Abstract
Background
Lifestyle factors such as diet and physical activity have been shown to modify the association between fat mass and obesity–associated (FTO) gene variants and metabolic traits in several populations; however, there are no gene-lifestyle interaction studies, to date, among Asian Indians living in India. In this study, we examined whether dietary factors and physical activity modified the association between two FTO single nucleotide polymorphisms (rs8050136 and rs11076023) (SNPs) and obesity traits and type 2 diabetes (T2D).
Methods
The study included 734 unrelated T2D and 884 normal glucose-tolerant (NGT) participants randomly selected from the urban component of the Chennai Urban Rural Epidemiology Study (CURES). Dietary intakes were assessed using a validated interviewer administered semi-quantitative food frequency questionnaire (FFQ). Physical activity was based upon the self-report. Interaction analyses were performed by including the interaction terms in the linear/logistic regression model.
Results
There was a significant interaction between SNP rs8050136 and carbohydrate intake (% energy) (Pinteraction = 0.04), where the ‘A’ allele carriers had 2.46 times increased risk of obesity than those with ‘CC’ genotype (P = 3.0 × 10−5) among individuals in the highest tertile of carbohydrate intake (% energy, 71 %). A significant interaction was also observed between SNP rs11076023 and dietary fibre intake (Pinteraction = 0.0008), where individuals with AA genotype who are in the 3rd tertile of dietary fibre intake had 1.62 cm lower waist circumference than those with ‘T’ allele carriers (P = 0.02). Furthermore, among those who were physically inactive, the ‘A’ allele carriers of the SNP rs8050136 had 1.89 times increased risk of obesity than those with ‘CC’ genotype (P = 4.0 × 10−5).
Conclusions
This is the first study to provide evidence for a gene-diet and gene-physical activity interaction on obesity and T2D in an Asian Indian population. Our findings suggest that the association between FTO SNPs and obesity might be influenced by carbohydrate and dietary fibre intake and physical inactivity. Further understanding of how FTO gene influences obesity and T2D through dietary and exercise interventions is warranted to advance the development of behavioral intervention and personalised lifestyle strategies, which could reduce the risk of metabolic diseases in this Asian Indian population.
Electronic supplementary material
The online version of this article (doi:10.1186/s12986-016-0098-6) contains supplementary material, which is available to authorized users.
Keywords: FTO gene variant, Asian Indian, BMI, Obesity, Type 2 diabetes, Carbohydrate intake, Dietary fibre, Physical activity, CURES
Background
Metabolic traits such as obesity and type 2 diabetes (T2D) are generally caused by overconsumption of energy, sedentary lifestyle and genetic susceptibility [1]. Candidate gene and genome-wide association (GWA) studies have identified several common genetic variants associated with obesity and diabetes [1–5]. Of these, the fat mass and obesity–associated gene (FTO) variants were found to be consistently associated with obesity-related traits in several populations and has been the strongest common genetic predictor of obesity known so far [1–3, 6–9]. To date, FTO has shown the strongest association with BMI, where the FTO SNPs increased the risk of obesity by 1.20–1.32 fold in Europeans [10] and by 1.25 fold in Asians [11]. A recent meta-analysis of data from eight Indian studies showed that FTO variant, rs9939609, increased the risk of obesity by 1.15 times, which is equivalent to BMI increase by 0.30 kg/m2 per effect allele [6]. Previous study in the South Indian population (CURES) has shown that the FTO SNPs (rs1588413 and rs8050136) increased obesity risk by 1.27 and 2.06 times, which is equivalent to BMI increase by 0.33 and 0.54 kg/m2 per effect allele, respectively [7]. The FTO gene is abundantly expressed in hypothalamus and has been shown to be involved in the control of satiety/appetite [12]. Given its role in T2D and obesity [11, 13–16], FTO was considered as a candidate for our gene-diet/-physical activity interaction study. Several studies have examined the influence of lifestyle factors such as diet and physical activity on the association between FTO variants and obesity traits [1, 9, 15, 17]; however, there are no studies to date among Asian Indians living in India.
India has the second largest number of people with T2D in the world, where 62.4 million people in India have T2D according to the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) Study [18]. Asian Indians have unique clinical and biochemical characteristics that are collectively referred to as the "South Asian" or "Asian Indian Phenotype" (higher waist circumference, higher levels of total and visceral fat, hyperinsulinemia, insulin resistance, and a greater predisposition to diabetes) [19] which confers an increased susceptibility to diabetes and premature cardiovascular disease. Although there is a strong genetic component [20–25], unhealthy diet and physical inactivity have also been shown to contribute to the increasing prevalence of metabolic diseases among Asian Indians [26, 27].
Studies in European populations have shown that physical activity and dietary intake may modify the association of the FTO variant with obesity-related traits [28, 29]. Increased physical activity levels have been shown to attenuate the effect of FTO variant on obesity traits in several populations [9, 17, 28]; however, FTO-diet interactions have shown conflicting results, where some studies found that high energy and fat intakes or low carbohydrate intake might strengthen the association of FTO variants with obesity [30, 31], while others failed to confirm such interactions [14]. These inconsistencies could be attributed to differences in the sample size, genetic heterogeneity and dietary assessments. In the present study, we examined whether dietary intake and physical activity levels modified the association of the two commonly studied FTO single nucleotide polymorphisms (SNPs) with obesity-related traits and T2D in 1,618 individuals in the Chennai Urban Rural Epidemiology Study (CURES).
