This article provides the first examination of skeletal muscle phosphatidylcholine-phosphatidylethanolamine (PC:PE) ratio at rest and in response to acute exercise in humans. In agreement with recent evidence in rodent models demonstrating a role for PC and PE in muscle metabolism, skeletal muscle PC:PE ratio was found to be inversely related to insulin sensitivity in obese individuals, adults with type 2 diabetes, and endurance-trained athletes; however, muscle PC:PE ratio was not altered in response to exercise.
Keywords: skeletal muscle, phospholipids, acute exercise, insulin sensitivity
Abstract
Several recent reports indicate that the balance of skeletal muscle phosphatidylcholine (PC) and phosphatidylethanolamine (PE) is a key determinant of muscle contractile function and metabolism. The purpose of this study was to determine relationships between skeletal muscle PC, PE and insulin sensitivity, and whether PC and PE are dynamically regulated in response to acute exercise in humans. Insulin sensitivity was measured via intravenous glucose tolerance in sedentary obese adults (OB; n = 14), individuals with type 2 diabetes (T2D; n = 15), and endurance-trained athletes (ATH; n = 15). Vastus lateralis muscle biopsies were obtained at rest, immediately after 90 min of cycle ergometry at 50% maximal oxygen consumption (V̇o2 max), and 2-h postexercise (recovery). Skeletal muscle PC and PE were measured via infusion-based mass spectrometry/mass spectrometry analysis. ATH had greater levels of muscle PC and PE compared with OB and T2D (P < 0.05), with total PC and PE positively relating to insulin sensitivity (both P < 0.05). Skeletal muscle PC:PE ratio was elevated in T2D compared with OB and ATH (P < 0.05), tended to be elevated in OB vs. ATH (P = 0.07), and was inversely related to insulin sensitivity among the entire cohort (r = −0.43, P = 0.01). Muscle PC and PE were altered by exercise, particularly after 2 h of recovery, in a highly group-specific manner. However, muscle PC:PE ratio remained unchanged in all groups. In summary, total muscle PC and PE are positively related to insulin sensitivity while PC:PE ratio is inversely related to insulin sensitivity in humans. A single session of exercise significantly alters skeletal muscle PC and PE levels, but not PC:PE ratio.
NEW & NOTEWORTHY
This article provides the first examination of skeletal muscle phosphatidylcholine-phosphatidylethanolamine (PC:PE) ratio at rest and in response to acute exercise in humans. In agreement with recent evidence in rodent models demonstrating a role for PC and PE in muscle metabolism, skeletal muscle PC:PE ratio was found to be inversely related to insulin sensitivity in obese individuals, adults with type 2 diabetes, and endurance-trained athletes; however, muscle PC:PE ratio was not altered in response to exercise.
for over 20 yr it has been known that skeletal muscle phospholipid acyl composition is related to insulin sensitivity in humans (5, 34, 45). More specifically, high levels of polyunsaturated fatty acids and other indices of unsaturation in skeletal muscle phospholipids are positively associated with estimates (e.g., fasting plasma insulin) and direct measures (e.g., hyperinsulinemic-euglycemic clamp) of insulin sensitivity (5, 34, 45). Evidence indicates that improved membrane fluidity and membrane-protein dynamics mediate these relationships, including enhanced insulin receptor kinetics (15, 30, 37).
Less well appreciated are the roles that different classes of phospholipids play in regulating membrane integrity, protein dynamics, and, perhaps, insulin action. Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are the principal phospholipid constituents that makeup cellular membranes, with PC accounting for ∼50% of the total phospholipid pool and PE accounting for another 20-30% (41). Mounting evidence indicates that hepatic PC:PE ratio is a key determinant of membrane integrity, and a clinically relevant predictor of fatty liver disease (2, 24, 25). Additionally, recent studies employing skeletal muscle-specific transgenic models have demonstrated a role for PC:PE ratio in contractile function and glucose metabolism (13, 14) and a possible role for PE in skeletal muscle growth and maintenance, mitochondrial biogenesis, oxidative capacity, and exercise performance (39). Together these reports suggest that PC:PE balance is important for regulation of muscle metabolism. Nevertheless, whether skeletal muscle PC:PE ratio may be related to insulin sensitivity in humans is not known.
Also of interest is whether skeletal muscle PC and PE may be regulated in response to exercise. Many reports in the literature have investigated the effect of chronic exercise training on skeletal muscle phospholipid acyl composition (1, 16, 20, 21, 27, 28, 33, 43). By contrast, no studies have examined the acute effect of a single session of exercise on skeletal muscle PC and PE in humans. This is important because even a single session of exercise is sufficient to significantly improve insulin sensitivity in obese humans, including those with type 2 diabetes (11, 26, 32). Thus it is of interest to determine whether a single session of exercise may regulate skeletal muscle PC and PE.
Herein we report the relationships between skeletal muscle PC and PE and insulin sensitivity, as well as acute exercise-induced changes in skeletal muscle PC and PE in humans. We also employ metabolomic and transcriptomic analysis to help characterize regulation of PC and PE in human skeletal muscle. In brief, we hypothesized that the skeletal muscle PC:PE ratio would be related to insulin sensitivity and altered in response to a single session of exercise.
METHODS
Study participants.
Samples from 14 obese sedentary adults (OB), 15 individuals with type 2 diabetes (T2D), and 15 endurance-trained athletes (ATH) were used in this study and have been used in previously published work (3, 4). ATH were excluded if they had a body mass index (BMI) <20 kg/m2 or >25 kg/m2, and OB and T2D were excluded if BMI <28 or >40 kg/m2. No participant had fasting triglycerides >150 mg/dl or any history of liver, kidney, thyroid, or lung disease. OB and T2D participants were sedentary, engaging in planned physical activity <2 h/wk. ATH were competitive cyclists, triathletes, and runners training on average 12 ± 1 (SE) h/wk for the past 10 ± 2 yr. Individuals with type 2 diabetes were excluded from the study if they used insulin and/or thiazolidinediones for the purpose of limiting complications of controlling blood glucose and influence on muscle lipid metabolism (42), respectively. All other medications were permissible, but washed out for 2 wk prior to metabolic testing to limit the potential day-to-day (and hour-by-hour) influence of medications on metabolism. OB and ATH were not taking medications. Participants were weight stable in the 6 mo prior to the study. All women were premenopausal and studied in the midfollicular phase of their menstrual cycle. The Colorado Multiple Institution Review Board at the University of Colorado Denver approved this investigation, and all participants gave informed, written consent prior to initiating the study.
