The doubly labeled water method is a precise method for measuring energy expenditure in free-living individuals. Its accuracy, however, is dependent on the ratio the relative dilution spaces of the two tracers. Herein we demonstrate that this ratio is not influenced by most subject characteristics and that a single constant can be used in most studies.
Keywords: doubly labeled water, dilution space, stable isotope, total energy expenditure
Abstract
Variation of the dilution space ratio (Nd/No) between deuterium (2H) and oxygen-18 (18O) impacts the calculation of total energy expenditure (TEE) by doubly labeled water (DLW). Our aim was to examine the physiological and methodological sources of variation of Nd/No in humans. We analyzed data from 2,297 humans (0.25-89 yr old). This included the variables Nd/No, total body water, TEE, body mass index (BMI), and percent body fat (%fat). To differentiate between physiologic and methodologic sources of variation, the urine samples from 54 subjects were divided and blinded and analyzed separately, and repeated DLW dosing was performed in an additional 55 participants after 6 mo. Sex, BMI, and %fat did not significantly affect Nd/No, for which the interindividual SD was 0.017. The measurement error from the duplicate urine sample sets was 0.010, and intraindividual SD of Nd/No in repeats experiments was 0.013. An additional SD of 0.008 was contributed by calibration of the DLW dose water. The variation of measured Nd/No in humans was distributed within a small range and measurement error accounted for 68% of this variation. There was no evidence that Nd/No differed with respect to sex, BMI, and age between 1 and 80 yr, and thus use of a constant value is suggested to minimize the effect of stable isotope analysis error on calculation of TEE in the DLW studies in humans. Based on a review of 103 publications, the average dilution space ratio is 1.036 for individuals between 1 and 80 yr of age.
NEW & NOTEWORTHY
The doubly labeled water method is a precise method for measuring energy expenditure in free-living individuals. Its accuracy, however, is dependent on the ratio the relative dilution spaces of the two tracers. Herein we demonstrate that this ratio is not influenced by most subject characteristics and that a single constant can be used in most studies.
the doubly labeled water (DLW) method is a unique tool for estimating total energy expenditure (TEE) in free-living condition with minimum constraints. The method was developed and validated by Lifson et al. in animals in 1955 (16). It was not until 27 yr later that Schoeller and van Santen applied first this method in humans (25). The DLW method has been validated against human calorimeter and/or food intake measurements in multiple experiments that targeted lean to obese subjects, infants to adults, and sedentary to exercise conditions (14a, 20, 22, 28, 30). The method has also been applied easily in hundreds of human studies because it provides a measure of TEE during habitual living conditions while imposing only a small subject burden only requiring that the subjects provide a modest number of specimens (any of urine, saliva, or plasma) before and after administration of DLW (usually by mouth) (14a, 28).
The DLW method makes use of the observation that the deuterium (2H) and oxygen-18 (18O) kinetics differ because 2H is eliminated from the body as water, while 18O is eliminated as water and carbon dioxide (16). Specifically, the rate of carbon dioxide production (rCO2) is calculated from the difference of the products of isotope elimination rate and isotope dilution space of the two stable isotopes (7, 8, 17). These isotope dilution spaces are close to that of total body water (TBW), but both 2H and 18O also exchange with nonaqueous body components and thus are slightly larger than TBW. The extent of the nonaqueous exchange is estimated to be approximately 4∼5 and 1% for 2H and 18O, respectively, in humans (13, 19). Although the variation around these estimates is small, any variation would propagate through the calculations and have a measureable effect on the calculated of rCO2, and hence TEE. For example, in the average adult human, if the actual dilution space ratio (Nd/No) was 0.01 lower than the Nd/No used in the calculation (1.027 vs 1.037), TEE is underestimated by 4–5%, which is not negligible for many applications of the method (18).
Currently, the most widely used equation for human subjects assumes Nd/No is a constant of 1.034 and not different between individuals (19); however, the individual values reported for this ratio have been reported to vary and there is concern that the variation might depend on physical or diet characteristics of the subjects or animals (10, 11). For example, female subjects have higher fat and slightly lower protein masses per unit of TBW, which may affect the fractional isotopic exchange into nonaqueous, organic compounds, and thus Nd/No. Indeed, Goran et al. (10) reported significantly higher Nd/No in males than in females (1.052 and 1.044, respectively). In contrast, Racette et al. (19) reported that females tended to have a higher Nd/No than males although neither difference was significant (P = 0.07). These studies, however, were based on a small sample size and might be biased by sampling or measurement technique (6).
The aim of this study was to examine the physiological and methodological effects on variation of Nd/No in human DLW studies and determine the Nd/No value and range using a large sample. In the past 30 yr, we have conducted quite a large number of the DLW studies providing a database large enough to detect smaller differences than in previous studies.
