Abstract
Background
Impairments in sleep and cognitive function have been observed in patients with substance abuse disorders and may be potential factors contributing to drug relapse. In addition, sleep disruption may itself contribute to cognitive deficits. In the present study we examined the impact of prolonged cocaine self-administration and abstinence on actigraphy-based measures of night-time activity in rhesus macaques as an inferential measure of sleep, and determined whether sleep-efficiency correlated with cognitive impairments in the same subjects on drug free days.
Methods
Actigraphy data was obtained from a group of rhesus macaques intravenously self-administering cocaine (n=6) and a control group (n=5). Periods were evaluated during which the mean cumulative doses of cocaine were 3.0+0.0 and 4.5+0.2 mg/kg/day for 4 days (Tuesday-Thursday) each week.
Results
Actigraphy-based sleep efficiency decreased during days of cocaine self-administration in a dose-dependent manner. Consistent with this observation, sleep became more fragmented. Activity-based sleep efficiency normalized during the weekend without cocaine prior to cognitive assessment on Monday. The magnitude of activity-based sleep disruption during self-administration did not correlate with the level of cognitive impairment on drug free days. With continued self-administration, the impact of cocaine on activity-based sleep efficiency declined indicating the development of tolerance.
Conclusions
Cocaine self-administration disrupted sleep efficiency in rhesus macaques as measured by actigraphy, but normalized quickly in the absence of cocaine. The cognitive impairment observed on drug free days was unlikely to be related to disruption of the nightly activity patterns on days of cocaine self-administration.
Keywords: cocaine, rhesus macaque, sleep efficiency, stimulant, cognition, actigraphy
1. INTRODUCTION
Sleep disruptions form an important component of psychiatric disorders including substance abuse disorders (Breslau et al., 1996; Garcia and Salloum, 2015; Hasler et al., 2012; Schierenbeck et al., 2008). Sleep disorders are more frequently observed in substance abusers compared to the general population (Brower, 2003; Mahfoud et al., 2009) and acutely, drugs of abuse disrupt sleep (Allen et al., 1993; Brower, 2003; Pace-Schott et al., 2005). Sleep disruption following prolonged withdrawal from drug use is regarded as an important potential contributor to drug relapse (Angarita et al., 2014; Brower, 2003; Gillin et al., 1994; Morgan and Malison, 2007) and may lead to impaired cognitive function frequently observed in addiction (Ersche et al., 2012; Morgan and Malison, 2007; Robbins et al., 2008).
Polysomnographic studies demonstrate that sleep onset is delayed and sleep efficiency is reduced following acute cocaine use (Kowatch et al., 1992; Pace-Schott et al., 2005). Reduced sleep efficiency and reduced REM sleep are observed following (prolonged) abstinence from cocaine and have been suggested to contribute to the risk of drug relapse (Chen et al., 2015; Morgan and Malison, 2007; Roth, 2009). Some reports indicate that sleep disruption may worsen with prolonged cocaine withdrawal over a 3 week period (Morgan et al., 2009; Pace-Schott et al., 2005). In addition to the difficulty of continued sleep monitoring following protracted drug withdrawal, particular challenges with human substance abuse studies are the lack of experimental control and frequent poly drug use. For these reasons preclinical studies are critical. Non-human primates exhibit sleep patterns as well as drug responses similar to humans (Bradberry, 2008; Daley et al., 2006; Hsieh et al., 2008; Masuda and Zhdanova, 2010). Recently, activity measures from compact accelerometers (Actical; Philips Respironics, Murrysville, PA) have been used to provide insight in sleep and activity patterns following stimulant self-administration (Andersen et al., 2013; Brutcher and Nader, 2013). These devices permit convenient non-invasive activity monitoring over prolonged periods. Although accelerometers cannot capture the detailed sleep architecture afforded by polysomnography, they do provide a valid index of human sleep (Sadeh, 2011). Several algorithms have been used to optimize sensitivity and specificity to characterize sleep in both humans and non-human primates (Andersen et al., 2013; Brutcher and Nader, 2013; Gaughan et al., 2014; Kripke et al., 2010; Paquet et al., 2007). NHP studies have indicated that methamphetamine self-administration worsens sleep but that sleep normalizes immediately following cessation of self-administration (Andersen et al., 2013) consistent with observations in human users. Similarly, low doses of cocaine self-administration reduce sleep quality although the consequences of withdrawal were not studied (Brutcher and Nader, 2013).
Because the disruption of sleep quality related to cocaine use and its potential impact on cognitive performance are clinically important as potential factors of drug relapse, in the present experiments we assessed the impact of cocaine use and abstinence on actigraphy-based sleep measures. In the same monkeys self-administering cocaine from which we previously reported impairment of cognitive performance (Porter et al., 2013; Porter et al., 2011), we assessed if any disruptions in activity-based sleep efficiency were related to these cognitive impairments.