Preliminary testing.
Participants reported to the Clinical Translational Research Center (CTRC) for screening procedures following a 12-h overnight fast, where they were given a health and physical examination, followed by a fasting blood draw. Body composition was determined using dual-energy X-ray absorptiometry (DEXA) analysis (Lunar DPX-IQ; Lunar).
After preliminary testing, insulin sensitivity was determined via intravenous glucose tolerance test (IVGTT) using standard methods (6). Briefly, after baseline samples, intravenous glucose (0.3 g/kg) was infused over 1 min, followed by insulin at 0.03 U/kg, 20 min after glucose administration. Blood samples were then frequently obtained, and whole body insulin sensitivity index (Si) was calculated using minimal model analysis software (6) (Millennium Version, MINMOD). Standard enzymatic assay was used to measure glucose (Olympus AU400e Chemistry Analyzer; Olympus America). Plasma insulin was measured using a radioimmunoassay (Diagnostic Systems Laboratories). After completing the IVGTT, participants were given a snack and then performed a maximal oxygen consumption (V̇o2 max) test on an electronically braked cycle ergometer (Lode Excalibur; Quinton Instruments) with respiratory gases measured via indirect calorimetry.
Participant diet and exercise.
All participants were given a prescribed diet for 3 days prior to admission to the CTRC for the exercise study (described below). Daily caloric requirement was estimated from the DEXA measurement of fat-free mass (FFM) using the equation daily energy intake = 1.4 kcal/day × [372 + (23.9 × FFM)] and analysis of dietary records, as previously reported (17). Composition of this diet was 55% carbohydrate, 30% fat, and 15% protein. The fat content of the diet was controlled with the composition of saturated, monounsaturated, and polyunsaturated fat in a 1:1:1 ratio. Participants were asked to refrain from planned physical activity for 48 h prior to the exercise intervention visit.
Exercise study.
After a 12-h overnight fast a resting percutaneous skeletal muscle biopsy was taken from the vastus lateralis, midway between the greater trochanter of the femur and the patella. Muscle was immediately flash frozen in liquid nitrogen and stored at −80°C until analysis.
After the resting muscle biopsy, participants exercised on a cycle ergometer for 1.5 h at 50% of V̇o2 max. Exercise intensity was verified using indirect calorimetry, while exercise workload was adjusted to maintain the same relative intensity throughout the exercise bout. A second muscle biopsy was taken from the same site used during rest within 1 min after stopping exercise. Participants then remained supine for 2 h of recovery after the exercise bout. At 2-h postexercise, a third and final muscle biopsy was obtained from the vastus lateralis of the contralateral leg. Only water consumption was allowed while participants remained fasted throughout rest, exercise, and recovery.
Muscle PC and PE analysis.
All skeletal muscle samples were dissected free of extramuscular fat on ice as previously described (18). Approximately 35 mg of human muscle sample were accurately weighed and placed into a glass lysis tube (MP Biomedicals) along with 20 μl of 2:1 chloroform-methanol per milligram of tissue and vortexed repeatedly to break up the tissue. An aliquot of 100 μl of this homogenate was combined with 250 μl of water and 50 μl internal standard solution (C14:0/C14:0 PC, C15:0/C15:0 PE, C15:0/C15:0 diacylglycerol, d5-tripalmitin triacylglycerol, C17:0 sphingomyelin, C17:0 ceramide, C15:0 lysophosphatidylcholine). Using a Beckman-Coulter Biomek FX, 240 μl methanol was added. The tissue, internal standard, water, and methanol solution was mixed automatically by aspirating and dispensing 200 μl of the solution 10 times. To each well 600 μl of chloroform was automatically added. The plate was sealed with a polypropylene cap and locked in place with an aluminum plate (Analytical Sales Products). Samples were agitated for 2 min using a Qiagen TissueLyser set at 20 Hz, then centrifuged at 805 g for 10 min. Samples were returned to the Biomek, and 400 μl of the lower layer were transferred to a fresh plate along with 800 μl of 20 mM solution of ammonium acetate in 1:1 methanol-isopropanol.
Lipidomic analysis was performed using electrospray infusion of samples in chloroform/methanol at 20 μl/min into an AB-Sciex 5600 QQ TOF mass spectrometer. The acquisition mode, MSMSall, is a method where the precursor mass is ramped at a 1-amu interval from low to high mass while capturing high-resolution product ions for each product mass. The bandwidth of the precursor mass is 0.7 Da, making it possible to discriminate double-bond differences in lipids. The resulting data are normalized with the internal standards, resulting in a height ratio output. The data set represents an array of the product ions of all precursor ions that can be mined for specific lipid classes. A set of predetermined precursor-product pairs has been created in MultiQuant (AB-Sciex) that essentially makes the identification and performs the normalization with the class-specific lipid. This analysis was performed on n = 13 OB, n = 13 T2D, and n = 6 ATH.
Using this method, a total of 33 distinct PC peaks and 21 PE peaks were reliably detected in greater than 75% of all skeletal muscle samples analyzed. An additional six PC peaks were excluded from the following analysis because of insufficient detection across all samples. Final peaks used in this analysis included 30:0, 30:2, 32:0, 32:1, 32:2, 32:3, 34:0, 34:1, 34:2, 34:3, 34:4, 34:5, 36:0, 36:1, 36:2, 36:3, 36:4, 36:5, 36:6, 38:0, 38:1, 38:2, 38:3, 38:4, 38:5, 38:6, 40:0, 40:1, 40:2, 40:3, 40:4, 40:5, 40:6 for PC and 34a:0, 34a:2, 34a:3, 36a:0, 36:1, 36a:2, 36a:3, 36a:4, 36p:4, 36:5, 38e:0, 38a:3, 38a:4, 38a:5, 38p:5, 38a:6, 40e:0, 40p:0, 40a:4, 40a:5, 40a:6 for PE, with X:Y referring to total carbon number:total double bonds. When present, a represents diacyl, p represents plasmalogen, and e represents ether.
