratrol was 400 mg/kg BW (Lagouge et al., 2006; Vingtdeux et al., 2010), and the total polyphenols from juice extract was 183 mg/kg BW (Krikorian et al., 2010). These doses were chosen based on the equivalent doses used in the studies that showed efficacy either in humans or in animal models for each component. Mice were given BDPP delivered through their drinking water for two weeks and the drinking solution was changed once every two days. For short-term dose finding experiments, animals were treated with different doses of quercetin (0.02, 0.2, 2, 20, and 200 mg/kg/day) or malvidin-3-O-glucoside (0.05, 0.5, 5, and 50 µg/kg/day) for 10 days. Animals were then sacrificed and brains were dissected for further analyses. For combinational polyphenol compound treatment, quercetin (0.2 mg/kg/day) and malvidin-3-O-glucoside (5 µg/kg/day) were delivered in the drinking water for 6 weeks. Drinking solution was changed once every two days. All animals were maintained on a 12:12-h light/dark cycle with lights on at 07:00 h in a temperature-controlled (20 ± 2 °C) vivarium, and all procedures were approved by the Mount Sinai ICAUC.
2.3. Sleep deprivation and cognitive assessment by contextual fear conditioning test
A contextual fear conditioning test was performed as previously described (Steele et al., 2013).
During conditioning, mice were trained and tested in conditioning chambers on 3 consecutive days in the cued and contextual fear conditioning paradigm. On Day 1, mice were placed into Context A (gray walls, grid floor, houselights at 50% with lemon scent) and allowed to explore for 180 s (baseline) prior to 3 tone/shock pairings (30 s 4.0 kHz pure tone co; terminating with a 2 s scrambled 0.6 mA foot shock). Each tone/shock pairing was separated by 30 s of exploration time, and animals were given 30 s to explore following the final tone/shock pairing (300 s total). After training session on Day 1, control mice were left undisturbed in their home cages, and sleep-deprived mice were kept awake in their home cages by gentle stroking (Ledoux et al., 1996). Specifically, immediately after day one fear conditioning test, two technicians used pencils to gently stroke the whiskers to keep the mice from asleep for 5 h In the event the mice did not respond to the gentle stroke, the technician gently picked up the mice to wake them. On Day 2, mice were replaced into Context A and allowed to explore for 180 s without the tone. On day 3, mice were placed into Context B (black/white checked walls, black plastic floor, houselights at 100% with vanilla scent) and allowed to explore for 180 s in the constant presence of the 4.0 kHz pure tone. Memory for the context (contextual memory) or the tone (cued memory) for each animal was obtained by subtracting the percent freezing during baseline from the percent freezing on Day 2 or Day 3, respectively. Freezing behavior was recorded remotely and analyzed using Video Freeze Fear Conditioning Software (MED Associates Inc.).
2.4. Brain accumulation of polyphenol metabolites following repeated dosing with BDPP
Grape-derived polyphenols, such as BDPP, are extensively metabolized during absorptive and post-absorptive xenobiotic metabolism (Lambert et al., 2007; Hribar and Ulrih, 2014), and are found in vivo primarily in their metabolite forms. Therefore, the information generated from naturally occurring “precursor” forms in in vitro bioactivity and mechanistic studies is largely physiologically irrelevant. Moreover, synaptic plasticity occurs in the brain including hippocampal formation. Therefore, it is important to determine which forms of polyphenol metabolites can penetrate the blood–brain barrier, accumulate in the brain, and exert their bioactivities. We conducted brain bioavailability studies to identify polyphenol metabolites in the brain following repeated dosing with the BDPP. In this study, we used Sprague Dawley rats. The choice of rats is based on their well established use as a model for bioavailability and metabolism of polyphenols in humans and based on the fact that both rats and mice possess similar xenobiotic enzyme systems, and similar metabolites, namely methylated and glucuronidated metabolites, are observed in both species in previous studies (Feng, 2006). Briefly, Sprague–Dawley rats were obtained from Harlan Sprague Dawley Inc. (Indianapolis, IN) and placed on a polyphenol free AIN-93M diet (Dyets, Bethlehem, PA), given deionized water ad lib, and allowed to acclimate for 3 days. Following the acclimation period, rats were given BDPP by intra-gastric gavage for 10 days. In order to reach proper dosage, rats were gavaged twice a day, with 8 h separation. The non-treatment group was gavaged with water. Bioavailability assessment was conducted on the tenth day of gavage. Rats were given a last dosage of treatment, anesthetized, and perfused with cold physiological saline. The brains were harvested, placed in 0.2% ascorbic acid in saline, and stored at −80 °C until analysis.