2. MATERIAL AND METHODS
All protocols were in accordance with the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
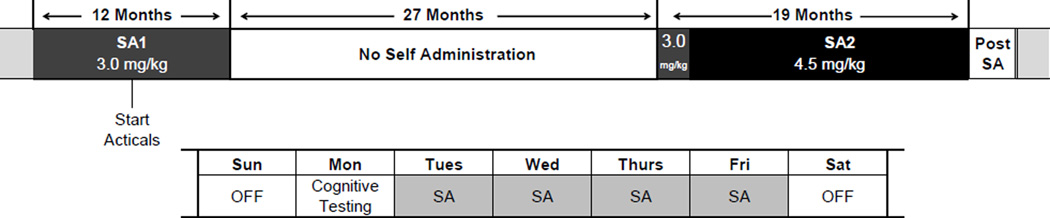
The experiments were started with a single cohort of 13 adult male rhesus macaques (7 subjects in the cocaine group and 6 in a control group; age at start of the self-administration period SA1: 5.8 ± 0.1 yr). Full details of the self-administration procedure for this cohort of monkeys have been published previously (Porter et al., 2013, 2011). Briefly, for 2 periods over a 5 year span (SA1: 12 months and SA2: 19 months separated by a 27 month period of abstinence), subjects in the cocaine group self-administered cocaine intravenously using an FR30 reinforcement schedule: up to 6 infusions 0.5 mg/kg/infusion each day 4 days/week (Tue-Fri) during SA1; the same schedule was used during the first part of SA2. After 5–7 weeks, the schedule was increased to up to 8 infusions 0.6mg/kg/infusion (Tue-Fri) during SA2. A timeline of the experiments is shown in fig. 1. We refer to the long 27 month period of abstinence as baseline for the cocaine subjects despite the previous period of self-administration (SA1), because the sleep measures were stable and did not differ between cocaine and control subjects. Subjects typically would take all infusions made available to them. Self-administration sessions took place between 07:30am and 02:30pm Tuesday-Friday. Based on the half-life of cocaine in rhesus macaques of approximately 60 min (calculated from data in (Bradberry et al., 2000)), cocaine was eliminated from the circulation by the time the light were turned off at 7pm.
Figure 1.
Timeline of cocaine self-administration (SA) experiments. Bottom shows the pattern of cocaine self-administration during a typical week with 4 days of intravenous self-administration at either 3.0 or 4.5 mg/kg, followed by a drug-free weekend and testing of cognitive function on Mondays.
Cognitive performance was tested on Mondays approximately 72hrs following the last cocaine infusion (Porter et al., 2013, 2011) and averaged for each self-administration period. The cumulative dose of cocaine for subjects at the end of SA1 was 524 ± 5 mg/kg; the cumulative dose at the end of SA2 was 1712 ± 8 mg/kg. Only 4/7 subjects in the cocaine group were available for the activity assessment at the end of the SA2 due to clinical issues unrelated to the study, and one control animal was transferred from the study after the SA1 period. Consequently, the number of subjects contributing to the group average declined during the course of the study as indicated by the decreasing N’s reported in the results and figure legends. The declining number of subjects did not alter the overall pattern of sleep disruption throughout the week (e.g., the decrease in activity-based sleep efficiency during the week of self-administration was 3.6±0.7% based on n=6, or 4.0±1.0% based on n=4, see results). The control subjects carried out similar touch screen-based tasks for water self-administration 4 days a week and with the same cognitive testing on Mondays (Porter et al., 2011).
The impairment in working memory function for each individual during the SA1 self-administration period was defined as the change of the slope of the accuracy versus delay plots before and during self-administration. The impairment in reversal learning during SA1 was defined as the difference of the slope of the accuracy versus trial number (following reversal) plots before and during the self-administration period (Porter et al., 2011). Behavioral scores for the SA2 period were calculated in a similar manner by subtracting the cognitive performance score for each animal during baseline from the same score during the self-administration period.
Subjects were housed in adjacent colony rooms (both control and cocaine subjects in each room) that were kept on a 12 hour light-dark cycle (lights on at 07:00am). Food (Labdiet 5038, PMI, Richmond, IN) was provided to maintain a healthy bodyweight and water intake was regulated at 25 ml/kg. Fresh fruit and treats as well as human interaction were provided daily. All subjects were single-housed throughout the SA2 period but 3 subjects in each group were pair-housed during SA1. The change in pairing status (due to social incompatibility) occurred largely during the 27 month period of abstinence between SA1 and SA2 while activity was not being monitored. In the single case where housing status changed while nightly activity was monitored we did not observe any significant change (p=0.32) in activity-based sleep efficiency.