MATERIALS AND METHODS
Subjects.
We analyzed the Nd/No data from 2,297 human subjects. Subjects included males and females with ages between 0.25 and 89 yr. All of the subjects lived in United States at the time of measurement. The subjects had been recruited to various research studies and the physical characteristics are summarized in Table 1. Each of the studies that contributed to the database as well as the creation of the deidentified database were approved by the IRBs of the institutions with which DAS was affiliated at the time of study (University of Wisconsin-Madison or the University of Chicago). Additional IRB approvals were obtained from the various institutions comprising the clinical sites at which the subjects were enrolled in the study.
Table 1.
Physical characteristics and body composition for human subjects
| Age, yr | Height, cm | BW, kg | BMI, kg/m2 | Body fat, % | FM, kg | FFM, kg | (n) |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| <1 yr | 62.1 ± 2.3 | 6.2 ± 0.8 | 17.7 ± 11.1 | 16.1 ± 1.3 | 1.1 ± 0.7 | 5.1 ± 0.7 | (20) |
| 2–5 yr | 94.4 ± 8.7 | 14.7 ± 3.1 | 24.1 ± 6.0 | 16.4 ± 1.2 | 3.7 ± 1.6 | 11.0 ± 1.8 | (44) |
| 6–11 yr | 130.5 ± 16.6 | 35.0 ± 17.9 | 27.3 ± 10.7 | 19.2 ± 5.2 | 11.1 ± 9.5 | 23.9 ± 9.1 | (44) |
| 12–19 yr | 174.0 ± 9.4 | 65.6 ± 10.4 | 22.5 ± 8.0 | 21.7 ± 3.4 | 15.1 ± 7.4 | 50.4 ± 7.6 | (81) |
| 20–29 yr | 179.1 ± 5.7 | 80.5 ± 16.8 | 21.6 ± 7.5 | 25.1 ± 5.0 | 18.1 ± 10.5 | 62.4 ± 9.2 | (36) |
| 30–39 yr | 181.4 ± 7.3 | 88.5 ± 17.9 | 24.7 ± 9.5 | 27.0 ± 5.8 | 23.0 ± 13.3 | 65.5 ± 7.9 | (21) |
| 40–49 yr | 177.9 ± 8.0 | 85.5 ± 15.1 | 29.8 ± 5.5 | 26.9 ± 4.0 | 25.9 ± 8.1 | 59.6 ± 9.2 | (111) |
| 50–59 yr | 176.4 ± 6.6 | 89.6 ± 15.7 | 32.7 ± 5.7 | 28.7 ± 4.6 | 29.8 ± 9.4 | 59.8 ± 8.5 | (102) |
| 60–69 yr | 175.8 ± 6.9 | 88.9 ± 15.7 | 34.6 ± 5.4 | 28.7 ± 4.2 | 31.3 ± 9.5 | 57.6 ± 7.8 | (81) |
| 70–79 yr | 173.4 ± 7.5 | 82.5 ± 13.2 | 33.4 ± 6.2 | 27.5 ± 4.2 | 28.0 ± 8.4 | 54.5 ± 7.0 | (148) |
| 80–89 yr | 172.0 ± 6.2 | 79.0 ± 13.4 | 34.0 ± 6.2 | 26.8 ± 4.5 | 27.4 ± 8.3 | 51.6 ± 6.9 | (54) |
| Total | 165.0 ± 28.6 | 73.7 ± 27.0 | 29.6 ± 8.3 | 25.4 ± 5.7 | 23.1 ± 12.0 | 50.6 ± 17.2 | (742) |
| Female | |||||||
| <1 yr | 60.9 ± 1.5 | 5.8 ± 0.5 | 23.2 ± 6.8 | 15.5 ± 1.1 | 1.3 ± 0.4 | 4.4 ± 0.5 | (25) |
| 2–5 yr | 93.3 ± 8.7 | 14.3 ± 3.1 | 25.7 ± 6.2 | 16.2 ± 1.4 | 3.8 ± 1.6 | 10.5 ± 1.8 | (46) |
| 6–11 yr | 129.4 ± 16.9 | 35.1 ± 19.1 | 32.8 ± 10.7 | 19.6 ± 6.4 | 13.1 ± 11.4 | 22.0 ± 8.6 | (39) |
| 12–19 yr | 164.7 ± 6.7 | 60.6 ± 10.4 | 33.4 ± 5.5 | 22.3 ± 3.3 | 20.5 ± 6.6 | 40.0 ± 5.4 | (151) |
| 20–29 yr | 164.7 ± 5.5 | 64.4 ± 13.5 | 33.2 ± 7.4 | 23.8 ± 5.2 | 22.0 ± 9.8 | 42.4 ± 6.1 | (87) |
| 30–39 yr | 166.3 ± 6.0 | 75.2 ± 19.1 | 36.6 ± 7.9 | 27.1 ± 6.4 | 28.5 ± 13.2 | 46.6 ± 7.8 | (42) |
| 40–49 yr | 164.3 ± 7.0 | 74.8 ± 20.4 | 38.7 ± 7.9 | 27.6 ± 7.0 | 30.2 ± 13.9 | 44.6 ± 7.8 | (115) |
| 50–59 yr | 163.3 ± 6.4 | 76.9 ± 18.2 | 42.7 ± 6.7 | 28.8 ± 6.3 | 33.8 ± 12.6 | 43.1 ± 6.8 | (121) |
| 60–69 yr | 161.9 ± 6.6 | 75.4 ± 15.3 | 42.8 ± 6.1 | 28.8 ± 5.7 | 33.0 ± 10.8 | 42.4 ± 5.7 | (359) |
| 70–79 yr | 160.4 ± 6.0 | 71.9 ± 14.7 | 42.3 ± 6.2 | 27.9 ± 5.5 | 31.0 ± 10.2 | 40.9 ± 5.8 | (461) |
| 80–89 yr | 158.5 ± 6.3 | 66.9 ± 11.4 | 40.2 ± 6.4 | 26.7 ± 4.7 | 27.4 ± 8.0 | 39.5 ± 5.1 | (109) |
| Total | 157.6 ± 19.0 | 67.8 ± 20.8 | 39.5 ± 8.1 | 26.5 ± 6.3 | 28.0 ± 12.6 | 39.8 ± 9.8 | (1,555) |
Values are the mean ± SD; n = number of subjects.