2.5. Analysis of polyphenol metabolites
Polyphenol metabolites from brains were extracted using SPE as previously described (Wang et al., 2014). Analysis of all polyphenol metabolites was performed on an Agilent 6400 Triple Quad under multiple reaction monitoring modes (MRM) as previously described (Wang et al., 2014). MRM mass transitions were 479 → 303 for methyl-O (MeO)-epicatechin (EC) glucuronides, 465 → 289 for EC-glucuronides, 289 → 137 for EC, 403 → 227 for resveratrol-glucuronide, and 227 → 143 for resveratrol under negative polarity on Triple Quad. MRM mass transitions were 493 → 317 for MeO-quercetin glucuronide, 479 → 303 for quercetin glucuronide, and 301 → 153 for quercetin aglycone under positive polarity. Quantification of all anthocyanin glucosides, except for cyanidin-3-O-glucoside, was estimated using calibration curves of malvidin-3-O-glucoside. Quantification of cyanidin-3-Oglucoside was achieved by a calibration curve constructed with cyanidin-3-O-glucoside standard.
2.6. Primary neuron preparation and treatments
Primary cortico-hippocampal neurons were prepared from Embryonic Day 15 (E15) mouse embryos as previously described (Wang et al., 2007). Neurons were seeded onto poly-d-lysine-coated 12-well plates at 5.0 × 105 cells per well in Neurobasal medium supplemented with 2% B27, 0.5 mM l-glutamine, and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). On Day 5, the neurons were treated for 72 h with either 250 nM of brain-targeting polyphenol compound or vehicle control. Cells were then lysed for further analysis.
2.7. Assessment of stress levels
Blood samples were collected 20 min after SD handling, and plasma corticosterone levels were measured using the Corticosterone ELISA kit (Enzo Lifesciences), according to the manufacturer's instructions.
2.8. Multi-pathway cell signaling assays and protein gel-blot analysis
Luminex xMAP multiplexed immunoassays (Millipore, Billerica, MA) were used to evaluate the levels of phosphorylated proteins in the hippocampal formation and in primary neuron cultures. The hippocampal tissue or primary neurons were lysed with MilliplexMAP Cell Signaling Universal Lysis Buffer containing phosphatase inhibitors including 1 mM sodium orthovanadate and freshly prepared 1× protease inhibitor cocktail (EMD Millipore). The phosphoprotein analyses used were: CREB (Ser133), AKT (Ser473), Erk1/2 (Thr185/Tyr187), JNK (Thr183/Tyr185), p70 S6 Kinase (Thr412), p38 (Thr189/Tyr182), STAT3 (Ser727), STAT5 (Tyr694/699), and NFκB (Ser536). 20 µg protein lysate was used for the assays, according to the procedure provided by the manufacturer. For protein gel-blot analysis, the same samples were used for SDS-PAGE protein separation, and antibodies specific for phosphomTOR and total mTOR are from Millipore, phospho-p70S6K is from Cell Signaling, total CaMKII, phospho-CaMKII and β-actin were obtained from Santa Cruz Biotechnology.