Methods
Study participants
CURES: The study participants were recruited from the urban component of the Chennai Urban Rural Epidemiology Study (CURES). CURES is a cross-sectional epidemiological study conducted on a representative sample of the population of Chennai (formerly Madras city) in Southern India [32]. The methodology of the study has been published elsewhere [32]. Briefly, in phase 1, 26,001 individuals were recruited based on a systematic random sampling technique. Participants with self-reported diabetes taking drug treatment for diabetes were classified as “known diabetes”. All individuals with known diabetes (n = 1529) were invited to visit the center for detailed studies. In addition, every 10th individual of the 26,001 individuals without known diabetes was invited to undergo oral glucose tolerance tests using a 75-g oral glucose load (dissolved in 250 ml of water) (Phase 3 of CURES). Those who were confirmed by oral glucose tolerance test to have 2-h plasma glucose value ≥ 11.1 mmol/l (200 mg/dl) based on World Health Organization (WHO) consulting group criteria were labelled as “newly detected diabetes” and those with 2-h plasma glucose value < 7.8 mmol/l (140 mg/dl) as being normal glucose tolerant (NGT) [33]. Informed consent was obtained from all study participants, and the study was approved by the Madras Diabetes Research Foundation Institutional Ethics Committee.
Phenotype measurements
Anthropometric measurements including weight, height, and waist were obtained using standardized techniques. The BMI was calculated as weight (in kg) divided by the square of height (in m). Biochemical analyses were done on a Hitachi-912 Auto Analyzer (Hitachi, Mannheim, Germany) using kits supplied by Roche Diagnostics (Mannheim). Fasting plasma glucose (glucose oxidase–peroxidase method), serum cholesterol (cholesterol oxidase-phenol-4-amino-antipyrene peroxidase method), serum triglycerides (glycerol phosphatase oxidase-phenol-4-amino-antipyrene peroxidase method), and high-density lipoprotein cholesterol (direct method; polyethylene glycol-pretreated enzymes) were measured. Low-density lipoprotein cholesterol was calculated using the Friedewald formula [34]. Glycated haemoglobin (HbA1c) was estimated by high-performance liquid chromatography using a Variant™ machine (Bio-Rad, Hercules, CA, USA). Serum insulin concentration was estimated using an enzyme-linked immunosorbent assay (Dako, Glostrup, Denmark).
Dietary assessment
Dietary intakes were assessed using a previously validated and published [35] interviewer administered semi-quantitative food frequency questionnaire (FFQ) containing 222 food items to estimate food intake over the past year and took between 20 and 30 min to complete. The nutritionists responsible for the data collection were well trained in the methodology to be used before the field work started. Individuals were asked to estimate the usual frequency (number of times per day, week, month or year/never) and the usual serving size of the portion of the various food items in the FFQ. Common household measures such as household cups, bowls, ladles, spoons (for the cooked foods like vegetables) wedges, circles of different diameter and visual atlas of different sizes of fruits (small, medium, large) were shown to assist the individuals in estimating portions. Information on total energy intake and macronutrients were obtained using the same FFQ. A detailed description of the development of FFQ and the data on reproducibility and validity had been published [35]. EpiNu, an in-house database was used to assess the average daily food and nutrient intake including dietary fibre intake.
Dietary glycemic load of the individual serving was calculated by multiplying the carbohydrate content (grams per serving) of each food by its glycemic index value. This glycemic load value was then multiplied by the frequency of consumption and these products were summed to obtain average daily dietary glycemic load [36].
Physical activity measurement: Physical activity was based upon a previously validated self-report questionnaire [37]. Based on exercise, leisure time activities and job related activities respondents were categorized into three groups indicating activity level (vigorously active, moderately active and sedentary). Individuals were graded as vigorously active if they did leisure time exercise and had physically demanding work, whereas individuals who either exercised or had physically demanding work were categorized as moderately active. All others were categorized as sedentary.
SNP selection and Genotyping
The two commonly studied FTO SNPs (rs8050136 and rs11076023) were chosen based on their previous association with obesity and T2D in the European and South Asian populations [7, 10, 14, 17, 38]. The SNP rs8050136 lies in intron 1 of the FTO gene which is highly conserved across species and is located in the putative transcriptional factor (Cutl- like 1, CUTL1) binding site [39]. The rs11076023 SNP is located in the 3′UTR region of the FTO gene and hence it can be speculated that it might have an effect on the stability of mRNA and gene expression. The SNPs were genotyped by polymerase chain reaction on a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA). The PCR products were digested with MluCI (rs8050136) and HinfI (rs11076023) restriction enzymes (New England Biolabs, Inc., Beverly, MA) and the digested products were resolved by a 3 % agarose gel electrophoresis. Based on the analysis of 200 blind duplicates (20 %), there was 100 % concordance in the genotyping. Furthermore, a few variants were confirmed by direct sequencing with an ABI 310 genetic analyzer (Foster City, CA). The FTO SNPs were in Hardy Weinberg equilibrium (P > 0.05).
Statistical analyses
Results from the descriptive analyses are presented as means and SD for continuous variables and as percentages for categorical variables. Generalized obesity was defined according to the World Health Organization Asia Pacific Guidelines for Asians as non-obese (BMI < 25 kg/m2) and obese (BMI ≥ 25 kg/m2) [40]. A goodness-of-fit chi-square test was performed to examine if the observed genotype counts were in Hardy-Weinberg equilibrium. Student t test as appropriate was used to compare groups for continuous variables. Given the low frequency of the rare homozygotes, dominant model was used (comparing common homozygotes with the combined group of rare homozygotes and heterozygotes). The genetic associations with the continuous and categorical outcomes were examined using linear and logistic regression models, respectively, adjusting for age and gender. When obesity/BMI was an outcome, T2D status was adjusted and when T2D was an outcome, obesity was adjusted in the model, in addition to age and gender. Participants were divided into tertiles of carbohydrate intake (% energy) (Means: first tertile, 58 %; second tertile, 65 %; third tertile, 71 %), glycemic load (Means: first tertile, 188; second tertile, 229; third tertile, 272) and dietary fibres (Means: first tertile, 21 g/d; second tertile, 31 g/d; third tertile, 44 g/d). Interactions between each SNP and dietary intake/physical activity were assessed using linear and logistic regressions and the likelihood ratio test. The interplay between FTO variants and dietary intake/physical activity was investigated by including an interaction term in the regression models. All analyses were carried out using STATA, version 13. A P value of less than 0.05 was considered to be statistically significant.