BW, body weight; BMI, body mass index; FM, fat mass; FFM, fat-free mass.
DLW administration and sample collection.
Subjects provided a baseline urine sample and were given an oral dose containing 18O- and 2H-labeled water. The dose was typically between 1.9 and 2.5 g/kg estimated TBW (10 atom% H218O) and between 0.01 and 0.016 g/kg estimated TBW (99.9 atom% 2H2O). Urine samples were collected at approximately 2, 3, and 4 h after dosing. Subjects were allowed a maximum of 250 ml of liquid after the 1-h postdose time point and up to 500 ml total water consumption through the 4-h time point but were usually less than 250 ml. These volumes were subtracted from the TBW values but not the dilution space. Urine samples were stored at −20°C until the stable isotope abundances of the physiologic samples were measured by isotope ratio mass spectrometry (IRMS). For geriatric populations, plasma or saliva samples were obtained and used for those who had evidence of delayed isotopic equilibration into urine likely caused by urine postvoid retention in the bladder (3). If no saliva was available, subjects for whom the dilution spaces at 3 and 4 h after the dose differed by more than 5% were dropped from the data set. Isotope dilution spaces were calculated by the plateau method according to Cole and Coward (5) using gravimetric dilutions of the dose water. These dilutions were performed in triplicate once for each batch of DLW dose water and those values used in the dilution space calculations for each individual whose dose water came from the batch. Two laboratory standards, one natural abundance and enriched to mimic postdose enrichments, were run daily to correct for day-to-day variations in the IRMS systems. Carbon dioxide production was calculated over ca. 7 or 14 days using the two-point DLW method with Eq. A6 of Schoeller et al. (23), as modified by Racette et al. (19), and energy expenditure using the modified Weir equation (29).
Stable isotope analysis.
Urine (<5 ml) was mixed with <200 mg carbon black to remove impurities and filtered through a 0.45-μm filter. Saliva was centrifuged (4°C, 1 h, relative centrifugal force: 12,000 g) to remove dense proteins. One milliliter of urine or saliva was placed into an autosampler vial and deuterium was measured by IRMS after reduction over chromium (Delta Plus mass spectrometer; Finnigan MAT, Bremen, Germany). From each 1 ml of sample, 1 μl was injected into a quartz tube packed with chromium powder held at 850°C to reduce the water to hydrogen gas. Each run included three injections of the sample with independent analysis. Data were corrected for both H3+ and memory. Results were corrected to the standard mean ocean water (SMOW) scale using laboratory-working standards. A second 1 ml of saliva or carbon black-cleaned urine was allowed to equilibrate with CO2 at 25°C for 48 h. The 18O concentration was measured by continuous flow IRMS (Delta V; Thermo Scientific, Bremen, Germany). Isotopic abundances were expressed on SMOW scale using calibrated laboratory standards.
Blind analysis for test-retest precision.
Urine samples from 54 subjects were blinded and analyzed twice. The samples were prepared by investigators outside of our institute. The samples were aliquoted into different tubes and labeled with a different ID. After all analyses had been finished, matching by IDs was done by an investigator who did not conduct any of the isotope analyses.
Redosing after 6 mo for intraindividual variability.