3. Results
3.1. BDPP treatment partially restored SD-mediated cognitive deficits, in part, through regulation of CREB and p70S6K signaling pathways
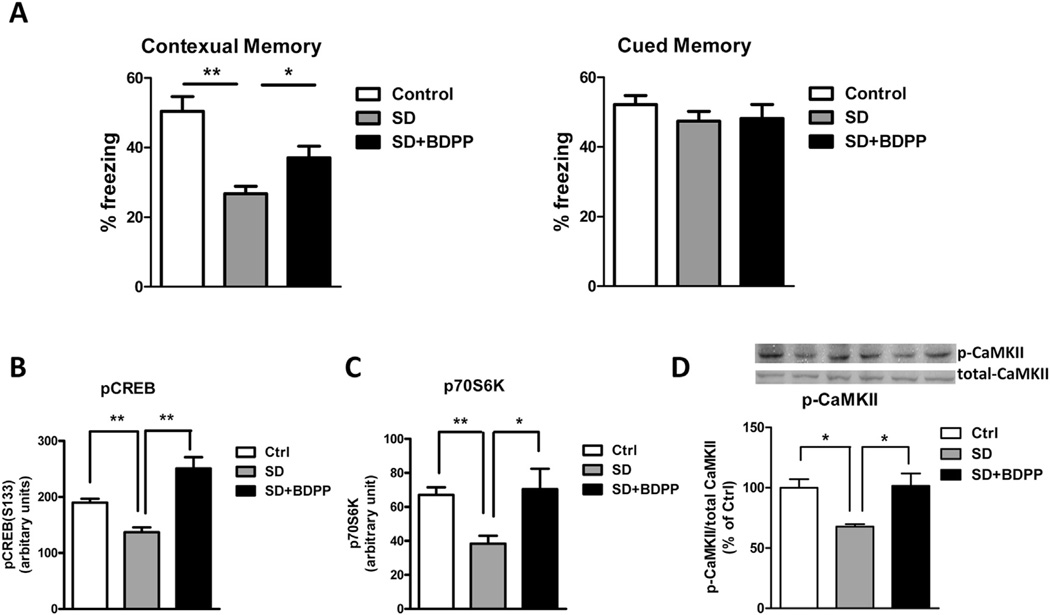
In order to test whether BDPP can prevent SD-mediated cognitive impairment, C57BL6 mice were randomly grouped into 3 groups: control group, SD group, and SD-BDPP group. Following two weeks' treatment, we performed behavioral testing for contextual fear conditioning memory. After the training session on Day 1, control mice were left undisturbed in their home cages and SD group and SD-BDPP group were kept awake in their home cages for 5 h by gentle stroking. We found that sleep deprivation led to significantly impaired contextual memory as compared to the control group (% of freezing: 26.75 ± 2.110 vs. 50.40 ± 4.288, respectively, p < 0.01). Treatment of BDPP partially restored the SD-induced contextual memory (% of freezing: 37.05 ± 3.333 vs. 26.75 ± 2.110, p < 0.05) (Fig. 2A). Using a multiplex ELISA, we found that BDPP treatment significantly rescued SD-induced decreased phosphorylation of CREB on Ser133 (p < 0.01, Fig. 2B) and p70S6K on Thr412 (p < 0.05, Fig. 2C) in the hippocampus tissue lysate, suggesting that BDPP might promote resilience against SD-induced cognitive impairment through activation of the CREB and p70S6K signaling pathways. Several kinases phosphorylate CREB at Ser133, including Ca2+/calmodulin-dependent protein kinase CaMKII, rasmitogen-activated protein kinase (MAPK/ERK), and cAMP-dependent protein kinases PKA and AKT/PKB. No change on the phosphorylation of ERK1/2 or AKT was detected (data not shown). Western blot analysis showed that SD decreased CaMKII phosphorylation in the hippocampal formation as compared to non-SD animals, and BDPP treatment restored the CaMKII phosphorylation (Fig. 2D).
Fig. 2.
BDPP provides resilience to SD-mediated cognitive deficit. C57BL/6 mice were treated with BDPP for two weeks. Immediately after fear conditioning training, mice were sleep deprived for 5 h. (A) Contextual memory and tone memory were measured 24 h after training. (B–D) Expression of phosphor-protein in the lsyate of hippocampus: (B) Phosphorylation of CREB on Ser133 (C) Phosphorylation of p70S6K on Thr412 and (D) Phosphorylation of CamKII. *p < 0.05; **p < 0.01 by two-tailed t-test; n = 5 per group.