Results
In the CURES population, 42 % of the individuals were overweight/obese (BMI > 25 kg/m2). As shown in Table 1. Participants with T2D had significantly higher BMI (P < 0.0001), waist circumference (P < 0.001), systolic and diastolic blood pressures (P < 0.0001), fasting plasma glucose and insulin levels (P < 0.0001), total cholesterol (P < 0.0001), triglycerides (P < 0.0001), high-density (P = 0.001) and low-density (P < 0.0001) lipoprotein cholesterols and HbA1c (P < 0.0001). FTO gene variants, rs8050136 and rs11076023, have been shown to be associated with obesity and T2D, respectively, in the CURES study [7].
Table 1.
Clinical and biochemical characteristics of the participants from the CURES study
| Clinical and biochemical parameters | Type 2 diabetic subjects (T2DM) | Normal glucose tolerance subjects (NGT) | P value* | ||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| (N = 407) | (N = 327) | (N = 509) | (N = 375) | ||
| Age (yrs) | 49.59 ± 10.49 | 50.74 ± 11.47 | 36.25 ± 11.21 | 36.54 ± 11.70 | <0.0001 |
| BMI (Kg/m2) | 26.33 ± 4.37 | 24.49 ± 3.94 | 23.57 ± 5.09 | 22.74 ± 4.25 | <0.0001 |
| WC (cm) | 90.08 ± 9.95 | 92.11 ± 9.93 | 80.76 ± 12.43 | 83.81 ± 11.85 | <0.0001 |
| Obese cases (%) | 243.00 (60.00) | 139.00 (42.50) | 179.00 (35.20) | 110.00 (29.40) | <0.0001 |
| Fasting plasma glucose (mg/dl) | 165.42 ± 73.22 | 155.71 ± 61.52 | 84.47 ± 8.36 | 84.71 ± 8.14 | <0.0001 |
| Fasting serum insulin (μIU/ml) | 12.68 ± 7.62 | 10.61 ± 6.95 | 8.48 ± 5.98 | 7.48 ± 4.88 | <0.0001 |
| Systolic blood pressure (mmHg) | 129.91 ± 21.82 | 128.11 ± 21.72 | 114.25 ± 17.54 | 116.25 ± 15.60 | <0.0001 |
| Diastolic blood pressure (mmHg) | 76.54 ± 12.07 | 78.03 ± 11.73 | 71.25 ± 11.38 | 74.28 ± 11.52 | <0.0001 |
| Total serum cholesterol (mg/dl) | 209.21 ± 43.71 | 193.66 ± 39.34 | 173.35 ± 35.15 | 171.99 ± 35.91 | <0.0001 |
| Fasting serum triglycerides (mg/dl) | 179.10 ± 118.48 | 189.25 ± 145.81 | 100.88 ± 53.71 | 123.67 ± 70.97 | <0.0001 |
| HDL cholesterol (mg/dl) | 43.68 ± 9.76 | 39.05 ± 8.66 | 45.47 ± 9.80 | 40.10 ± 9.49 | <0.0001 |
| LDL cholesterol (mg/dl) | 131.76 ± 36.19 | 120.93 ± 33.66 | 107.90 ± 30.17 | 107.47 ± 29.78 | <0.0001 |
| Glycated Haemoglobin (HbA1c) | 8.65 ± 2.39 | 8.76 ± 2.32 | 5.54 ± 0.50 | 5.55 ± 0.49 | <0.0001 |
| PAL [Sedentary (%); Moderate (%); Vigorous (%)] | 263 (77.10); 76 (22.30); 2 (0.60) | 193 (46.80); 60 (20.10); 45 (15.10) | 362 (80.80); 80 (17.90); 6 (1.30) | 240 (64.70); 112 (30.20); 19 (5.10) | 0.001 |
| Total Carbohydrate energy % | 65.50 ± 5.43 | 64.08 ± 6.27 | 64.60 ± 5.89 | 63.09 ± 6.84 | 0.003 |
| Fat energy % | 23.23 ± 4.51 | 23.58 ± 4.76 | 23.81 ± 4.79 | 23.88 ± 4.65 | 0.05 |
| Protein energy % | 11.47 ± 1.20 | 11.43 ± 1.25 | 11.25 ± 1.12 | 11.35 ± 1.25 | 0.009 |
| Energy adjusted glycemic index | 62.76 ± 0.14 | 62.16 ± 0.18 | 62.94 ± 0.12 | 62.65 ± 0.14 | 0.02 |
| Energy adjusted glycemic load | 233.52 ± 1.64 | 226.12 ± 2.40 | 231.63 ± 1.74 | 224.32 ± 0.36 | 0.41 |
| Dietary fibre (g) | 28.62 ± 0.54 | 34.91 ± 0.69 | 29.84 ± 0.42 | 35.73 ± 0.56 | 0.10 |
NGT, Normal Glucose Tolerance; BMI, Body Mass Index; WC, Waist Circumference; HDL, High Density Lipoproteins; LDL, Low Density Lipoproteins; PAL, Physical Activity Level
Data shown are represented as means ± SD, wherever appropriate
*P values for the differences in the means/proportions between Type 2 diabetes cases and NGT participants
Interactions between FTO variants and dietary intake on obesity traits and T2D
There was a significant interaction between FTO SNP rs8050136 and carbohydrate intake % energy) (Pinteraction = 0.04) on obesity, where the ‘A’ allele carriers of the SNP rs8050136 had 2.46 times increased risk of obesity than those with ‘CC’ genotype (P = 1.0 × 10−5) among individuals in the highest tertile of carbohydrate intake (% energy, 71 %) (Fig. 1). We also found a borderline interaction between SNP rs8050136 and energy adjusted glycemic load (Pinteraction = 0.07) on obesity (Additional file 1: Figure S1), where the ‘A’ allele carriers of the SNP rs8050136 had 2.31 times increased risk of obesity than those with ‘CC’ genotype among individuals in the highest tertile of energy adjusted glycemic load (P = 6.0 × 10−5).