A total of 55 subjects received the second DLW experiment after 6 mo. The DLW administration and sample collection protocols were identical to those of the first study. The samples were analyzed after being labeled by an investigator outside our institute to blind the analysis and an investigator who did not perform any of the analyses matched the data after all analyses had been completed.
Statistical analysis.
The subjects were categorized by body mass index (BMI) into underweight (<18.5 kg/m2), normal healthy weight (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), class I obese (30–34.99 kg/m2), class II obese (35–39.99 kg/m2), and class III obese (>40 kg/m2). The subjects were also categorized by age group based on date of birth.
Results were presented as mean ± SD. Two-way ANOVA was conducted for Nd/No with BMI category and sex as between-subject factor. Regression analysis was conducted for Nd/No with age, sex, BMI, and body fat (%fat) as independent variables. All analyses were performed using IBM SPSS 23.0 for Mac (IBM, New York, NY). For all of the analyses, an α of 0.05 was used to denote statistical significance.
RESULTS
Physiological effects.
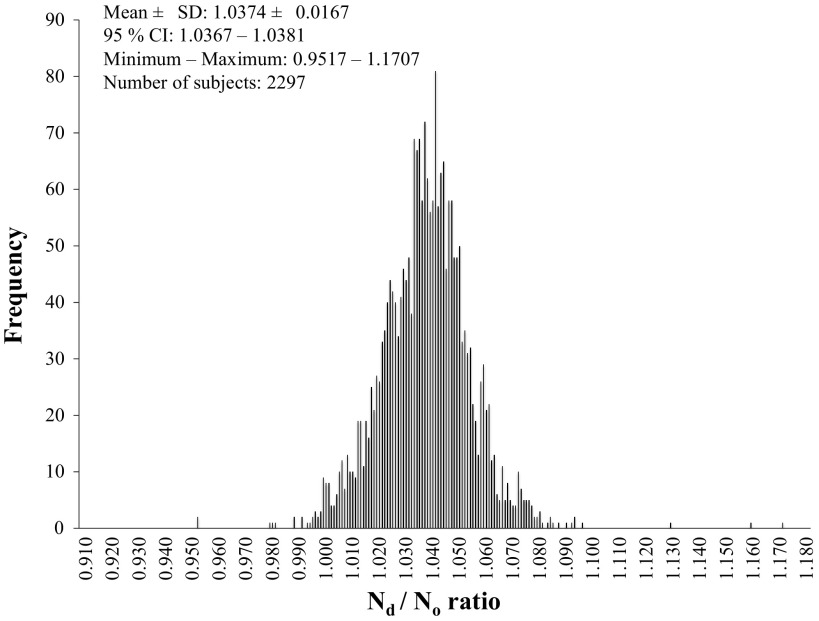
Figure 1 displays the frequency distribution Nd/No of all subjects, which was normally distributed. The mean Nd/No for all human subjects (n = 2,297) is 1.0374 ± 0.0167 and 95% confidence interval (CI) for the mean was 1.0367–1.0381 (Table 2). The mean Nd/No and 95% CI are shown for each of the 11 age groups and sex in Table 2.
Fig. 1.
Dilution space ratio (Nd/No) histogram for all human subjects. CI, confidence interval.
Table 2.
Nd/No ratio for human subjects
| Age | Male (n) | Female (n) | All (n) | 95% CI |
|---|---|---|---|---|
| <1 yr | 1.0363 ± 0.0230 (20) | 1.0342 ± 0.0164 (25) | 1.0351 ± 0.0194 (45) | 1.0295–1.0408 |
| 2–5 yr | 1.0505 ± 0.0149 (44) | 1.0419 ± 0.0223 (46) | 1.0461 ± 0.0194 (90) | 1.0421–1.0501 |
| 6–11 yr | 1.0382 ± 0.0138 (44) | 1.0412 ± 0.0237 (39) | 1.0396 ± 0.0190 (83) | 1.0355–1.0437 |
| 12–19 yr | 1.0364 ± 0.0130 (81) | 1.0324 ± 0.0121 (151) | 1.0338 ± 0.0126 (232) | 1.0322–1.0354 |
| 20–29 yr | 1.0385 ± 0.0145 (36) | 1.0375 ± 0.0156 (87) | 1.0378 ± 0.0152 (123) | 1.0351–1.0405 |
| 30–39 yr | 1.0377 ± 0.0158 (21) | 1.0437 ± 0.0153 (42) | 1.0417 ± 0.0156 (63) | 1.0378–1.0456 |
| 40–49 yr | 1.0385 ± 0.0178 (111) | 1.0364 ± 0.0168 (115) | 1.0374 ± 0.0173 (226) | 1.0352–1.0397 |
| 50–59 yr | 1.0350 ± 0.0182 (102) | 1.0375 ± 0.0200 (121) | 1.0363 ± 0.0192 (223) | 1.0338–1.0388 |
| 60–69 yr | 1.0433 ± 0.0147 (81) | 1.0420 ± 0.0127 (359) | 1.0422 ± 0.0131 (440) | 1.0410–1.0434 |
| 70–79 yr | 1.0325 ± 0.0185 (148) | 1.0366 ± 0.0162 (461) | 1.0356 ± 0.0168 (609) | 1.0342–1.0369 |
| 80–89 yr | 1.0282 ± 0.0169 (54) | 1.0320 ± 0.0180 (109) | 1.0308 ± 0.0177 (163) | 1.0281–1.0335 |
| Total | 1.0370 ± 0.0174 (742) | 1.0376 ± 0.0163 (1,555) | 1.0374 ± 0.0167 (2,297) | 1.0367–1.0381 |
Values are the mean ± SD; n = number of subjects.