3.2. Identification of brain-targeting polyphenol metabolites
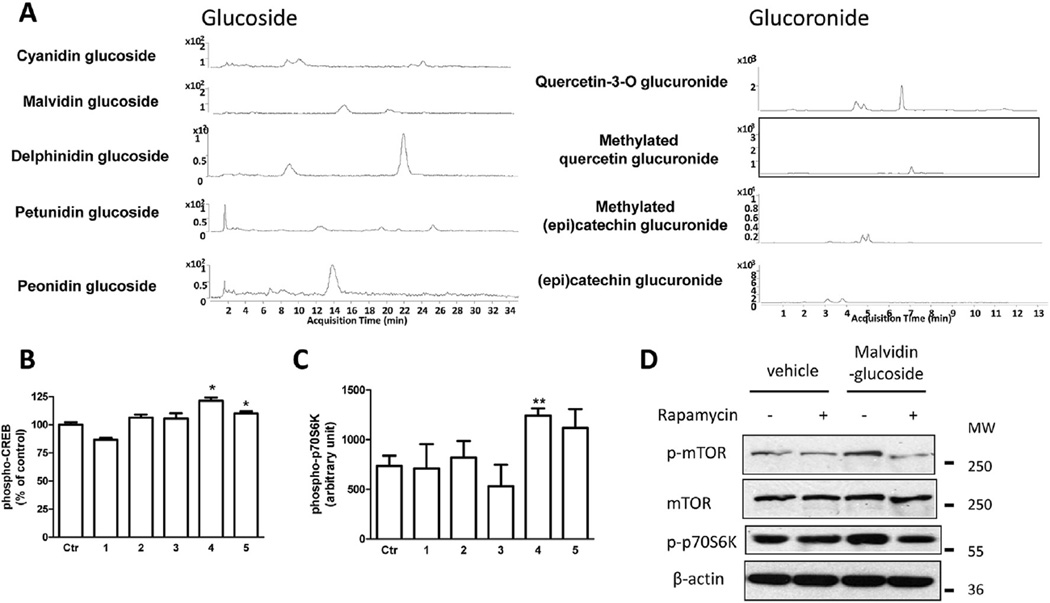
Consistent with previous findings (Wang et al., 2014), the metabolites found in the brain following 10 days of BDPP treatment were (Fig. 3A): catechin and epicatechin from GSE led to the accumulation of proanthocyanidins (PAC) glucuronides (Gluc) including catechin-O-β-Gluc, 3′-O-methyl-catechin-O-β-Gluc, epicatechin-O-β-Gluc, and 3′-O-methyl-epicatechin-O-β-Gluc; quercetin from juice resulted in the accumulation of quercetin-3-O-Gluc, O-methyl-quercetin-Gluc, malvidin-3-O-glucoside (Glc), petunidin-Glc, delphinidin-Glc, and peonidin-Glc; cyanidin-Glc from juice led to the plasma and brain accumulation of malvidin-3-O-glucoside (Glc), petunidin-3-O-Glc, delphinidin-3-O-Glc, peonidin-3-O-Glc, and cyanidin-3-O-Glc; resveratrol led to the accumulation of resveratrol and resveratrol-Gluc in the brain.
Fig. 3.
Select brain-available polyphenol metabolites increase CREB and mTOR signaling in primary neurons. (A) HPLC diagram of brain-available polyphenol metabolites. Effect of commercially available polyphenol metabolites on the phosphorylation of (B) CREB on Ser133 and (C) p70S6K on Thr412. (D) Effect of malvidin-3-O-glucoside on mTOR/p70S6K signaling. 1: 3′-O-methyl-epicatechin-O-β-Glucuronide; 2: cyanidin-3-O-glucoside; 3: delphinidin-3-O-glucoside; 4: malvidin-3-O-glucoside; 5: quercetin-3-O-glucuronide. *p < 0.05, **p < 0.01 by two-tailed t-test.
3.3. Select polyphenol metabolites activate CREB and mTOR signaling in primary neurons
In order to identify the bioactive polyphenol metabolites that exert their bioactivities by promoting CREB signaling and downstream mTOR signaling, we treated primary hippocampal-cortico neurons with commercially available brain-targeting polyphenol compounds identified in our brain bioavailability experiment (Fig. 3A), and measured the levels of CREB and p70S6K phosphorylation. We found that treatment with 250 nM of malvidin-3-O-glucoside and quercetin-3-O-glucuronide significantly increases CREB phosphorylation (Fig. 3B). Interestingly, malvidin-glucoside also significantly increases the level of phospho-p70S6K on Thr412 (Fig. 3C). Western blot analysis revealed that treatment with malvidin-glucoside in primary neurons increases the phosphorylation of p70S6K and its upstream kinase, mTOR, and the activation of mTOR and p70S6K can be blocked by rapamycin, an mTOR signaling-specific inhibitor (Fig. 3D).