Fig. 1.
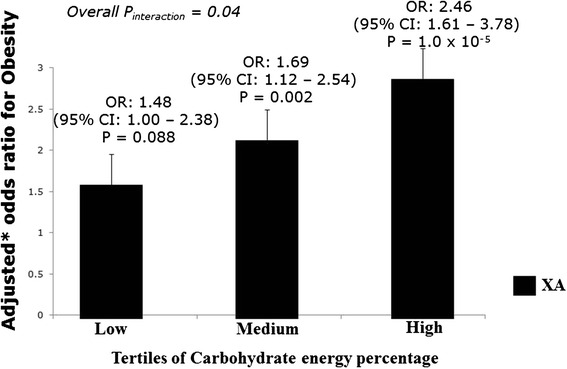
Interaction of the FTO gene polymorphism (rs8050136) with carbohydrate energy percentage on obesity. The ‘A’ allele carriers (XA = CA + AA) who are in the 3rd tertile of carbohydrate energy percentage have 2.46 times increased risk of obesity (P = 1.0 × 10−5). *Odds ratio adjusted for age, gender and diabetes
With the SNP rs11076023, we observed a significant interaction (Pinteraction = 0.0008), where individuals with ‘AA’ genotype who are in the 3rd tertile of dietary fibre intake (44 g/d) had 1.62 cm decrease in waist circumference than those with ‘T’ allele carriers (P = 0.02) (Fig. 2a). A similar interaction trend, but less stronger, was observed with BMI (Pinteraction = 0.03), where individuals with ‘AA’ genotype who are in the 3rd tertile of dietary fibre intake (44 g/d) had 0.50 kg/m2 decrease in BMI than those with ‘T’ allele carriers (P = 0.07) (Fig. 2b). A borderline interaction (Pinteraction = 0.09) was also seen, where the ‘A’ allele carriers of the SNP rs11076023 in the highest tertile of carbohydrate (% energy, mean: 71 %) had 1.57 times increased risk of T2D than those with ‘TT’ genotype (P = 0.002) (Additional file 1: Figure S2), while the first (mean: 58 %) and second (mean: 65 %) tertiles did not show any evidence for an association of the SNP rs11076023 with T2D.
Fig. 2.
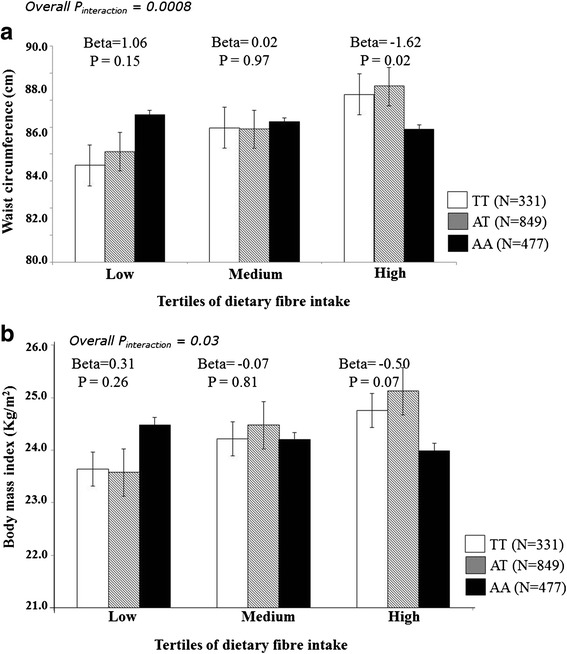
a Interaction of the FTO gene polymorphism (rs11076023) with dietary fibre intake on waist circumference. The individuals with AA genotype who are in the 3rd tertile of dietary fibre intake have 1.62 cm decrease in waist circumference compared to those with ‘T’ allele carriers (P = 0.02). b Interaction of the FTO gene polymorphism (rs11076023) with dietary fibre intake on body mass index. The individuals with AA genotype who are in the 3rd tertile of dietary fibre intake have 0.50 kg/m2 decrease in body mass index compared to those with ‘T’ allele carriers (P = 0.07)
None of the other interactions between the FTO SNPs and fat and protein energy percentages, glycemic index and dietary fibre on obesity traits and T2D were statistically significant (Pinteraction > 0.05 for all interactions) (Tables 2 and 3).
Table 2.