CI, confidence interval.
As shown in Table 3, there were no significant age groups-by-sex interactions for BMI classification Nd/No (adults: 20–89 yr, P = 0.386). There were no significant BMI group main effects (P = 0.576) or sex main effect (P = 0.080).
Table 3.
Nd/No ratio for human adult subjects in categorized BMI
| BMI Classification | Male (n) | Female (n) | All (n) | 95% CI |
|---|---|---|---|---|
| Underweight | 1.0306 ± 0.0295 (5) | 1.0344 ± 0.0098 (19) | 1.0336 ± 0.0151 (24) | 1.0275–1.0396 |
| Normal | 1.0376 ± 0.0168 (164) | 1.0376 ± 0.0168 (451) | 1.0376 ± 0.0168 (615) | 1.0363–1.0390 |
| Overweight | 1.0345 ± 0.0181 (244) | 1.0384 ± 0.0166 (435) | 1.0370 ± 0.0173 (679) | 1.0357–1.0383 |
| Class I obese | 1.0374 ± 0.0184 (105) | 1.0384 ± 0.0152 (234) | 1.0381 ± 0.0162 (339) | 1.0363–1.0398 |
| Class II obese | 1.0353 ± 0.0152 (25) | 1.0378 ± 0.0132 (96) | 1.0373 ± 0.0136 (121) | 1.0349–1.0397 |
| Class III obese | 1.0309 ± 0.0148 (10) | 1.0392 ± 0.0167 (57) | 1.0380 ± 0.0166 (67) | 1.0340–1.0420 |
| Total | 1.0359 ± 0.0177 (553) | 1.0381 ± 0.0161 (1,292) | 1.0374 ± 0.0166 (1,845) | 1.0367–1.0382 |
Values are the mean ± SD; n = number of subjects.
Nd/No, dilution space ratio. The subjects were categorized by BMI into underweight (<18.5 kg/m2), normal weight (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), class I obese (30–34.99 kg/m2), class II obese (35–39.99 kg/m2), and class III obese (>40 kg/m2). Values were calculated by two-way ANOVA. There were no significant BMI groups-by-sex interactions for (P = 0.386), main effect of BMI (P = 0.576), and main effect of sex (P = 0.080).
The potential factors (sex, age, BMI, and %fat) that might influence the Nd/No were investigated by linear regression analysis for all subjects. None of these correlations with sex, BMI, and %fat were statistically significant. There was poor negative correlation for age (r = −0.050, P = 0.017, slope = −0.00003, and intercept = 1.0391; Table 4). The effect was most apparent at the extreme ends of the age spectrum (Table. 2).
Table 4.
Correlation coefficients between dilution space ratio and selected physiological parameter
| r | P | ||
|---|---|---|---|
| Sex (male: 742; female: 1,555) | 0.019 | 0.375 | |
| Age, yr | 52.3 ± 25.4 | −0.050 | 0.017 |
| BMI, kg/m2 | 26.2 ± 6.1 | −0.010 | 0.619 |
| Body fat, % | 36.3 ± 9.4 | −0.020 | 0.335 |
Values are the mean ± SD; n = 2,297.
P, significance level; r, correlation coefficient.
Methodological effects.
The intraindividual and measurement variability (n = 54, BMI = 26.7 ± 5.4, and %fat = 38.4 ± 8.8) were determined by two repeated methods analyses (blind analysis for test-retest precision and redosing after 6 mo). Blind analysis test-retest precision shows that inter- and intraindividual SD (0.013 and 0.010, respectively). In addition, redosing after 6 mo for intraindividual variability (n = 55, BMI = 26.7 ± 5.9, and %fat = 32.7 ± 10.1) shows that intraindividual SD over time and measurement SD were 0.015 and 0.013, respectively. Because both of these studies were analyzed as using the same determination of the gravimetric DLW dose dilutions, they underestimate the analytical variation compared with that cross-sectional data analyses, which involved ∼50 DLW dose batches. The SD for the gravimetric dilutions expressed, as a SD of Nd/No, was 0.008. Thus the intraindividual SD and analytical SD including adjustment for the gravimetric dilutions were 0.017 and 0.015, respectively (Table 5).