3.4. Combination treatment of quercetin and malvidin-glucoside partially restored SD-mediated cognitive deficits
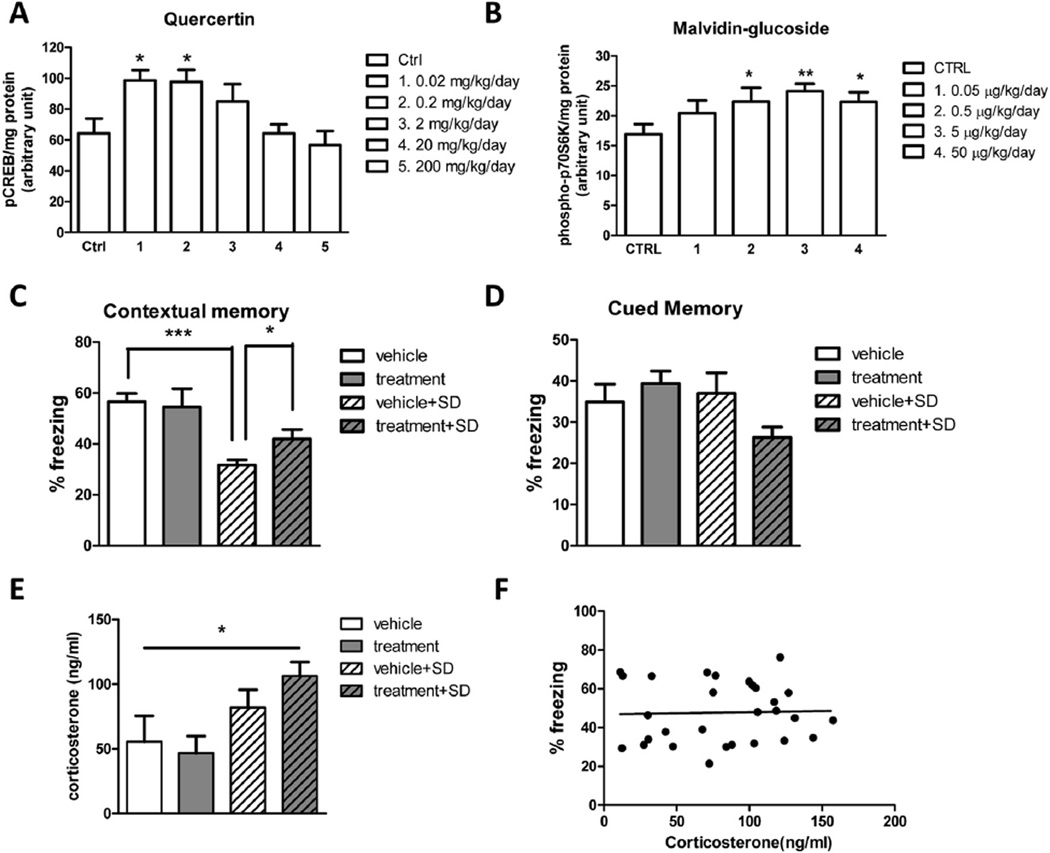
Based on the above in vitro mechanistic studies, as a proof of concept, we tested whether a combination of quercetin and malvidin can attenuate SD-mediated cognitive deficits. Since rodents have UDP-glucuronosyltransferase but lack glycosidase, in this study, we used quercetin, which can be metabolized to quercertin-3-O-glucuronide in vivo, and malvidin-glucoside for in vivo animal studies. We first performed dose finding experiments in C57BL6 mice. We found that quercetin significantly induces CREB phosphorylation at concentrations of 0.02 and 0.2 mg/kg/day (Fig. 4A). Malvidin-glucoside significantly increases p70S6K phosphorylation at concentrations of 0.5, 5, and 50 µg/kg/day (Fig. 4B). Based on these results, we chose 0.2 mg/kg/day quercetin and 5 µg/kg/day malvidin-3-O-glucoside for the efficacy study.
Fig. 4.