Metabolomic analysis.
Skeletal muscle samples were lyophilized, reweighed, added to 1 ml iced MeOH, and homogenized for 1 min on ice (Omni TH; Omni International). Total lipids were then extracted as previously described (38). Samples were shaken on a rotational mixer for 1.5 h at 4°C, then spun at 3,000 relative centrifugal force (rcf) for 15 min to separate phases. The polar layer was saved and run for metabolomic analysis.
Metabolomic analysis was completed as recently described (9, 31). In brief, 20 μl of polar fraction extracts were injected onto an ultra performance liquid chromatography (UPLC) system (Ultimate 3000; Thermo) and separated during a 3-min isocratic run at 250 μl/min (mobile phase: 5% acetonitrile, 95% 18 mΩ H2O, 0.1% formic acid) in a Kinetex C18 column (150 × 1-mm ID, 1.7-μm particle size; Phenomenex). The UPLC system was coupled online with a QExactive system (Thermo), scanning in Full MS mode (2 microscans) at 70,000 resolution in the 60–900 m/z range, 4-kV spray voltage, 15 sheath gas and 5 auxiliary gas, operated either in negative or positive ion mode (separate runs). Calibration was performed before each analysis against positive or negative ion mode calibration mixes (Piercenet; Thermo Fisher) to ensure sub ppm error on the intact mass. Metabolite assignments were performed through Maven software (7) upon conversion of .raw files into .mzXML format through MassMatrix (46). Maven allows for peak picking, feature detection, and metabolite assignment against the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (22). Assignments were further confirmed against chemical formula determination (as gleaned from isotopic patterns and accurate intact mass), and retention times against a library of 619 standard compounds (SIGMA Aldrich; MLSMS, IROATech). Relative quantitation was performed by normalizing integrated peak areas of extracted ion chromatograms to the tissue sample weight. This analysis was performed on n = 8 OB, n = 8 T2D, and n = 8 ATH.
Transcriptomic analysis.
Skeletal muscle samples were lysed in Trizol (Invitrogen) using a FastPrep instrument and Lysing Matrix D tubes (MP Biomedical). RNA was isolated using chloroform extraction and ethanol precipitation. The isolated RNA was further purified utilizing RNeasy columns (Qiagen) and was treated with DNase prior to elution into RNase free water. RNA integrity was assessed on an Agilent Technologies 2100 Bioanalyzer using an RNA integrity number ≥6.0. RNA was then used for Affymterix Human Genome U133 Plus 2.0 Array chip analysis according to manufacturer instructions (10). This analysis was performed on n = 8 OB, n = 10 T2D, and n = 9 ATH.
Statistical analysis.
All comparisons of resting values among groups were made using a one-way ANOVA. Analysis of PC, PE, and PC:PE ratio during rest, exercise, and recovery were made using one-way repeated measures ANOVA within each group. For ANOVA comparisons, significant F-tests (where P < 0.05) were evaluated using Fisher's least significant difference post hoc analysis. Relationships between variables of interest were examined via Pearson's correlation coefficient analysis. Significance was set at the P < 0.05 level. For metabolomic analysis, comparisons were examined using a two-way (group × time) ANOVA with Tukey's post hoc analysis corrected for multiple comparisons. For transcriptomic analysis, Partek Genomics Suite software was used to identify differentially expressed transcripts using a one-way ANOVA model with a false discovery rate of <0.05 to control for multiple testing (Partek). Ingenuity pathway analysis (IPA) was then used to visualize the data (Qiagen). No sex-specific differences were found for primary outcome variables and thus are not reported here. All data are presented as means ± SE. Statistical analysis was performed and figures were generated using GraphPad Prism 6.0 (GraphPad Software).
RESULTS
Participant characteristics.
Characteristics of the study participants are provided in Table 1. There was no mean age difference among groups. By design, OB and T2D had significantly greater BMI, percent body fat, and fat mass compared with ATH (all P < 0.01). However, none of these measures differed between OB and T2D (all P > 0.05). Also by design, relative V̇o2 max (ml·kg−1·min−1) of ATH was approximately double that of OB and T2D (both P < 0.01). Insulin sensitivity determined via IVGTT was also significantly greater in ATH compared with OB and T2D (both P < 0.001).
Table 1.
Participant characteristics
| OB (n = 14) | T2D (n = 15) | ATH (n = 15) | |
|---|---|---|---|
| Male/female | 9/5 | 11/4 | 11/4 |
| Age, yr | 40 ± 2 | 43 ± 1 | 41 ± 1 |
| Body mass, kg | 94.3 ± 3.3 | 93.7 ± 5.8 | 72.7 ± 2.9* |
| BMI, kg/m2 | 32.1 ± 0.9 | 32.3 ± 1.8 | 23.9 ± 1.2* |
| Body fat, % | 33.2 ± 2.0 | 33.8 ± 1.8 | 17.0 ± 1.6* |
| Fat mass, kg | 31.1 ± 2.2 | 31.7 ± 2.8 | 12.1 ± 1.1* |
| Fat-free mass, kg | 79.3 ± 2.6 | 79.3 ± 4.8 | 67.1 ± 2.9* |
| V̇o2max, ml·kg−1·min−1 | 25.7 ± 1.8 | 24.0 ± 1.1 | 51.2 ± 1.7* |
| Si, (mU/l)/min | 2.93 ± 0.23 | 2.02 ± 0.28 | 8.93 ± 1.07* |
Data are presented as means ± SE.