Interaction of the FTO SNP (rs8050136) with dietary factors on BMI, obesity, waist circumference and Type 2 diabetes
| SNP * Fat energy percentage | SNP * Protein energy percentage | SNP * Carbohydrate energy percentage | SNP * energy adjusted glycemic load | SNP * energy adjusted glycemic index | SNP * dietary fibre |
|---|---|---|---|---|---|
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on BMI | |||||
| −0.06 ± 0.06 (0.27) | −0.16 ± 0.23 (0.49) | 0.02 ± 0.04 (0.67) | 0.002 ± 0.007 (0.81) | 0.05 ± 0.09 (0.60) | −0.01 ± 0.02 (0.55) |
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on obesity | |||||
| −0.05 ± 0.03 (0.07) | −0.11 ± 0.11(0.29) | 0.04 ± 0.02 (0.04) | 0.005 ± 0.003 (0.08) | 0.05 ± 0.04 (0.21) | −0.008 ± 0.01 (0.45) |
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on waist circumference | |||||
| −0.14 ± 0.14 (0.34) | −0.27 ± 0.57 (0.64) | 0.04 ± 0.11 (0.68) | 0.004 ± 0.02 (0.83) | 0.20 ± 0.23 (0.38) | −0.03 ± 0.06 (0.57) |
| Beta coefficients ± standard error (Pinteraction)** for interaction on Type 2 diabetes | |||||
| 0.01 ± 0.03 (0.73) | −0.03 ± 0.12 (0.78) | 0.007 ± 0.02 (0.73) | 0.00008 ± 0.003 (0.98) | −0.02 ± 0.05 (0.70) | 0.03 ± 0.01 (0.01) |
¥ P values adjusted for age, gender and type 2 diabetes
**P values adjusted for age, gender and obesity
Table 3.
Interaction of the FTO SNP (rs11076023) with dietary factors on BMI, obesity, waist circumference and Type 2 diabetes
| SNP * Fat energy percentage | SNP * Protein energy percentage | SNP * Carbohydrate energy percentage | SNP * energy adjusted glycemic load | SNP * energy adjusted glycemic index | SNP * dietary fibre |
|---|---|---|---|---|---|
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on BMI | |||||
| 0.009 ± 0.04 (0.79) | 0.10 ± 0.14 (0.44) | 0.004 ± 0.03 (0.89) | 0.003 ± 0.004 (0.52) | 0.03 ± 0.06 (0.57) | −0.03 ± 0.01 (0.03) |
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on obesity | |||||
| 0.0004 ± 0.01 (0.98) | −0.04 ± 0.06 (0.50) | 0.004 ± 0.01 (0.74) | 0.001 ± 0.002 (0.59) | 0.01 ± 0.03 (0.59) | −0.01 ± 0.007 (0.17) |
| Beta coefficients ± standard error (Pinteraction)¥ for interaction on waist circumference | |||||
| −0.12 ± 0.0.09 (0.18) | −0.32 ± 0.34 (0.34) | 0.11 ± 0.07 (0.12) | 0.02 ± 0.01 (0.04) | 0.25 ± 0.14 (0.08) | −0.12 ± 0.04 (0.0008) |
| Beta coefficients ± standard error (Pinteraction)** for interaction on Type 2 diabetes | |||||
| −0.03 ± 0.02 (0.15) | −0.08 ± 0.07 (0.27) | 0.02 ± 0.01 (0.09) | 0.003 ± 0.002 (0.16) | 0.02 ± 0.03 (0.53) | −0.007 ± 0.007 (0.39) |
¥ P values adjusted for age, gender and type 2 diabetes
**P values adjusted for age, gender and obesity
Interactions between FTO variants and physical activity levels on obesity traits and T2D
We observed interactions between the SNP rs8050136 and physical activity on BMI, waist circumference and obesity (Additional file 1: Figure S3a, b and c). Even though the overall interaction P values were not statistically significant (P > 0.11 for all comparisons), sub-group analysis within each levels of physical activity revealed stronger associations of the SNP rs8050136 with obesity traits among those who were physically inactive. Among those who were physically inactive, the ‘A’ allele carriers of the SNP rs8050136 had 0.95 kg/m2 per minor allele increase in BMI (P = 0.002), 3.06 cm per minor allele increase in waist circumference (P = 2 × 10−4) and 1.89 times increased risk of obesity (P = 4.0 × 10−5), compared to those with ‘CC’ genotype. Given that the overall interaction was not significant and the effect was observed only after stratification based on physical activity levels, we tested for the association between the SNP and physical activity levels to avoid spurious interaction effects due to collider bias [41]. There was no association between the SNP rs8050136 and physical activity levels (P = 0.53) suggesting that the observed interaction is not spurious.
We also observed a statistically significant interaction of the SNP rs8050136 with physical activity on T2D (Pinteraction = 0.003) (Table 4). None of the other interactions with physical activity levels on obesity traits and T2D were statistically significant (Pinteraction > 0.05 for all interactions).
Table 4.
Interaction of the FTO gene polymorphisms (rs8050136 and rs11076023) with physical activity levels on BMI, obesity and Type 2 diabetes
| Beta coefficients ± standard error (Pinteraction *) for interaction on BMI | |
| rs8050136 | −0.76 ± 0.48 (0.11) |
| rs11076023 | −0.32 ± 0.29 (0.27) |
| Beta coefficients ± standard error (Pinteraction *) for interaction on obesity | |
| rs8050136 | −0.21 ± 0.24 (0.38) |
| rs11076023 | 0.002 ± 0.15 (0.99) |
| Beta coefficients ± standard error (Pinteraction *) for interaction on waist circumference | |
| rs8050136 | −1.86 ± 1.29 (0.14) |
| rs11076023 | −0.29 ± 0.77 (0.70) |
| Beta coefficients ± standard error (Pinteraction **) for interaction on Type 2 diabetes | |
| rs8050136 | 0.91 ± 0.32 (0.003) |
| rs11076023 | 0.24 ± 0.18 (0.18) |
*P values adjusted for age, gender and type 2 diabetes
** P values adjusted for age, gender and obesity
Discussion
Our study provides the first gene-diet interaction data on obesity-related traits and T2D in an Asian Indian population. The present study shows that carbohydrate and dietary fibre intake may modify the association of the FTO SNPs, rs8050136 and rs11076023, with obesity traits, with the effect of the SNP being more pronounced among those who consumed high levels of carbohydrate and dietary fibre. The study also provides evidence that low levels of physical activity may accentuate the risk of obesity by the FTO SNP rs8050136. Given the high prevalence of T2D in South Asia and as 28–44 % of Asians carry at least one copy of the FTO risk allele [11], our findings hold significant implications for public health.