Combination treatment of quercetin and malvidin-glucoside attenuates SD-induced cognitive impairment. (A) Effect of quercetin doses on the phosphorylation of CREB in the hippocampal formation. (B) Effect of malvidin-3-O-glucoside doses on the phosphorylation of p70S6K in the hippocampal formation. Combination treatment of quercetin and malvidin-3-O-glucoside provides resilience to SD-induced (C) contextual memory deficits without affecting (D) tone memory. (E) Levels of corticosterone were measured after sleep deprivation handling. (F) Association analysis of plasma corticosterone levels and percentage of freezing. n = 10 per group. *p < 0.05, **p < 0.01 by two-tailed t-test.
C57BL6 mice were treated with quercetin and malvidin-3-O-glucoside. Animals were randomly divided into 4 groups: vehicle, treatment, vehicle-SD, and treatment-SD. We found that the treatment was well tolerated and we did not observed any adverse effects in response to 6-weeks treatments as reflected by normal drinking, eating, grooming behavior and normal body weight (data not shown). After 6 weeks' treatment, we performed behavioral testing for contextual fear conditioning memory. Following training session on Day 1, the vehicle group and the treatment group were left undisturbed in their home cages, and the vehicle-SD group and the treatment-SD group were kept awake in their home cages for 5 h by gentle stroking. We found that SD leads to significantly impaired contextual memory compared to the vehicle group (% of freezing: 31.66 ± 2.001 vs. 56.66 ± 3.248, respectively, p < 0.001). Combination treatment of quercetin and malvidin-3-O-glucoside restored the SD-induced contextual memory (% of freezing: 41.87 ± 3.780 vs. 31.66 ± 2.001, p < 0.05) (Fig. 4C). No significant change in tone memory was detected (Fig. 4D). Notably, there is a trend of increase in plasma corticosterone levels after 5 h SD handling compared to non-SD groups (Fig. 4E), suggesting that the SD procedure may increase stress levels in animals. However, association analysis revealed that there is no correlation between plasma corticosterone levels and percentage of freezing in all animal tested (Fig. 4F), suggesting that the beneficial effects of quercetin and malvidin-3-O-glucoside on cognitive performance is not stress-related.
4. Discussion
Cognitive decline is one of the consequences of brain dysfunction induced by sleep loss. The critical role of CREB signaling as a cellular mechanism underlying synaptic plasticity makes it a potential therapeutic target for memory disorders. Recent studies using natural plant extracts delayed memory decline by maintaining normal levels of active CREB. For example, green tea catechins attenuate age-related memory impairment by increasing CREB phosphorylation (Li et al., 2009a,b). Procyanidins enhance phosphorylation of CREB, ERK1/2, and CaMKIV in aged-memory-impaired rats (Xu et al., 2010). Grape seed extract significantly improves cognitive function through the activation of the CREB signaling pathway in a mouse model of AD (Wang et al., 2012). Abundant evidence has linked mTOR signaling to synaptic change, memory, and neurological disorders. However, contradictory effects of polyphenols targeting the mTOR signaling pathway have been reported. For example, it has been found that resveratrol inhibits inflammation in microglial cells via mTOR activation (Zhong et al., 2012). On the other hand, application of piceatannol in prostate cancer cells results in down-regulation of mTOR and its upstream and downstream effector proteins, AKT and eIF-4E-BP1 (Hsieh et al., 2012).
Our study demonstrated that SD-mediated cognitive dysfunction was associated with reduced expressions of pCREB and p70S6K in the hippocampal formation in the mouse model of acute SD. Our data suggest that oral administration of BDPP may attenuate SD-induced memory impairment, in part, through activation of the CREB and mTOR signaling pathways. Furthermore, we demonstrated that the brain-bioavailable polyphenol metabolites quercetin-3-O-glucuronide and malvidin-3-O-glucoside can, respectively, promote the phosphorylation of CamKII/CREB and enhance mTOR/p70S6K signaling, which are potential underlying mechanisms associated with cognitive function in the context of sleep deprivation (Fig. 5). Our preclinical efficacy studies further demonstrate that combination treatment with quercetin and malvidin-3-O-glucoside can effectively attenuate SD-induced cognitive impairments in mouse models. Future studies will focus on further dissecting the effects of polyphenol compounds on CREB/mTOR upstream regulators, downstream targets, and synaptic growth changes.