Starting during the first cocaine self-administration period (SA1), activity data was collected in 1 minute sampling epochs from Actical accelerometers located inside custom made aluminum collars. Due to the limited number of available units, accelerometers were switched between the cocaine and control groups. Data were downloaded following approximately 40 days of recording using Actical 2.12. All further data analyses were performed using custom scripts written in Matlab 2012. Sleep was defined according to a standard Actiware algorithm (essentially a weighted, sliding average; Paquet et al., 2007) with a threshold optimized for night time activity of non-human primates (threshold of 14 instead of 20 for the standard Actiware algorithm; Gaughan et al., 2014). A limitation of the use of these algorithms is that sleep is inferred from periods of inactivity, which tends to overestimate the amount of time slept during the night and underestimate nighttime sleep disruptions (Gaughan et al., 2014; Kripke et al., 2010; Paquet et al., 2007; Sadeh, 2011). We opted to use the algorithm with a reduced threshold because of its greater sensitivity to night time sleep disruption and to minimize type II errors based on an extensive ROC analysis of threshold parameter settings in combination with infrared video analyses of rhesus monkeys (Gaughan et al., 2014). Different published algorithms, including the standard Actiware algorithm, largely provided similar results (data not shown; Herringa et al., 2009; Paquet et al., 2007). We will refer to periods of inactivity categorized by the algorithm as sleep as activity-based sleep although we acknowledge the lack of EEG data to confirm this.
Sleep efficiency was quantified as the total number of minutes slept each night as the percentage of the lights off period (7pm-7am) similar to previous studies in NHPs (Andersen et al., 2013; Barrett et al., 2009; Brutcher and Nader, 2013; Hsieh et al., 2008). It is important to note however, that this definition of sleep efficiency in the pre-clinical literature differs from that used in the clinical literature where sleep efficiency is defined as the total sleep time as a percentage of the time in bed which is controlled by each individual (Morgan et al., 2006, 2009; Pace-Schott et al., 2005; Paquet et al., 2007). In addition to sleep efficiency, the number of bouts of uninterrupted sleep and the average duration of these bouts were calculated. Sleep onset latency was defined as time between lights off and the first minute characterized as sleep. Unless otherwise mentioned, activity-based sleep parameters were averaged over 3 weeks of activity scores at the beginning of each period and compared to parameters for the same duration immediately prior to it.
Based on the pattern of sleep disruption across days of the week, the magnitude of the sleep disruption was calculated as the difference between the activity-based sleep efficiency on the last 2 days prior to self-administration (mean of Sun and Mon) and the last 2 days with self-administration (mean of Thursday and Friday). Because a worsening pattern of sleep disruption was observed across the four consecutive days of cocaine use per week, only data from complete weeks were analyzed, and weeks in which other study-related sleep disruptions occurred (anesthesia for TB testing or imaging procedures) were excluded from analysis. All data are reported as the mean ± SEM and were analyzed with repeated measures ANOVA (rm-ANOVA) using α= 0.05 and weekday and treatment or dose as factors. For post-hoc comparisons critical levels were adjusted for multiple comparisons according to Holm–Sidak (Sigmastat 3.5, Systat Software, Richmond, CA, USA) and multiplicity adjusted P-values are reported in the manuscript.
3. RESULTS
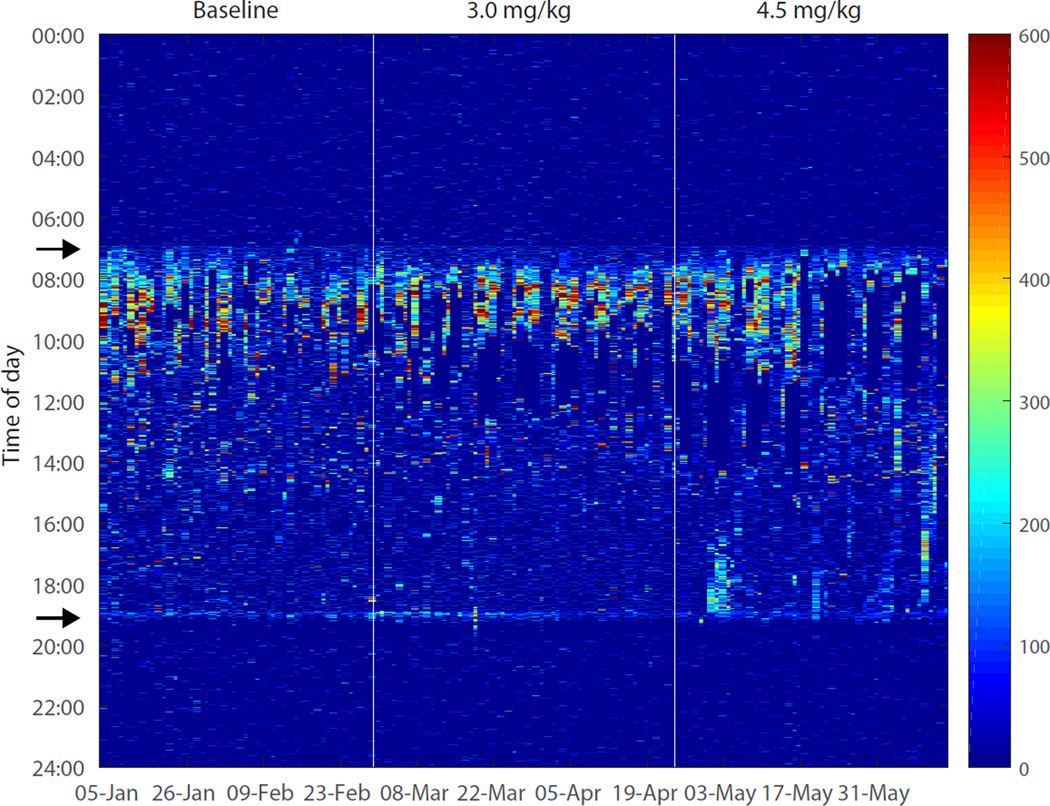
The activity records demonstrated a regular diurnal pattern with the majority of activity occurring during the daytime and little activity during nights. Activity scores were zero during behavioral testing and cocaine self-administration periods because animals were chaired (with their collar immobilized) during these procedures. Upon initiation of cocaine self-administration sessions, no gross disruption of activity patterns was observed (fig. 2).