P < 0.05 for ATH compared with OB and T2D. No differences were found between OB and T2D. Si, insulin sensitivity index.
Basal skeletal muscle PC and PE.
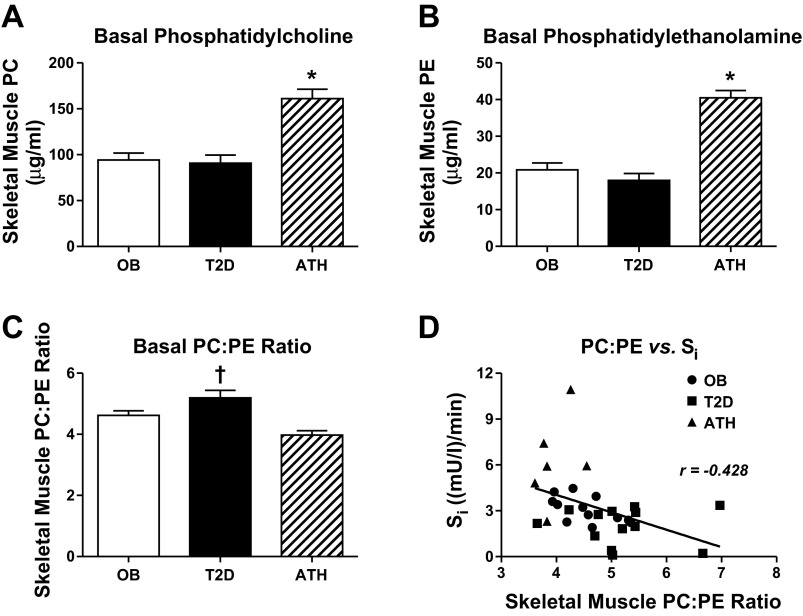
Total basal levels of skeletal muscle PC and PE were similar between OB and T2D, but significantly lower compared with ATH (P < 0.01, Fig. 1, A and B, respectively). Notably, total levels of skeletal muscle PC and PE were positively related to insulin sensitivity among all participants (r = 0.54, P < 0.01 for PC; r = 0.62, P < 0.01 for PE). Basal skeletal muscle PC:PE ratio was elevated in T2D compared with ATH and OB (P < 0.05) and tended to be elevated in OB compared with ATH (P = 0.07, Fig. 1C). Accordingly, basal PC:PE ratio was negatively related to insulin sensitivity among all participants (r = −0.43, P = 0.01; Fig. 1D).
Fig. 1.
Basal skeletal muscle phosphatidylcholine (A), phosphatidylethanolamine (B), PC:PE ratio (C), and PC:PE ratio vs. insulin sensitivity (Si) (D). Data are means ± SE. *P < 0.05 for ATH compared with OB and T2D. †P < 0.05 for T2D compared with OB and ATH.
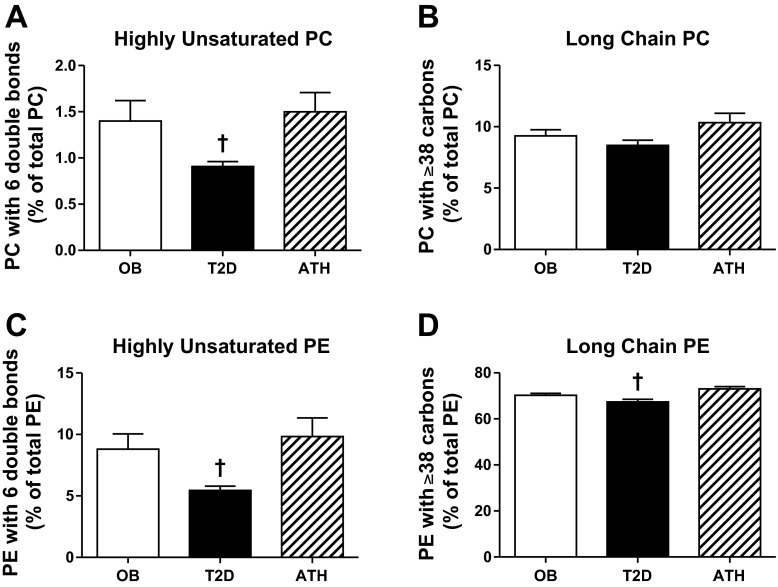
The contribution of highly unsaturated phospholipids (i.e., 6 total double bonds) to total PC and PE was significantly lower in T2D compared with OB and ATH (all P < 0.05, Fig. 2, A and C, respectively). T2D also had lesser contribution of phospholipids containing long-chain acyl moieties (i.e., ≥38 total carbons) to total PE compared with OB and ATH (P < 0.05, Fig. 2D). A similar relationship was observed for long-chain PC, but did not achieve significance (P = 0.10, Fig. 2B).
Fig. 2.
Basal skeletal muscle PC containing six double bonds (A), PC containing ≥38 carbons (B), PE containing six double bonds (C), and PE containing ≥38 carbons (D). Data are means ± SE. †P < 0.05 for T2D compared with OB and ATH.
Examination of individual PC and PE revealed that between-group differences in total skeletal muscle PC and PE were the result of a similar pattern exhibited for virtually all PC and PE. That is, ATH exhibited greater levels of nearly all PC and PE compared with OB and T2D, while OB and T2D tended to have similar levels of nearly all PC and PE (data not shown).
Participant exercise results.
All participants successfully performed 90 min of cycle ergometry exercise at the same relative target intensity (50 ± 2, 49 ± 2, and 51 ± 1% V̇o2 max for OB, T2D, and ATH, respectively, P = 0.69) with a similar respiratory exchange ratio (0.87 ± 0.01, 0.88 ± 0.01, and 0.84 ± 0.01 for OB, T2D, and ATH, respectively, P = 0.11). However, ATH expended significantly greater energy during the bout of exercise (475 ± 48, 451 ± 38, and 783 ± 67 kcal for OB, T2D, and ATH, respectively, P < 0.01 for ATH compared with OB and T2D).
Skeletal muscle PC and PE after exercise.