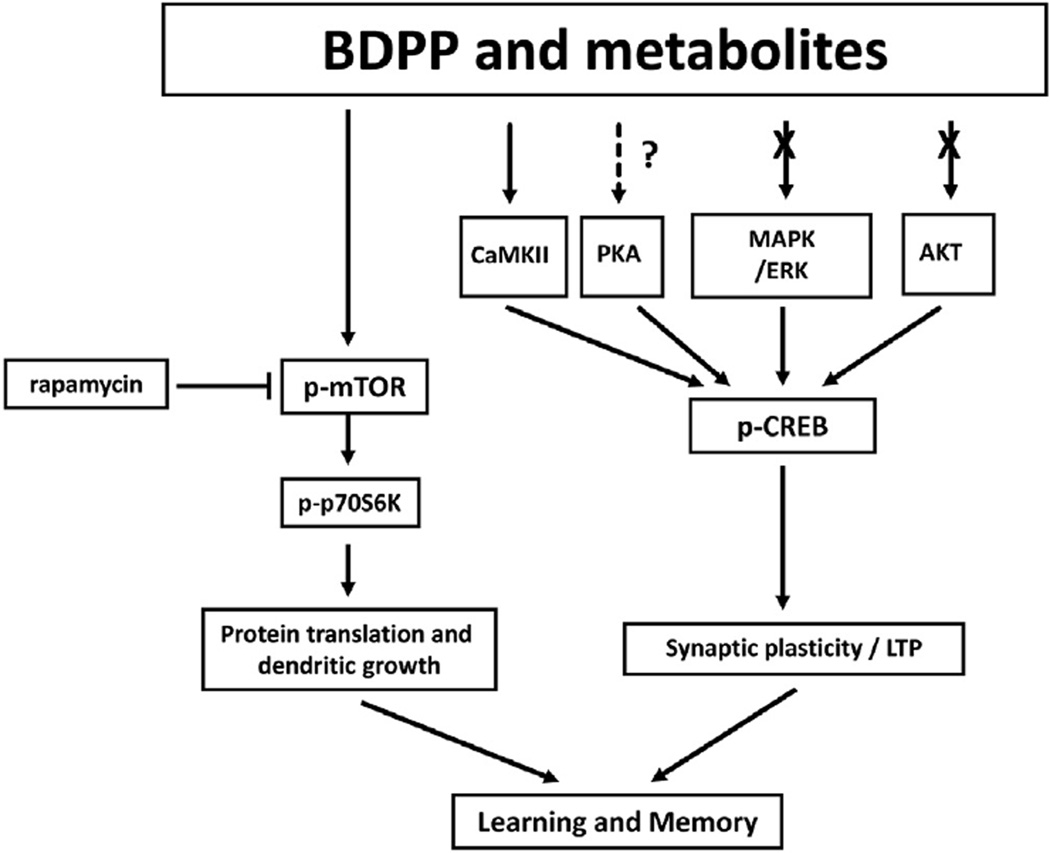
Fig. 5.
BDPP and metabolites enhance synaptic plasticity through mTOR signaling and CamKII-dependent CREB signaling.
5. Conclusions
Collectively, our study demonstrates that application of novel polyphenol compounds can prevent SD-mediated cognitive impairment, possibly through the activation of two independent yet interrelated pathways: the CREB signaling pathway and the mTOR signaling pathway. Given the safety and tolerability of these two compounds, our preclinical study has provided a basis for potential translational application of novel polyphenol compounds in promoting resilience to SD-induced cognitive deficits by targeting the CREB and mTOR signaling pathways.
Acknowledgments
This study was supported by discretionary funding from the Icahn School of Medicine at Mount Sinai to Dr. Giulio Pasinetti. In addition, Dr. Pasinetti holds a Career Scientist Award in the Research and Development unit and is the Director of the Basic and Biomedical Research and Training Program, GRECC, James J. Peters Veterans Affairs Medical Center. We acknowledge that the contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This publication was supported by Grant Number P50 AT008661-01, titled “Dietary Botanicals in the Preservation of Cognitive and Psychological Resilience,” from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, ODS, or the National Institutes of Health.