Figure 2.
Activity scores for each minute of an individual subject before and after the start of cocaine self-administration. The first vertical line indicates the day on which self-administration was started at a daily dose of 3.0mg/kg and the second vertical line indicates the day on which the daily dose was increased to 4.5mg/kg. The arrows (at 7am and 7pm on y-axis) indicate the time of light onset and offset, respectively.
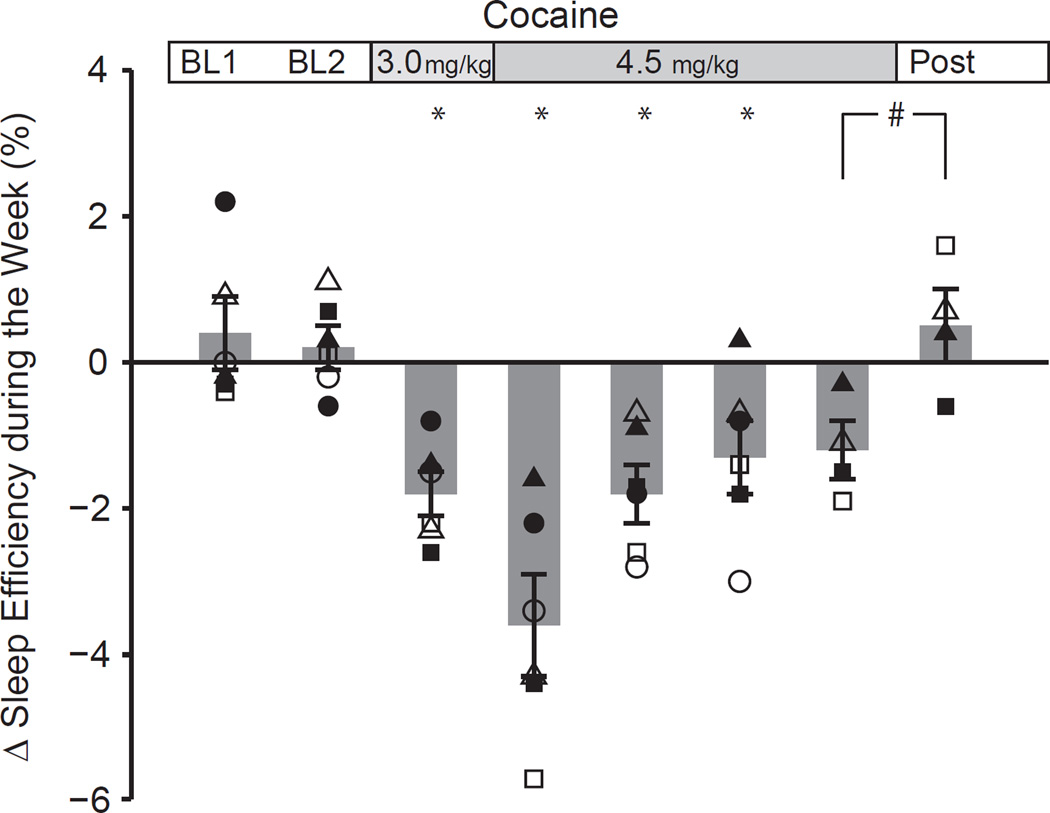
Activity-based sleep efficiency, defined preclinically as the time asleep during the lights-out period (see Material and Methods), of subjects in the cocaine and control groups was similar during the baseline period prior to the start of self-administration (the mean ± SEM for the cocaine (n=6) and control (n=5) groups were 91±1% and 91±3%, respectively). Upon initiating cocaine self-administration (SA2 cumulative daily dose 3.0±0.0 mg/kg, n=6), their sleep efficiency showed a subtle but significant decline across the week (difference between Sun/Mon and Thurs/Fri) during cocaine self-administration (−1.8±0.3% (n=6) compared to +0.2±0.3% for these subjects prior to self-administration, fig.3). After 5–7 weeks animals were permitted to administer a greater number of infusions (with a higher unit dose), resulting in a higher daily cumulative dose of 4.5±0.2 mg/kg (n=6), whereupon activity-based sleep efficiency across the week declined further (−3.6±0.7% mean of the first 3 weeks; n=6) resulting in a significant effect of dose (1-way rm-ANOVA P<0.001). Post-hoc comparisons demonstrated that the magnitude of activity-based sleep disruption at both the lower dose and the higher dose were different from the baseline period (P=0.011 and P<0.001, respectively) and that the magnitude of disruption differed between the lower and the higher dose (P=0.011). When followed over time, the magnitude of sleep disruption was the greatest in the first 3 week block at the high dose and gradually decreased to a smaller magnitude over the subsequent blocks at this dose (−1.3±0.5%; n=6; P=0.031 vs baseline; P=0.001 vs first 3 weeks at 4.5 mg/kg). Later in the study, a smaller number of subjects (n=4) was available as described in Material and Methods but the activity patterns of these subjects could be observed over a much longer period of self-administration. Despite the smaller number of subjects, the same patterns remained (−4.0±1.0%; n=4 during the first 3 week block, followed by a decline to −1.2±0.4% many months later, in the last 3 weeks at the high dose during SA2; n=4). Upon cessation of cocaine self-administration, activity-based sleep efficiency normalized within the first 