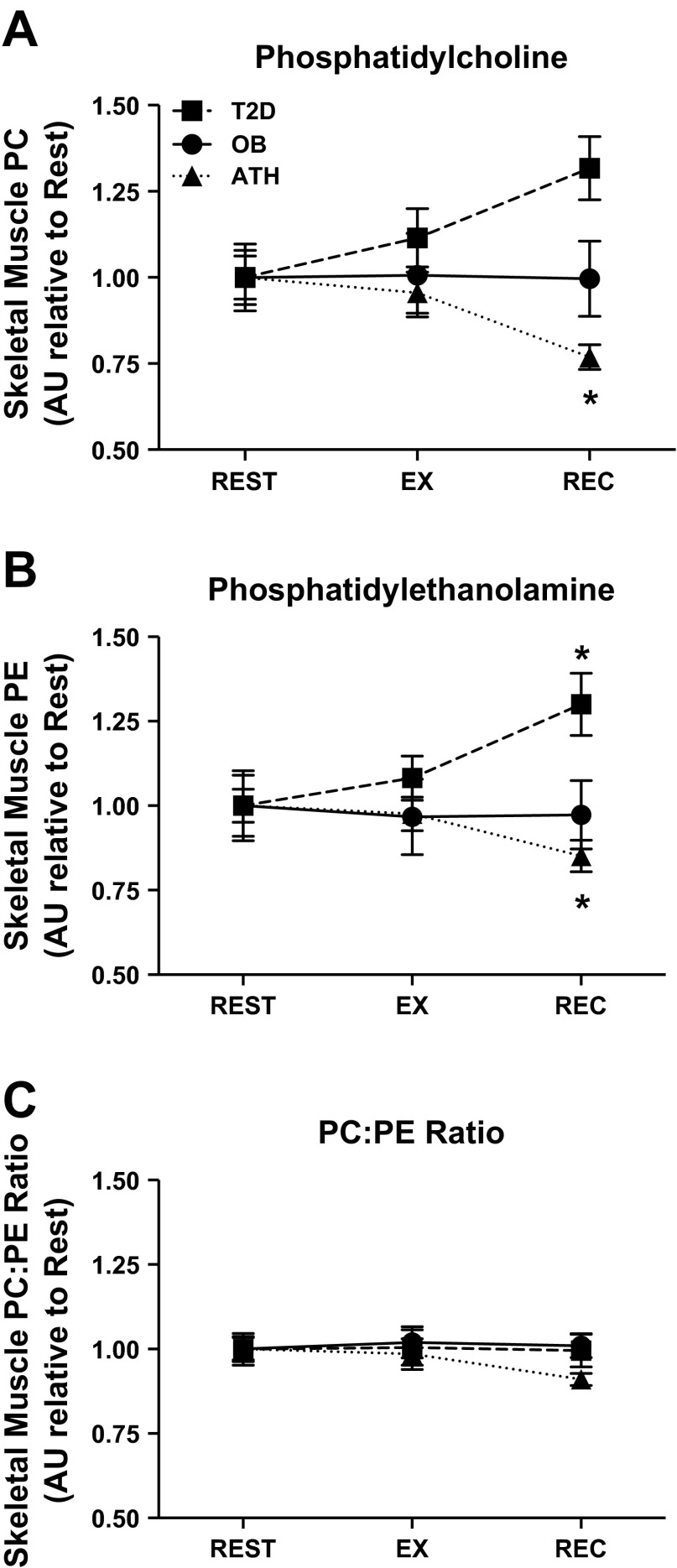
Skeletal muscle PC and PE were appreciably altered in response to exercise. To account for differences in resting concentration between groups and focus our analysis on changes with exercise and recovery, all PC and PE data were normalized to mean basal values for each group. In ATH, total PC and PE were significantly attenuated in recovery (both P < 0.01 compared with rest; Fig. 3, A and B, respectively). Conversely, total PE was significantly increased (P < 0.01) and total PC tended to be increased (P = 0.07) for T2D in recovery compared with rest (Fig. 3, B and A, respectively). In contrast to these responses, total skeletal muscle PC and PE remained unchanged after exercise in OB. Despite dynamic regulation of skeletal muscle PC and PE in response to exercise, PC:PE ratio remained unchanged after exercise in all groups (Fig. 3C). Akin to basal PC and PE, between-group differences in total skeletal muscle PC and PE after exercise and in recovery were the result of similar dynamic patterns exhibited for virtually all PC and PE within each group (data not shown).
Fig. 3.
Skeletal muscle phosphatidylcholine (A), phosphatidylethanolamine (B), and PC:PE ratio (C) during rest, exercise (EX), and recovery (REC). Data are normalized to resting values within each group and presented as means ± SE. *P < 0.05 for recovery compared with rest and exercise within group. AU, arbitrary units.
Metabolomic and transcriptomic analysis of skeletal muscle PC and PE biosynthesis.
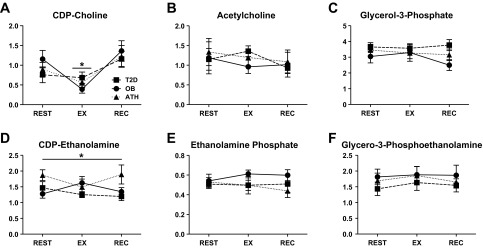
To gain insight into possible explanations for group- and exercise-related differences in PC and PE, we used metabolomic and transcriptomic analysis to examine components of phospholipid biosynthesis pathways. In brief, basal levels of detected glycerolipid synthesis intermediates were similar among groups (Fig. 4, A–F). However, there was a main effect of ATH having significantly greater skeletal muscle cytidine diphosphate (CDP)-ethanolamine than T2D (P = 0.01), and a similar trend for ATH compared with OB (P = 0.08, Fig. 4D). No other group effects were found, and exercise resulted in minimal changes in these metabolites.
Fig. 4.