While carbohydrates do not constitute the bulk of the energy in the West (~45 %) [42], the diet is high in carbohydrates among South Asian countries such as India (65.6 %) [36, 43]. Furthermore, higher dietary carbohydrates and glycemic load have been shown to be associated with the risk of T2D among urban south Indians [43]. In the present study, we found a significant interaction between the FTO SNP rs8050136 and carbohydrate energy percentage on obesity, where the minor allele carriers had higher risk of obesity among those in the highest tertile of carbohydrate energy percentage. While our findings are in contrast to the large European studies which failed to find an interaction between FTO and dietary carbohydrate intake on obesity [14, 44, 45], a study in 4,895 Swedish individuals [30] showed a significant interaction (Pinteraction = 0.0004); however, the effect allele of the FTO SNP rs9939609 (strong LD with rs8050136) increased the risk of obesity among those with low carbohydrate and high fat intakes. In the present study, the total carbohydrate intake was much higher (first tertile, 276 g/d; third tertile, 560 g/d) compared with that reported in the Western population (first quintile, 162 g/d; fifth quintile, 238 g/d) [46] and in another Asian population (first quintile, 233.3 g/d; fifth quintile, 321.9 g/d) [47]. This might be one of the reasons why the previous European studies on obesity failed to identify an interaction with carbohydrate intake and also this finding partly explains the role of varying dietary patterns in contributing to the heterogeneity in identifying and replicating gene-diet interactions.
The positive interaction that was observed between SNP rs11076023 and dietary fibre intake on BMI and waist circumference in our study (mean dietary fibre intake, our study: ~32 g/d) is consistent with the previous report from the Finnish Diabetes Prevention Study (mean dietary fibre intake, Finnish: ~20 g/d) [48], where higher BMI was observed in those who had a diet low in dietary fibre. Furthermore, a recent study from the CURES also showed an inverse association between dietary fibre intake and cardiovascular disease (CVD) risk factors among those with T2D, where individuals who consumed dietary fibre <29 g/d had a higher levels of total and LDL cholesterol [49]. In our study, the interaction between dietary fibre intake and SNP rs11076023 was more pronounced on waist circumference than on BMI, which suggests that effects are likely to be on central obesity rather than obesity per se. This was indeed shown in another Asian (Japanese) population where dietary fibre intake was associated with reduced prevalence of abdominal obesity after multivariate adjustments including obesity in 4,399 individuals [50].
The prevalence of physical inactivity among South East Asians (17 %) has been shown to be lower than that of Europeans (34.8 %) [51]. However, a recent study in 14,227 individuals has shown that 54.4 % of the Indian population is physically inactive [26]. Significant interaction between FTO and physical inactivity on obesity has been reported in several studies from Europe and Asia [9, 17]; however, there are no studies, to date, among Asian Indians living in India. In the present study, we found that among those who were physically inactive, the minor allele carriers of the FTO SNP rs8050136 had higher risk of obesity. Our finding is in accordance with the previous large studies among Europeans [9, 17] where physical activity was shown to attenuate the risk of FTO risk allele on obesity.
Our study has several limitations. We performed a cross-sectional study and hence, we are unable to infer causality between the consumption of carbohydrate-rich foods/physical inactivity and development of obesity. Although confounders were adjusted in all our analyses, residual confounding due to unknown factors cannot be excluded. Interactions were significant only with total carbohydrate intake (energy %) but not when split as total and added sugars and refined and whole cereals (data not shown). Even though our study has a small sample size compared to the large European cohorts, we were still able to confirm the previously reported interactions between FTO variants and dietary intake and physical activity on obesity traits in this Asian Indian population. The small sample size might be one of the reasons why there was no consistency in the gene–diet interactions, where SNP rs8050136 showed an interaction with carbohydrate intake (energy %) while SNP rs11076023 showed an interaction with dietary fibre. However, we cannot rule out the fact that complex traits such as obesity are caused by multiple gene variants and lifestyle factors and hence, it is not necessary for all the variants in the gene to show interaction with the same dietary factor. Given the questionnaire based assessment of physical activity levels, measurement error cannot be completely excluded. Finally, even though we adjusted for age in all the analyses, it is possible that the unmatched age in cases and controls can introduce a bias in the study. Two main strengths of our study is the validated FFQ, which has shown high reproducibility and validity for total carbohydrates, dietary fibre, glycemic index and glycemic load, and the analysis of interactions in a well characterised population.
Conclusions
In summary, this is the first study to provide evidence for a gene-diet and gene-physical activity interaction on obesity and T2D in an Asian Indian population. Our findings suggest that the effect of the FTO SNPs, rs8050136 and rs11076023, on obesity is influenced by high carbohydrate and dietary fibre intakes and low physical activity levels. Given that India leads the world in diabetes [52], our study highlights the need to consume foods with low carbohydrate content and high dietary fibre and to increase physical activity levels, as these could substantially reduce the genetic risk of obesity and diabetes. Further understanding of how FTO gene influences obesity and T2D through dietary and exercise interventions is warranted to advance the development of behavioral intervention and personalised lifestyle strategies, which could reduce the risk of metabolic diseases in this Asian Indian population.