3 weeks (fig 3; magnitude +0.5±0.5%; n=4; paired t-test P=0.079 versus 3 weeks immediately prior to cessation). Given the subtle effect after a prolonged period of self-administration and the limited statistical power associate with the reduced number of subjects, it is difficult to determine an exact time of recovery. Our observations of normalization of activity-based sleep efficiency after each drug-free weekend suggest however, that the sleep disruptions do not last very long following the current paradigm of cocaine self-administration.
Figure 3.
The magnitude of the decline in activity-based sleep efficiency during the week over the course of the study was calculated as the difference between the sleep efficiency on the last 2 days prior to self-administration (Sun and Mon) and the last 2 days of cocaine use (Thursday and Friday). Open and closed symbols reflect data points for individual subjects over 3 week periods. Each bar represents the average (±SEM) of the all subjects (n=6 with the exception of the last 2 bars on the right, which represent the average of 4 subjects). BL1 and BL2 refer to consecutive periods during the baseline condition which is followed by subsequent 3 week periods during cocaine-self administration.
* Significantly different from last 3 weeks of baseline: P<0.05 (n=6);
# P=0.079 versus last 3 weeks of cocaine self-administration (n=4).
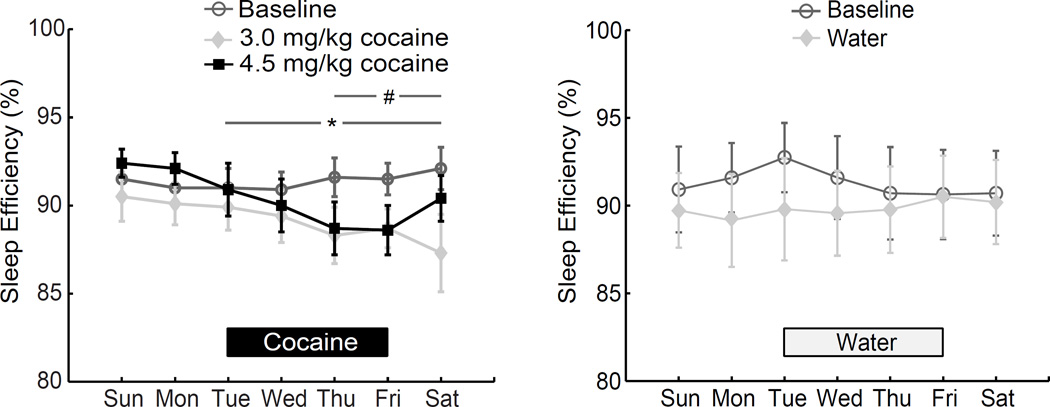
When daily activity-based sleep efficiency was examined across days of the week, there was a significant dose × weekday interaction (P<0.001; fig. 4). Follow-up 1-way rm-ANOVA demonstrated a significant effect of weekday during both the low dose self-administration period (3.0mg/kg daily dose; same three weeks as bar 3 in fig. 3) and the later period with higher cocaine dose (first three weeks with 4.5 mg/kg daily dose; same three weeks as bar 4 in fig. 3) (P=0.002 and P<0.001, respectively) but not during the baseline period (p=0.583). Posthoc tests indicated that activity-based sleep efficiency was significantly reduced compared to Sunday during low dose self-administration on Thursdays, Fridays, and Saturdays, and from Tuesday-Saturday during the higher daily dose of cocaine. A similar pattern of reduced activity-based sleep efficiency across the week was observed during the first period of cocaine self-administration (SA1) in which only the 3mg/kg daily dose was tested: (−3.4±0.4% (n=7); study period × day of the week interaction P=0.005; data not shown). No changes in actigraphy-based sleep efficiency across days of the week were observed in the control group (n=5; study period × day of the week interaction P=0.187).