Table 5.
Sources of variation in Nd/No dilution space ratio in blind repeat test for same samples and repeat experiment after 6 mo
| Source | SD |
|---|---|
| Interindividual (total) | 0.0167 |
| Measurement error | 0.010 |
| Duplicate samples | 0.008 |
| Intraindividual (6 mo) | 0.013 |
| Intraindividual physiologic | 0.002 |
| Interindividual physiologic | 0.011 |
For same samples, n = 54; for repeat experiment after 6 mo, n = 55 by ANOVA.
Literature review.
To avoid any bias from the average dilution space ratio in our single laboratory, we reviewed reported dilution space ratios from 103 published articles (see Supplemental Material; Supplemental Material for this article is available online at the Journal website). For individuals between 1 and 80 yr, the average dilution space ratio was 1.036. For newborns and infants <1 yr of age, the average was 1.028, and for individuals over 80 yr was 1.024.
DISCUSSION
We found the mean Nd/No and variance of all subject groups categorized by age, BMI status, or sex were tightly clustered and that none of these variables displayed a significant correlation with Nd/No. The group mean Nd/No for 2,297 human subjects was 1.037, which was quite similar to the value of 1.034 that Racette et al. reported previously (19), although there was a significant difference between two. The difference between these two estimates translates to a 1.5% difference in rCO2 and TEE for studies in which the deuterium elimination rate 25% larger than oxygen-18 elimination rate.
By deduction, our findings that common physiologic variables do not explain the cross-sectional variability in interindividual Nd/No suggest that the variation is mainly due to analytical error. Far more direct evidence, however, is that our adjusted SD for Nd/No of 0.015 is only slightly smaller than the interindividual SD of 0.0167 from the cross-sectional analysis. The difference corresponds to the fact that the physiologic variability in Nd/No under the above conditions would propagate to a SD of 3% in rCO2 and TEE.
Other investigations regarding the variation of Nd/No have given divergent results. Król and Speakman (15) conducted a careful study in which they examined the variation in Nd/No using 2H, tritium (3H) and 18O in mice. They hypothesized that if the variation of Nd/No is dependent on physiological factors rather than analytical error, the dilution space ratio of 2H and 18O would be highly correlated with the dilution space ratio of 3H and 18O. They observed the significant correlation between these two-dilution space ratios and concluded the variation of the observed Nd/No was mostly physiological rather than analytical. In contrast, Reilly and Fedak (21) and Arnould et al. (1) examined the dilution space difference between 2H and 3H using desiccation method to measure actual TBW in seals (Halichoerus grypus and Arctocephalus gazella, respectively). The dilution spaces of 2H and 3H, relative to TBW, were not correlated, which, if one assumes that most physiological variation in Nd/No is derived from Nd, suggests that the variability of the dilution space ratio is mostly analytical. Our study is consistent with the latter. We can suggest two possible explanations for this disagreement. It may be that larger animals have slower weight-normalized metabolic flux rates and thus lesser influences of any metabolic reactions that result in exchange or incorporation of the isotopes into nonaqueous pools, thereby a more constant dilution space ratio. The other possibility is there may have been a type II error in one or both of these prior studies because the sample size in both these studies was small (Halichoerus grypus: 4; Arctocephaus gazelle: 5; and mice: 26, respectively). In the present study, we conducted careful experiments that could examine whether the variability of Nd/No is dependent on physiological or analytical factors in humans, using blinded test-retest analysis and reexperimentation of DLW dosing after 6 mo and a propagation of error analysis to differentiate between analytical and physiological variation.
As summarized by Racette et al. from our laboratory (19), the variability of Nd/No in humans from the majority of DLW studies reported by others was small. Thus we would conclude the variability of Nd/No in larger animals such as human is mostly the result of random analytical error. It is possible, however, that for small animals such as mice (<40 g) there might be physiological variation, just as there is a difference in the best fit equation for calculating rCO2 in small animals that results from a difference in the nonaqueous routes of loss for the stable isotope tracers (27).
Most other reports did not test for differences as a function of age, BMI, or sex. There was a trend, however, with adults male and females having average Nd/No values that were only 0.2% different, which was not statistically significant (1.0359 and 1.0381, respectively, P = 0.080). Thus there is a trend for sex effect, but it is not statically significant despite the large sample size and we therefore do not support use of different values for males and females. More importantly, the difference between 1.0359 and 1.0381 would only induce a difference of approximately 0.7% in TEE, which is negligible for most DLW applications.