Abbreviations
- AD
Alzheimer's disease
- BDPP
bioactive dietary polyphenol preparation
- BW
body weight
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP-response element-binding protein
- EC
epicatechin
- Glc
glucoside
- Gluc
glucuronide
- GSE
grape seed extract
- LTP
long-term potentiation
- MeO
Methyl-O
- MRM
multiple reaction monitoring
- mTOR
mammalian target of rapamycin
- PAC
proanthocyanidins
- PKA
protein kinase A
- SD
sleep deprivation
References
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Blount JW, Ferruzzi M, Raftery D, Pasinetti GM, Dixon RA. Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders. Biochem. Biophys. Res. Commun. 2012;417:457–461. doi: 10.1016/j.bbrc.2011.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J. Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Feng WY. Metabolism of green tea catechins: an overview. Curr. Drug Metab. 2006;7(7):755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- Fishbein W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav. 1971;6:279–282. doi: 10.1016/0031-9384(71)90155-7. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr. Scand. Suppl. 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der WL, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann. Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu C, Tufik S, Bergamaschi CT, Tenorio NM, Araujo P, Andersen ML. Sleep pattern in an experimental model of chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2010;299:F1379–F1388. doi: 10.1152/ajprenal.00118.2010. [DOI] [PubMed] [Google Scholar]
- Ho L, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer's disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar U, Ulrih NP. The metabolism of anthocyanins. Curr. Drug Metab. 2014;15(1):3–13. doi: 10.2174/1389200214666131211160308. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Lin CY, Lin HY, Wu JM. AKT/mTOR as novel targets of polyphenol piceatannol possibly contributing to inhibition of proliferation of cultured prostate Cancer cells. ISRN Urol. 2012;2012:272697. doi: 10.5402/2012/272697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem. The National Academies Press; 2006. [PubMed] [Google Scholar]
- Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep. Med. 2012;13:327–334. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25:289–296. [PubMed] [Google Scholar]
- Knutson KL, Van CE. Associations between sleep loss and increased risk of obesity and diabetes. Ann. N. Y. Acad. Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Sang S, Lu AY, Yang CS. Metabolism of dietary polyphenols and possible interactions with drugs. Curr. Drug Metab. 2007;8:499–507. doi: 10.2174/138920007780866870. [DOI] [PubMed] [Google Scholar]
- Ledoux L, Sastre JP, Buda C, Luppi PH, Jouvet M. Alterations in c-fos expression after different experimental procedures of sleep deprivation in the cat. Brain Res. 1996;735:108–118. doi: 10.1016/0006-8993(96)00599-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009a;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Li Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1–42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009b;163:741–749. doi: 10.1016/j.neuroscience.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafian J, Mohamadifard N, Siadat ZD, Sadri G, Rahmati MR. Association between sleep duration and diabetes mellitus: Isfahan healthy heart program. Niger. J. Clin. Pract. 2013;16:59–62. doi: 10.4103/1119-3077.106756. [DOI] [PubMed] [Google Scholar]
- Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr. Pharm. Des. 2013;19:2409–2419. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam SM. Legal issues in accidents caused by sleepiness. J. Hum. Ergol. (Tokyo) 2001;30:107–111. [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JW, et al. Latrepirdine improves cognition and arrests progression of neuropathology in an Alzheimer's mouse model. Mol. Psychiatry. 2013;18:889–897. doi: 10.1038/mp.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat. Rev. Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol. Genom. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert. Rev. Respir. Med. 2012;6:557–566. doi: 10.1586/ers.12.44. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer's disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014;6:42. doi: 10.3389/fnagi.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Brain-targeted proanthocyanidin metabolites for Alzheimer's disease treatment. J. Neurosci. 2012;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J. Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Santa-Maria I, Ho L, Ksiezak-Reding H, Ono K, Teplow DB, Pasinetti GM. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer's disease. J Alzheimers. Dis. 2010;22:653–661. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Role of standardized grape polyphenol preparation as a novel treatment to improve synaptic plasticity through attenuation of features of metabolic syndrome in a mouse model. Mol. Nutr. Food Res. 2013;57:2091–2102. doi: 10.1002/mnfr.201300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, et al. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:933–940. doi: 10.1093/gerona/glq094. [DOI] [PubMed] [Google Scholar]
- Zhong LM, Zong Y, Sun L, Guo JZ, Zhang W, He Y, Song R, Wang WM, Xiao CJ, Lu D. Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One. 2012;7:e32195. doi: 10.1371/journal.pone.0032195. [DOI] [PMC free article] [PubMed] [Google Scholar]