Figure 4.
Sleep efficiency as determined by actigraphy in cocaine and control subjects over the course of the week. Activity-based sleep efficiency decreased during cocaine self-administration (n=6; left) compared to Sunday. During the initial 3 weeks at the 3.0mg/kg dose, the reduction was significant from Thursday-Saturday (# P<0.05), whereas during the initial 3 weeks at 4.5mg/kg, activity-based sleep efficiency was significantly reduced from Tuesday-Saturday (* P<0.05). No significant patterns were found in the control group during water self-administration (n=5 right).
During SA1, there was no correlation between the magnitude of a subject’s sleep disruption (change in activity-based sleep efficiency during the week) and their contemporaneous performance impairment on either the visual working memory task or the reversal task (both p>0.25, n=7; Porter et al., 2011). The cognitive performance during SA2 was assessed on slightly different tasks: a modified version of the working memory task (Porter et al., 2014), and tasks assessing attention (modified after; Robbins, 2002; Young et al., 2009) and inhibitory control (stop signal reaction time task (Liu et al., 2009). In contrast to the earlier period of self-administration, we found no impairment on any of the tasks evaluated during SA2 compared to the performance during the baseline period. There was no correlation between the (lack of) change in behavioral performance and the magnitude of activity-based sleep disruption during SA2 (p>0.5).
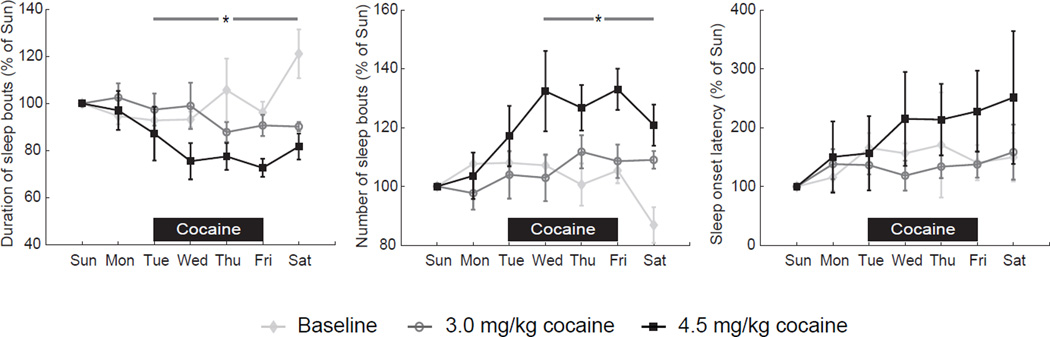
Consistent with the reduced activity-based sleep efficiency during the self-administration period (SA2), sleep became more fragmented. There was a study period by weekday interaction for duration of sleep bouts (P<0.001; n=6; fig. 5) showing a significant reduction during the week at the higher but only at the trend level at the lower daily dose (P=0.002 and 0.052, respectively). There was also a significant study period by weekday interaction for the number of sleep bouts (P<0.001) showing a significant increase during the week at the higher but not the lower daily dose (P<0.001 and 0.210, respectively). Sleep onset commenced later across days of the week only at the higher daily dose (1way rm-ANOVA on ranks: p=0.044).
Figure 5.
Duration and number of sleep bouts during the week and sleep onset latency. Data for the initial 3 weeks at each dose (n=6) are normalized to Sunday night for graphical purposes. The average duration of sleep bouts on Sunday nights was 32±2 (baseline), 29±4 (3.0 mg/kg), and 37±5 min (4.5mg/kg), respectively. The average number of sleep bouts on Sunday nights was 21±1 (baseline), 25±3 (3.0 mg/kg), and 20±3 (4.5mg/kg), respectively and the onset latency on Sunday nights was 6±2 (baseline), 8±3 (3.0 mg/kg), and 7±2min (4.5mg/kg), respectively.
# P<0.05 versus Sunday (3.0mg/kg); * P<0.05 versus Sunday (4.5mg/kg).
The cumulative activity scores during the day light (measured between 4–7 pm) were not significantly altered during the cocaine self-administration periods. It should be noted that the amount of activity varied substantially between subjects and from day to day.
4. DISCUSSION
The present experiments report the impact of chronic cocaine self-administration and abstinence in rhesus macaques on their daily activity patterns as measured by actigraphy. There was a small but significant reduction in activity-based sleep efficiency across repeated days of self-administration in a week. This impairment of activity-based sleep efficiency was associated with increased sleep fragmentation as indicated by an increased number of sleep bouts of shorter duration. Actigraphy-based sleep efficiency rebounded during the weekends when no self-administration took place. The decrease in activity-based sleep efficiency was larger with greater cocaine intake and there was evidence of the development of tolerance with ongoing self-administration. Upon cessation of self-administration, activity-based sleep efficiency normalized within the first 3 weeks. We observed a less robust effect of cocaine self-administration on sleep onset latency, but this measure was highly variable and could be greatly impacted by day to day dynamics within the colony room, in particular during the brief period of activation after the lights were turned off (fig 2).