The mechanism that results in the isotope dilution spaces being larger than the TBW is due isotope exchange and/or incorporation of tracer into nonaqueous pools (24). In the case of Nd, it is known that the hydrogen in water exchanges with the labile protons in protein and estimates of the number of labile protons suggest that the Nd space would be ∼5% larger than TBW (9). In the case of oxygen, the primary route of exchange into inorganic pools is during the formation of ester bonds, with the most rapid being movement into phosphate groups during oxidative phosphorylation (12). We have estimated that these routes would expand the 18O space relative to TBW by 0.7 to 1% (24).
Validation studies of the DLW method discuss whether to use the measured (normalized observed value) or constant (fixed value) for Nd/No. Previous research has shown that using the individual ratios does not enhance the fit of individual data to the reference methods (2, 4, 19, 23, 26). Moreover, the DLW validation studies of Coward and Prentice (7), Wong et al. (31), and Bevan et al. (2) suggested there was an improved fit of DLW data to the reference method when individual ratios were used. Herein, the notion that the variation is physiological is supported by propagating error from the mass spectrometric determinations of hydrogen and oxygen enrichment. Such error propagation calculations indicate that most of the observed variation is analytical, and thus we predict that using individual data will actually increase the variability in TEE and recommend again against it to minimize error in TEE (18). Thus the use of the constant value of Nd/No (1.036) for each subject is suggested to minimize the effect of stable isotope analysis error on calculation of TEE in the DLW studies in humans. We reviewed the literature and found measured Nd/No ratio in 103 references (Supplemental Table S1). The values was 1.036 in 158 groups of subjects, which excluded <1 and >80 age groups. This constant is not very different from the previous observation of 1.034 by Racette et al. (19) from our laboratory but is preferable to that of Racette because it is based on a much larger sample and is probably not biased by specific analytical methods used in a single laboratory. We recommend the 1.036 as revised constant value of Nd/No for individuals between 1 and 80 yr of age, 1.029 for those <1 yr of age, and 1.025 for those >80 yr of age. The effect of using 1.036 in place of 1.034 on TEE is ∼1% for most conditions. If a laboratory is finding its average dilution space ratios does not agree with what is reported here or our variance is out of range reported here, we recommend it reevaluates its analytical methodologies. We suggest that the differences for by age results from systematic change in the water to protein ratio as both groups have slightly higher hydration of their fat-free mass (14).
Using data from our single laboratory and thus minimizing systematic error due to differences in laboratory methods, we found no differences in the Nd/No ratio due to sex, gender, or BMI status and only a small difference due to age between 0.25 and 89 yr. Thus we recommend the use of a single value 1.036 for the Nd/No ratio in calculating rCO2 and TEE from DLW with the exception of infants and advanced elderly, since this value was based on measures of Nd/No from our large subjects and multiple laboratories literature.
GRANTS
These investigators were supported by multiple National Institutes of Health Grants. The data analysis was supported by a Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists Research Grant (14J11761; to H. Sagayama).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.S., Y.Y., N.M.R., T.C.S., and D.A.S. analyzed data; H.S., Y.Y., and D.A.S. interpreted results of experiments; H.S., Y.Y., and D.A.S. prepared figures; H.S., Y.Y., and D.A.S. drafted manuscript; H.S., Y.Y., N.M.R., and D.A.S. edited and revised manuscript; H.S., Y.Y., N.M.R., T.C.S., and D.A.S. approved final version of manuscript; Y.Y. and D.A.S. conception and design of research; N.M.R., T.C.S., and D.A.S. performed experiments.
Supplementary Material
ACKNOWLEDGMENTS
We thank the many investigators who provided these specimens over the three decades.