Skeletal muscle metabolites of phosphatidylcholine and phosphatidylethanolamine biosynthesis, including CDP-choline (A), acetylcholine (B), glycerol-3-phosphate (C), CDP-ethanolamine (D), ethanolamine phosphate (E), and glycerol-3-phosphoethanolamine (F). All panels are presented in arbitrary units relative to dry muscle weight; data are means ± SE. In A, *P < 0.05 for a main effect of EX compared with REST. In D, *P < 0.05 for a main effect of ATH compared with T2D.
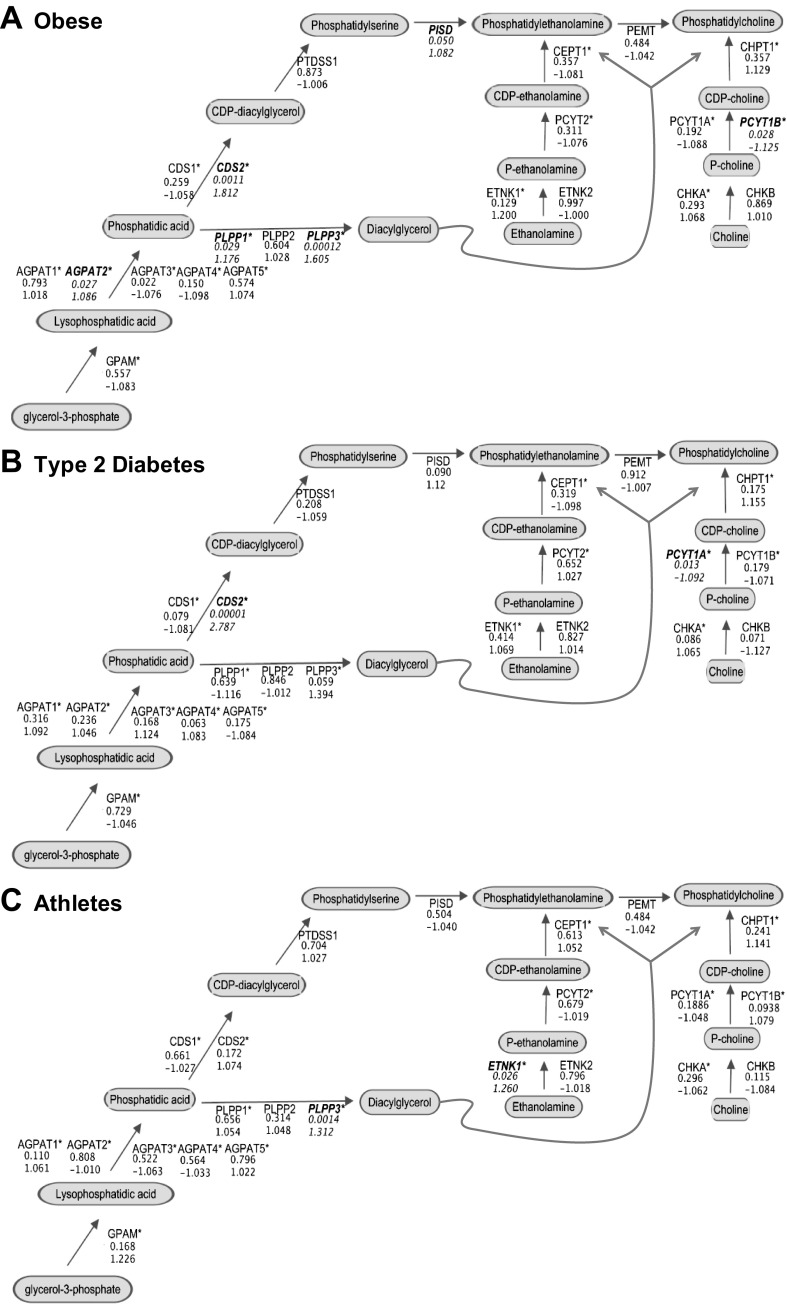
Examination of 63 transcripts that encode enzymes in PC and PE biosynthesis pathways revealed no significant differences between OB and T2D in the resting condition. By contrast, both CHKA and ETNK2 were increased in ATH compared OB and T2D at rest (P = 0.026 and P < 0.001, respectively). Respectively, these genes encode enzymes responsible for catalyzing the first steps of PC and PE synthesis within the Kennedy pathway. Exercise-related changes in all PC and PE biosynthesis transcripts were evaluated within group, in recovery compared with rest (Fig. 5). In brief, transcript level changes did not appear to be broadly coordinated (i.e., systematic up/down-regulation) or remarkably robust; however, several significant differences were found. Within OB, transcripts were increased for several glycerolipid synthesis enzymes in recovery compared with rest (AGPAT2, PLPP1, PLPP3, CDS2, all P < 0.05, Fig. 5A). CDS2 was also significantly increased in T2D in recovery compared with rest (P < 0.001, Fig. 5B). PCYT1A and PCYT1B catalyze the rate-limiting step of PC synthesis and were significantly lowered in recovery compared with rest in T2D and OB, respectively. Only ETNK1, regulating the initial step of PE synthesis, and PLPP3, regulating the formation of diacyglycerol from phosphatidic acid, were significantly increased in recovery compared with rest in ATH (both P < 0.05, Fig. 5C).
Fig. 5.
Comparison of skeletal muscle transcripts for enzymes regulating phosphatidylcholine and phosphatidylethanolamine biosynthesis during recovery compared with rest in OB (A), T2D (B), and ATH (C). For each gene the multiple comparisons-adjusted P value is reported (top value), followed by the fold change in recovery compared with rest (bottom value). In all panels significant differences for recovery vs. rest comparisons are highlighted using bold, italicized text for the gene symbol, whereas * denotes multiple transcripts were examined for the same gene. AGPAT, 1-acylglycerol-3-phophate acyltransferase; CDS, CDP-diacylglycerol synthase; CEPT, choline/ethanolamine phosphotransferase; CHK, choline kinase; CHPT, choline phosphotransferase; ETNK, ethanolamine kinase; GPAM, glycerol-3-phosphate acyltransferase; PCYT1, choline phosphate cytidylyltransferase; PCYT2, ethanolamine phosphate cytidylyltransferase; PEMT, phosphatidylethanolamine N-methyltransferase; PISD, phosphatidylserine decarboxylase; PLPP, phospholipid phosphatase; PTDSS, phosphatidylserine synthase.