Acknowledgements
Dr Karani S Vimaleswaran acknowledges support from the British Nutrition Foundation. The study was supported by Lady Tata Memorial Trust, Mumbai. The Chennai Wellingdon Corporate Foundation supported the CURES field studies (CURES-79).
Abbreviations
- CURES
Chennai Urban Rural Epidemiological Study
- FTO
Fat mass and obesity associated
- NGT
Normal glucose tolerance
- T2D
Type 2 diabetes
Additional file
Figure S1: Interaction of the FTO gene polymorphism (rs8050136) with energy adjusted glycemic load on obesity. The ‘A’ allele carriers had 2.31 times increased risk of obesity than those with ‘CC’ genotype among individuals in the highest tertile of energy adjusted glycemic load (P=6.0x10-5). Figure S2: Interaction of the FTO gene polymorphism (rs11076023) with carbohydrate energy percentage on Type 2 diabetes. The ‘A’ allele carriers who are in the 3rd tertile of carbohydrate energy percentage have 1.66 times increased risk of Type 2 diabetes (P=0.002). *Odds ratio adjusted for age, gender and obesity. Figure S3a: Interaction of the FTO gene polymorphism (rs8050136) with physical activity level on body mass index (BMI). The ‘A’ allele carriers who are physically inactive have 0.95 kg/m2 increase in BMI compared to those with ‘C’ allele carriers (P = 0.002). Figure S3b: Interaction of the FTO gene polymorphism (rs8050136) with physical activity level on waist circumference. The ‘A’ allele carriers who are physically inactive have 2.90cm higher waist circumference compared to those with ‘C’ allele carriers (P = 2 × 10-4). Figure S3c: Interaction of the FTO gene polymorphism (rs8050136) with physical activity level on obesity. The ‘A’ allele carriers who are physically inactive have 1.89 times increased risk of obesity compared to those with ‘C’ allele carriers (P = 4 x 10-5). *Odds ratio adjusted age, gender and Type 2 diabetes. (DOCX 259 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VKS conceived the study, performed the statistical analysis and drafted the manuscript; LN, BD and SV assisted with the statistical analysis; MV, ARM, SV and RV designed the CURES study; RV designed the genetic study; RK and BD performed the genotyping analysis; BD, LN, ARM, SV, JAL, SK, MV and RV critically reviewed the manuscript. All authors contributed to and approved the final version of the manuscript.
References
- 1.Vimaleswaran KS, Loos RJ. Progress in the genetics of common obesity and type 2 diabetes. Expert Rev Mol Med. 2010;12 doi: 10.1017/S1462399410001389. [DOI] [PubMed] [Google Scholar]
- 2.Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44:526–31. doi: 10.1038/ng.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–9. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Replication DIG, Meta-analysis C, Asian Genetic Epidemiology Network Type 2 Diabetes C, South Asian Type 2 Diabetes C, Mexican American Type 2 Diabetes C, Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples C et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–44. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasan SK, Karpe F, Gu HF, Brismar K, Fall CH, Ingelsson E, et al. FTO genetic variants and risk of obesity and type 2 diabetes: a meta-analysis of 28,394 Indians. Obesity (Silver Spring) 2014;22:964–70. doi: 10.1002/oby.20606. [DOI] [PubMed] [Google Scholar]
- 7.Ramya K, Radha V, Ghosh S, Majumder PP, Mohan V. Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79) Diabetes Technol Ther. 2011;13:33–42. doi: 10.1089/dia.2010.0071. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AE, Sandeep MN, Janipalli CS, Giambartolomei C, Evans DM, Kranthi Kumar MV, et al. Associations of FTO and MC4R Variants with Obesity Traits in Indians and the Role of Rural/Urban Environment as a Possible Effect Modifier. J Obes. 2011;2011:307542. doi: 10.1155/2011/307542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Kilpelainen TO, Liu C, Zhu J, Liu Y, Hu C, et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia. 2012;55:981–95. doi: 10.1007/s00125-011-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–8. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, et al. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–52. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vimaleswaran KS, Angquist L, Hansen RD, Van Der AD, Bouatia-Naji N, Holst C, et al. Association between FTO variant and change in body weight and its interaction with dietary factors: the DiOGenes study. Obesity (Silver Spring) 2012;20:1669–74. doi: 10.1038/oby.2012.49. [DOI] [PubMed] [Google Scholar]
- 15.Roswall N, Angquist L, Ahluwalia TS, Romaguera D, Larsen SC, Ostergaard JN, et al. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: influence of FTO and TCF7L2 loci. Am J Clin Nutr. 2014;100:1188–97. doi: 10.3945/ajcn.114.089706. [DOI] [PubMed] [Google Scholar]
- 16.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vimaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw KT, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr. 2009;90:425–8. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 18.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohan V, Deepa R. Adipocytokines and the expanding 'Asian Indian Phenotype'. J Assoc Physicians India. 2006;54:685–6. [PubMed] [Google Scholar]
- 20.Vimaleswaran KS, Radha V, Ramya K, Babu HN, Savitha N, Roopa V, et al. A novel association of a polymorphism in the first intron of adiponectin gene with type 2 diabetes, obesity and hypoadiponectinemia in Asian Indians. Hum Genet. 2008;123:599–605. doi: 10.1007/s00439-008-0506-8. [DOI] [PubMed] [Google Scholar]