In the present experiments, we demonstrated impairment in activity-based sleep efficiency at 2 different cocaine doses (3.0 and 4.5 mg/kg) which are both higher than the cumulative daily doses estimated for a previous study (Brutcher and Nader, 2013). In those experiments, decreases in Actigraphy-based sleep efficiency were observed at and around the “lowest preferred dose” of cocaine, which varied per subject, and appeared to yield a U-shaped dose response relationship. Although the overall magnitude of sleep impairment as determined by actigraphy in the present study was smaller than that observed by Brutcher and co-workers, we found greater disruption of sleep efficiency with the higher dose of cocaine. The smaller magnitude of sleep disruption compared to Brutcher et al. could be related to the fact that the present study analyzed sleep parameters over a longer period (3 weeks versus “at least 5 sessions”) and that tolerance appears to develop with ongoing cocaine exposure. The development of tolerance may also have contributed to the apparent non-linear dose-response relationship observed as a result of the random dose-order of testing in Brutcher et al. Regardless, at either dose that we used, the impact of cocaine self-administration on activity-based sleep efficiency appears smaller than similar experiments using methamphetamine self-administration (cumulative daily dose 0.2–1.0 mg/kg; Andersen et al., 2013).
The decrease in activity-based sleep efficiency returned to pre-self-administration levels during each weekend without access to cocaine. Furthermore, after complete cessation of self-administration, sleep efficiency returned to the same levels as prior to self-administration. We found no evidence of progressive worsening of sleep measures with abstinence and these data do not parallel the clinical observations of worsening sleep with prolonged abstinence (Chen et al., 2015; Morgan and Malison, 2007). It is not clear whether this discrepancy is related to differences in technical approach, species, or dose or pattern of cocaine exposure. The pattern of repeated self-administration for 4 days each week followed by the repeated lack of access to cocaine over the weekend (and cognitive testing on Mondays), may have reduced the impact of prolonged abstinence from cocaine in the present study. Accelerometer-based measurements are incapable of detecting differences in sleep architecture upon abstinence as described for cocaine users (Morgan and Malison, 2007; Morgan et al., 2006, 2008; Pace-Schott et al., 2005), although differences in sleep efficiency can clearly be detected using both techniques. The subjects in the present study were always in the same environment throughout all phases of the study eliminating concerns about the novelty and adaptation to the laboratory environment associated with human addiction studies.
A limitation of the use of accelerometers is that they measure activity and that sleep is inferred from periods of inactivity using a variety of algorithms. The different algorithms tend to overestimate the amount of time slept during the night and underestimate nighttime sleep disruptions (Gaughan et al., 2014; Kripke et al., 2010; Paquet et al., 2007; Sadeh, 2011). Despite the lower activity threshold used in the present algorithm to increase sensitivity to disruptions of sleep (i.e. reduce false positive minutes characterized as sleep of awake but non-moving subjects), we potentially still overestimated the sleep efficiency (~91 ± 1 % at baseline for the cocaine and control groups combined (n=11)) in the present study compared to classical electrophysiological techniques (70–90%; Daley et al., 2006; Hsieh et al., 2008)) and may have underestimated the amount of sleep disruption caused by cocaine exposure. Nevertheless, using the present algorithm, sleep efficiency decreased with multiple days of cocaine exposure indicating that it was sufficiently sensitive to detect the sleep disruption. In addition, for the present data set we found essentially the same result of progressive sleep impairments after multiple days of self-administration that recovered during a drug-free weekend, regardless of the algorithm used (Herringa et al., 2009; Paquet et al., 2007).
The present results indicate that the activity-based sleep disruptions we observed following multiple days of cocaine self-administration normalized during a drug-free weekend. Importantly, sleep efficiency as determined by actigraphy was similar on the night prior to (drug free) cognitive testing sessions, in which we previously demonstrated impaired visual working memory and reversal learning performance (Porter et al., 2013, 2011). Furthermore, the magnitude of activity-based sleep disruption did not correlate with the level of impairment of cognitive performance in the same subjects. As a result, the cognitive impairments we observed after the drug-free weekends are likely unrelated to poor sleep as assessed by actigraphy. Nevertheless, it cannot be excluded that the lack of correlation of sleep and cognitive measures is related to a limited number of subjects. Future studies with polysomnography could address whether individual sleep stages show similar or potentially more protracted disruptions with repeated cocaine self-administration (Irwin et al., 2016).
Highlights.
Repeated cocaine self-administration leads to sleep impairment as determined by actigraphy in rhesus macaques.
Activity-based sleep disruption normalizes during weekends without access to cocaine.