REFERENCES
- 1.Arnould JP, Boyd IL, Speakman JR. Measuring the body composition of Antarctic fur seals (Arctocephalus gazella): Validation of hydrogen isotope dilution. Physiol Zool 69: 93–116, 1996. [Google Scholar]
- 2.Bevan RM, Speakman JR. Daily energy expenditure of tufted ducks: a comparison between indirect doubly calorimetry, double labelled water and heart rate. Funct Ecol 9: 40–47, 1995. [Google Scholar]
- 3.Blanc P, Colligan AS, Trabulsi J, Harris T, Everhart JE, Bauer D, Schoeller DA, Colligan AS, Trabulsi J, Harris T, Everhart JE, Bauer D, Schoeller DA. Influence of delayed isotopic equilibration in urine on the accuracy of the 2H218O method in the elderly. J Appl Physiol 1571: 1036–1044, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Boyd IL, Woakes AJ, Butler PJ, Davis RW, Williams TM. Validation of heart rate and doubly labelled water as measures of metabolic rate during swimming in California sea lions. Funct Ecol 9: 151, 1995. [Google Scholar]
- 5.Cole T, Coward W. Precision and accuracy of doubly labeled water energy-expenditure by multipoint and two-point method. Am J Physiol Endocrinol Metab 263: E965–E973, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Coward W, Ritz P, Cole T. Revision of calculations in the doubly labeled water method for measurement of energy-expenditure in humans. Am J Physiol Endocrinol Metab 267: E805–E807, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Coward WA, Prentice AM. Isotope method for the measurement of carbon dioxide production rate in man. Am J Clin Nutr 41: 659–663, 1985. [DOI] [PubMed] [Google Scholar]
- 8.Coward WA, Roberts SB, Cole TJ. Theoretical and practical considerations in the doubly-labelled water (2H218O) method for the measurement of carbon dioxide production rate in man. Eur J Clin Nutr 42: 207–212, 1988. [PubMed] [Google Scholar]
- 9.Culebras JM, Moore FD. Total body water and the exchangeable hydrogen. I. Theoretical calculation of nonaqueous exchangeable hydrogen in man. Am J Physiol Regul Integr Comp Physiol 232: R54–R59, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Goran MI, Poehlman ET, Nair KS, Danforth E. Effect of gender, body composition, and equilibration time on the 2H-to-18O dilution space ratio. Am J Physiol Endocrinol Metab 263: E1119–E1124, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Guidotti S, Meijer HA, van Dijk G. Validity of the doubly labeled water method for estimating CO2 production in mice under different nutritional conditions. Am J Physiol Endocrinol Metab 305: E317–E324, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Hackney DD, Boyer PD. Evaluation of the partitioning of bound inorganic phosphate during medium and intermediate phosphate in equilibrium water oxygen exchange reactions of yeast inorganic pyrophosphatase. Proc Natl Acad Sci USA 75: 3133–3137, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggarty P, McGaw BA, Franklin MF. Measurement of fractionated water loss and CO2 production using triply labelled water. J Theor Biol 134: 291–308, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: implications for body composition assessment. Am J Physiol Endocrinol Metab 265: E88–E95, 1993. [DOI] [PubMed] [Google Scholar]
- 14a.International Atomic Energy Agency. Assessment of body composition, and total energy expenditure in humans using stable isotope technique. International Atomic Energy Agency Vienna International Centre Human Health Series No. 3. Vienna, Austria: 2009. [Google Scholar]
- 15.Król E, Speakman JR. Isotope dilution spaces of mice injected simultaneously with deuterium, tritium and oxygen-18. J Exp Biol 202: 2839–2849, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lifson N, Gordon GB, McClintock R. Measurement of total carbon dioxide production by means of D2O18. J Appl Physiol 7: 704–710, 1955. [DOI] [PubMed] [Google Scholar]
- 17.Lifson N, McClintock R. Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol 12: 46–74, 1966. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DE, Gilker CD. Impact of 2H and 18O pool size determinations on the calculation of total energy expenditure. Obes Res 3, Suppl 1: 21–29, 1995. [PubMed] [Google Scholar]
- 19.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol Endocrinol Metab 267: E585–E590, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Ravussin E, Harper IT, Rising R, Bogardus C. Energy expenditure by doubly labeled water: validation in lean and obese subjects. Am J Physiol Endocrinol Metab 261: E402–E409, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Reilly JJ, Fedak MA. Measurement of the body composition of living gray seals by hydrogen isotope dilution. J Appl Physiol 69: 885–891, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Roberts SB, Coward WA, Schlingenseipen KH, Nohria V, Lucas A. Comparison of the doubly labeled water (2H218O) method with indirect calorimetry and a nutrient-balance study for simultaneous determination of energy expenditure, water intake, and metabolizable energy intake in preterm infants. Am J Clin Nutr 44: 315–322, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jéquier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol Regul Integr Comp Physiol 250: R823–R830, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 33: 2686–2693, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Schoeller DA, van Santen E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol 53: 955–959, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Speakman JR, Nair KS, Goran MI. Revised equations for calculating CO2 production from doubly labeled water in humans. Am J Physiol Endocrinol Metab 264: E912–E917, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Speakman JR. Doubly Labelled Water: Theory and Practice. New York: Springer, 1997. [Google Scholar]
- 28.Speakman JR. The history and theory of the doubly labeled water technique. Am J Clin Nutr 68: 932S–938S, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Weir de V JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerterp KR, Brouns F, Saris WH, ten Hoor F. Comparison of doubly labeled water with respirometry at low- and high-activity levels. J Appl Physiol 65: 53–6, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Wong WW, Cochran WJ, Klish WJ, O'Brian Smith E, Lee LS, Klein PD. In vivo isotope-fractionation factors and the measurement of deuterium- and oxygen-18-dilution spaces from plasma, urine, saliva, respiratory water vapor, and carbon dioxide. Am J Clin Nutr 47: 1–6, 1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.