- 21.Vimaleswaran KS, Radha V, Mohan V. Thr54 allele carriers of the Ala54Thr variant of FABP2 gene have associations with metabolic syndrome and hypertriglyceridemia in urban South Indians. Metabolism. 2006;55:1222–6. doi: 10.1016/j.metabol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Vimaleswaran KS, Radha V, Jayapriya MG, Ghosh S, Majumder PP, Rao MR, et al. Evidence for an association with type 2 diabetes mellitus at the PPARG locus in a South Indian population. Metabolism. 2010;59:457–62. doi: 10.1016/j.metabol.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Sathyanarayana Rao MR, Mohan V. Uncoupling protein 2 and 3 gene polymorphisms and their association with type 2 diabetes in asian indians. Diabetes Technol Ther. 2011;13:19–25. doi: 10.1089/dia.2010.0091. [DOI] [PubMed] [Google Scholar]
- 24.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Rao MR, Mohan V. A haplotype at the UCP1 gene locus contributes to genetic risk for type 2 diabetes in Asian Indians (CURES-72) Metab Syndr Relat Disord. 2010;8:63–8. doi: 10.1089/met.2009.0039. [DOI] [PubMed] [Google Scholar]
- 25.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Deepa R, Babu HN, et al. Peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene polymorphisms and their relationship to Type 2 diabetes in Asian Indians. Diabet Med. 2005;22:1516–21. doi: 10.1111/j.1464-5491.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 26.Anjana RM, Pradeepa R, Das AK, Deepa M, Bhansali A, Joshi SR, et al. Physical activity and inactivity patterns in India - results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5] Int J Behav Nutr Phys Act. 2014;11:26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan V, Spiegelman D, Sudha V, Gayathri R, Hong B, Praseena K, et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther. 2014;16:317–25. doi: 10.1089/dia.2013.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpelainen TO, Franks PW. Gene-physical activity interactions and their impact on diabetes. Med Sport Sci. 2014;60:94–103. doi: 10.1159/000357339. [DOI] [PubMed] [Google Scholar]
- 29.Ortega-Azorin C, Sorli JV, Asensio EM, Coltell O, Martinez-Gonzalez MA, Salas-Salvado J, et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol. 2012;11:137. doi: 10.1186/1475-2840-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90:1418–25. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 31.Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr. 2011;141:2219–25. doi: 10.3945/jn.111.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, et al. The Chennai Urban Rural Epidemiology Study (CURES)--study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- 33.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 35.Sudha V, Radhika G, Sathya RM, Ganesan A, Mohan V. Reproducibility and validity of an interviewer-administered semi-quantitative food frequency questionnaire to assess dietary intake of urban adults in southern India. Int J Food Sci Nutr. 2006;57:481–93. doi: 10.1080/09637480600969220. [DOI] [PubMed] [Google Scholar]
- 36.Radhika G, Ganesan A, Sathya RM, Sudha V, Mohan V. Dietary carbohydrates, glycemic load and serum high-density lipoprotein cholesterol concentrations among South Indian adults. Eur J Clin Nutr. 2009;63:413–20. doi: 10.1038/sj.ejcn.1602951. [DOI] [PubMed] [Google Scholar]
- 37.Mohan V, Sandeep S, Deepa M, Gokulakrishnan K, Datta M, Deepa R. A diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians - the Chennai Urban Rural Epidemiology Study (CURES-38) Diabetes Obes Metab. 2007;9:337–43. doi: 10.1111/j.1463-1326.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 38.Rees SD, Islam M, Hydrie MZ, Chaudhary B, Bellary S, Hashmi S, et al. An FTO variant is associated with Type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet Med. 2011;28:673–80. doi: 10.1111/j.1464-5491.2011.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratigopoulos G, Padilla SL, Leduc CA, Watson E, Hattersley AT, McCarthy MI, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1185–96. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organisation WPR . The Asia-Pacific Perspective. Redefining Obesity and its Treatment. Melbourne: WHO/IASO/IOTF: International Diabetes Institute; 2000. [Google Scholar]
- 41.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens J, Ahn K. Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care. 2002;25:1715–21. doi: 10.2337/diacare.25.10.1715. [DOI] [PubMed] [Google Scholar]
- 43.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59) Br J Nutr. 2009;102:1498–506. doi: 10.1017/S0007114509990468. [DOI] [PubMed] [Google Scholar]
- 44.Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23:6961–72. doi: 10.1093/hmg/ddu411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi Q, Downer MK, Kilpelainen TO, Taal HR, Barton SJ, Ntalla I, et al. Dietary Intake, FTO Genetic Variants, and Adiposity: A Combined Analysis of Over 16,000 Children and Adolescents. Diabetes. 2015;64:2467–76. doi: 10.2337/db14-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560–6. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 47.Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med. 2007;167:2310–6. doi: 10.1001/archinte.167.21.2310. [DOI] [PubMed] [Google Scholar]
- 48.Lappalainen T, Lindstrom J, Paananen J, Eriksson JG, Karhunen L, Tuomilehto J, et al. Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr. 2012;108:1859–65. doi: 10.1017/S0007114511007410. [DOI] [PubMed] [Google Scholar]
- 49.Narayan S, Lakshmipriya N, Vaidya R, Bai MR, Sudha V, Krishnaswamy K, et al. Association of dietary fiber intake with serum total cholesterol and low density lipoprotein cholesterol levels in Urban Asian-Indian adults with type 2 diabetes. Indian J Endocrinol Metab. 2014;18:624–30. doi: 10.4103/2230-8210.139215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujii H, Iwase M, Ohkuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 52.Sicree R, Shaw J, Zimmet P. Diabetes and impaired glucose tolerance. In: Gan D, editor. Diabetes Atlas International Diabetes Federation. 3. Belgium: International Diabetes Federation; 2006. pp. 15–103. [Google Scholar]