DISCUSSION
Substantial evidence indicates that skeletal muscle phospholipid composition is related to insulin sensitivity in humans. However, less is known about the balance of phospholipid classes in skeletal muscle and how PC and PE may relate to insulin sensitivity. In this study, we determined relationships between skeletal muscle PC and PE and insulin sensitivity and sought to determine whether a single session of exercise may remodel skeletal muscle PC and PE in humans. The main findings of this investigation are as follows: 1) muscle PC:PE ratio was inversely related to insulin sensitivity among all study participants; 2) endurance-trained athletes had significantly greater skeletal muscle PC and PE compared with individuals who are obese or have type 2 diabetes; 3) muscle PC and PE levels were altered in response to exercise, in a highly group-specific manner; however, 4) muscle PC:PE ratio was unchanged in response to exercise. Furthermore, our finding that low levels of polyunsaturation and long-chain acyl groups in muscle phospholipids are associated with insulin resistance is in agreement with previous reports (5, 34, 45).
The a priori assumption of this investigation was that differences observed between ATH and OB and/or T2D would (in part) reflect adaptation to regular exercise training in ATH. The principal limitation of this model is that without a sedentary lean group we cannot dissociate leanness from adaptation to exercise. However, by characterizing the response to an acute bout of exercise our intent was to gain insight into the mechanisms underlying possible training-related adaptation and a stimulus known to enhance insulin sensitivity (11, 26, 32). To this end, we are the first to report that skeletal muscle PC:PE ratio is inversely related to insulin sensitivity among endurance-trained athletes, sedentary obese adults, and individuals with type 2 diabetes. However, muscle PC:PE ratio remained unchanged in all groups in response to exercise despite significant group-specific changes in PC and PE (for ATH and T2D). We interpret this finding to suggest that the lower muscle PC:PE ratio in athletes compared with our sedentary groups may represent adaptation to chronic exercise training, rather than the (repeated) acute effect of a single session of exercise, per se. Multiple recent investigations support this notion.
Two reports from Funai and colleagues (13, 14) indicate that elevated muscle PC:PE ratio may be detrimental for endurance exercise performance. Briefly, these authors have shown that selective skeletal muscle knockout of fatty acid synthase (FAS) or choline/ethanolamine phosphotransferase 1 (CEPT1) in mice resulted in lowered PE synthesis and increased PC:PE ratio in the sarcoplasmic reticulum (SR). These animals have impaired sarco/endoplasmic Ca2+ ATPase (SERCA) activity and attenuated endurance exercise capacity. In agreement, it is known that PE in lipid bilayers increases the affinity of calcium for SERCA (19). Thus increased PE and lowered muscle PC:PE ratio may favor improved SERCA activity, contractile function, and endurance exercise capacity. In a separate report, disruption of PE synthesis via selective skeletal muscle deletion of CTP:phosphoethanolamine cytidylyltransferase produced a complicated phenotype that, among many attributes, was associated with reduced skeletal muscle mass (39). Taken together, these findings suggest that increased PE and lower muscle PC:PE ratio may represent adaptation to chronic endurance training for the purpose of enhanced skeletal muscle growth, maintenance, and contractile function.
In agreement with our finding that endurance-trained athletes have elevated skeletal muscle PC and PE content compared with OB and T2D, exercise training was recently shown to induce similar skeletal muscle remodeling in mice (40). This effect was partly PGC1α dependent, as skeletal muscle overexpression of PGC1α was sufficient to increase muscle PC and PE levels, though skeletal muscle PGC1α knockout ablated the training-induced increase of some, but not all, muscle PC and PE species. Importantly, the training-induced increase in PC and PE was evident in the glycolytic extensor digitorum longus (EDL), but not the oxidative soleus. This is presumably due in part to greater training-related mitochondrial biogenesis in the EDL compared with the soleus. Mitochondrial membranes are relatively enriched with PE (39), which may also help to explain lower muscle PC:PE ratio in ATH compared with OB and T2D.
It is less clear as to whether skeletal muscle PC:PE ratio may have direct implications for insulin action. The observation that muscle PC:PE ratio was lower in OB compared with T2D (P = 0.04) and tended to be inversely related to insulin sensitivity among these groups (P = 0.07) provides support for the idea, as this difference occurred in the absence of any adaptation to chronic exercise training. Previous studies indicate that the balance of PC to PE could be expected to influence membrane integrity (24) and even susceptibility to cytokine-induced inflammation (8, 23, 29). Furthermore, skeletal muscle PC and PE are critical determinants of several aspects of muscle metabolism, including glucose uptake and mitochondrial biogenesis (13, 14, 39). It is therefore feasible that a relationship between muscle PC:PE ratio and insulin action may be causal. In agreement with this possibility, sarcoplasmic reticulum (SR) PC:PE ratio was recently shown to be increased in insulin-resistant primary muscle cells cultured from severely obese humans compared with insulin sensitive cells cultured from lean humans (35). Conversely, mouse models with increased SR PC:PE ratio are protected against high-fat diet (HFD)-induced insulin resistance (13, 14). However, this protective phenotype (during HFD) was not due to preservation of proximal insulin signaling, but instead enhanced Ca2+-dependent activation of AMP-activated protein kinase (AMPK) due to impaired SERCA-dependent Ca2+ uptake. In each of these reports comparing SR PC:PE ratio to insulin action, whole muscle PC and PE levels and PC:PE ratio were not presented (13, 14, 35). Nevertheless, our findings in whole muscle, combined with those of Paran et al. (35), indicate that elevated PC:PE ratio in skeletal muscle from humans (and human myocytes) is associated with impaired insulin sensitivity. The influence of compartment-specific phospholipid remodeling on muscle insulin action will need to be addressed in future investigations, as localization of lipids is clearly important for regulation of muscle metabolism, including insulin sensitivity (4).