Sleep disruption is likely unrelated to cognitive impairment in the same subjects.
Acknowledgments
This research was supported by NIH/NIDA DA025636 and VA Medical Research Award VA BLR&D 1IO1BX000782
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Role of the funding source:
Nothing declared
Contributors:
GG, CWB, and HPJ designed the experiments
JAC, GG, CAE, KG, JN collected the data
JAC, GG, CWB, and HPJ analyzed data
CWB and HPJ wrote manuscript
All authors have approved the final manuscript.
Conflict of Interest:
None. All authors declare that they have no conflict of interest.
REFERENCES
- Allen RP, McCann UD, Ricaurte GA. Persistent effects of (+/−)3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") on human sleep. Sleep. 1993;16:560–564. doi: 10.1093/sleep/16.6.560. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Diaz MP, Murnane KS, Howell LL. Effects of methamphetamine self-administration on actigraphy-based sleep parameters in rhesus monkeys. Psychopharmacology (Berl.) 2013;227:101–107. doi: 10.1007/s00213-012-2943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angarita GA, Canavan SV, Forselius E, Bessette A, Pittman B, Morgan PT. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depend. 2014;134:343–347. doi: 10.1016/j.drugalcdep.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34:1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J. Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol. Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med. Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brutcher RE, Nader MA. The relationship between cocaine self-administration and actigraphy-based measures of sleep in adult rhesus monkeys. Psychopharmacology (Berl.) 2013;229:267–274. doi: 10.1007/s00213-013-3101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang Y, Liu X, Liu Z, Dong Y, Huang YH. Sleep regulates incubation of cocaine craving. J. Neurosci. 2015;35:13300–13310. doi: 10.1523/JNEUROSCI.1065-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JT, Turner RS, Freeman A, Bliwise DL, Rye DB. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta. Sleep. 2006;29:221–231. [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am. J. Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review. Am. J. Addict. 2015;24:590–598. doi: 10.1111/ajad.12291. [DOI] [PubMed] [Google Scholar]
- Gaughan TJ, Kay DB, Pongibove M, Kreider B, Rockcastle N, Ryan ND, Cameron JL. Society For Neuroscience, 45th Annual Meeting. Washington, D.C.: 2014. Validation Of An Assessment Strategy To Accurately Measure Sleep In Non-Human Primates From Actigraphy Data. [Google Scholar]
- Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biol. Psychiatry. 1994;35:843–849. doi: 10.1016/0006-3223(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med. Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Ryan ND, Dahl RE, McCann T, Cameron JL. Sleep patterns in the rhesus monkey: links to physiological and psychological traits Society for Neuroscience. Chicago, IL: 2009. [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep. 2008;31:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Bjurstrom MF, Olmstead R. Polysomnographic measures of sleep in cocaine dependence and alcohol dependence: implications for age-related loss of slow wave, stage 3 sleep. Addiction epub ahead of print. 2016 doi: 10.1111/add.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK. Electroencephalographic sleep and mood during cocaine withdrawal. J. Addict. Dis. 1992;11:21–45. doi: 10.1300/J069v11n04_03. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Hahn EK, Grizas AP, Wadiak KH, Loving RT, Poceta JS, Shadan FF, Cronin JW, Kline LE. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J. Sleep Res. 2010;19:612–619. doi: 10.1111/j.1365-2869.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J. Neurosci. Methods. 2009;177:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoud Y, Talih F, Streem D, Budur K. Sleep disorders in substance abusers: how common are they? Psychiatry (Edgmont) 2009;6:38–42. [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Zhdanova IV. Intrinsic activity rhythms in Macaca mulatta: their entrainment to light and melatonin. J. Biol. Rhythms. 2010;25:361–371. doi: 10.1177/0748730410379382. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Cocaine and sleep: early abstinence. Scientific World Journal. 2007;7:223–230. doi: 10.1100/tsw.2007.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep architecture, cocaine and visual learning. Addiction. 2008;103:1344–1352. doi: 10.1111/j.1360-0443.2008.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Paliwal P, Malison RT, Sinha R. Sex differences in sleep and sleep-dependent learning in abstinent cocaine users. Pharmacol. Biochem. Behav. 2009;93:54–58. doi: 10.1016/j.pbb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl.) 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30:1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Gurnsey K, Jedema HP, Bradberry CW. Latent vulnerability in cognitive performance following chronic cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl.) 2013;226:139–146. doi: 10.1007/s00213-012-2903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Minhas D, Lopresti BJ, Price JC, Bradberry CW. Altered cerebellar and prefrontal cortex function in rhesus monkeys that previously self-administered cocaine. Psychopharmacology (Berl.) 2014;231:4211–4218. doi: 10.1007/s00213-014-3560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J. Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl.) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann. N. Y. Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roth T. Does effective management of sleep disorders reduce substance dependence? Drugs. 2009;69(Suppl. 2):65–75. doi: 10.2165/11531120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med. Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med. Rev. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]