A single session of exercise resulted in highly group-specific alterations in skeletal muscle levels PC and PE. The group-specific changes observed in our study are similar to an early observation from Dohm et al. (12). These authors previously reported that a single session of exhaustive exercise acutely decreased mitochondrial phospholipid content in trained rats, but acutely increased mitochondrial phospholipid content in untrained rats. The reason for this training-related difference is unclear, but is similar to the exercise-induced changes we observed in ATH and T2D. It is possible that high rates of muscle triacylglycerol utilization (and associated phospholipid degradation) during exercise among athletes (44), compared with less robust triacylglycerol utilization in sedentary obese populations (36), may help to explain the significant reduction in muscle PC and PE found only in the athletes. Increased energy expenditure during exercise in ATH compared with OB and T2D would also exacerbate this effect. Nevertheless, the observed changes in PC and PE suggest that the persistent effect of exercise was not captured in muscle samples collected 2 h after cessation of exercise. It would otherwise be paradoxical that ATH exhibited elevated resting levels of skeletal muscle PC and PE, yet both PC and PE were significantly lowered in response to a bout of endurance exercise. This suggests that the decrease in muscle PC and PE observed after 2 h of recovery in athletes is reversed at a later time point that was not examined here. It is equally possible that the response to exercise observed in obese and type 2 diabetes participants may not be fully captured. Still, muscle PC:PE ratio remained unchanged in all groups, suggesting that a single session of exercise may not be sufficient to alter this ratio.
Elevated CDP-ethanolamine and transcripts for PC and PE biosynthesis enzymes in ATH compared with OB and T2D may help to explain elevated basal levels of muscle PC and PE in ATH. It is also likely that diet may contribute to basal differences in PC and PE observed among groups (43). By contrast, metabolomic and transcriptomic analysis offered little explanation for the diverse response to exercise observed in this study. However, these analyses are not without limitation. Both methods employed nontargeted analysis, and neither high-throughput method (metabolomics, transcriptomics) resulted in 100% coverage of the PC and PE biosynthesis pathways. Furthermore, static concentrations of metabolites cannot capture rates of flux, and transcripts do not necessarily translate to protein levels or account for posttranslational modification. Nevertheless, the absence of coordinated or robust transcript-level changes in PC and PE pathway enzymes in response to exercise indicates that the acutely observed changes in skeletal muscle PC and PE levels are not transcriptionally regulated events.
In summary, our study demonstrates that endurance-trained athletes maintain a unique level and ratio of PC and PE compared with sedentary individuals that are obese or have type 2 diabetes. Skeletal muscle PC:PE ratio was inversely related to insulin sensitivity among the entire cohort. A single session of exercise may not be sufficient to remodel skeletal muscle PC:PE ratio. Whether the relationships demonstrated here may be causally related to muscle insulin action in humans is unknown, but mounting evidence suggests that the balance of PC and PE is a key determinant of muscle physiology and metabolism. Future studies should be directed at understanding the transient vs. sustained effect of exercise on muscle PC and PE and compartment-specific analysis of PC and PE as it relates to insulin sensitivity in humans.
GRANTS
S. A. Newsom was supported by National Institutes of Health training award DK007658. This work was supported by National Institutes of Health grants DK048520, DK089170, and DK066219.
DISCLOSURES
Lipidomic analysis in this study was supported by Eli Lilly and Company. Several authors are employees of Eli Lilly and Company (see author affiliations). This affiliation had no influence on the work presented. The authors have no other relevant conflicts of interest to report.
AUTHOR CONTRIBUTIONS
S.A.N., L.P., and B.C.B. conception and design of research; S.A.N., L.P., and B.C.B. performed experiments; S.A.N., J.T.B., K.K.-V., A.N.S., S.D.B., A.A.K., H.H.B., P. Sanders, P. Siddall, T.W., M.T., M.S.K., T.N., A.D., K.C.H., L.P., and B.C.B. analyzed data; S.A.N., J.T.B., K.K.-V., A.N.S., S.D.B., A.A.K., H.H.B., P. Sanders, P. Siddall, T.W., M.T., M.S.K., T.N., A.D., K.C.H., L.P., and B.C.B. interpreted results of experiments; S.A.N., K.K.-V., L.P., and B.C.B. prepared figures; S.A.N., L.P., and B.C.B. drafted manuscript; S.A.N., J.T.B., K.K.-V., A.N.S., S.D.B., A.A.K., H.H.B., P. Sanders, P. Siddall, T.W., M.T., M.S.K., T.N., A.D., K.C.H., L.P., and B.C.B. edited and revised manuscript; S.A.N., J.T.B., K.K.-V., A.N.S., S.D.B., A.A.K., H.H.B., P. Sanders, P. Siddall, T.W., M.T., M.S.K., T.N., A.D., K.C.H., L.P., and B.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the participants for their enthusiastic participation in this investigation, as well as the helpful medical staff at the University of Colorado Clinical Translational Research Center.
REFERENCES
- 1.Andersson A, Sjödin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol Endocrinol Metab 274: E432–E438, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Arendt BM, Ma DWL, Simons B, Noureldin SA, Therapondos G, Guindi M, Sherman M, Allard JP. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab 38: 334–340, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS, Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 309: E398–E408, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55: 1140–1150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 328: 238–244, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinformatics 37:14.11: 14.11.1–14.11.23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curry FE. Modulation of venular microvessel permeability by calcium influx into endothelial cells. FASEB J 6: 2456–2466, 1992. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, Slaughter A, Fragoso M, Hansen KC, Silliman CC, Banerjee A. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol 308: R1034–R1044, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalma-Weiszhausz DD, Warrington J, Tanimoto EY, Miyada CG. The affymetrix GeneChip platform: an overview. Methods Enzymol 410: 3–28, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 36: 434–439, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Dohm GL, Barakat H, Stephenson TP, Pennington SN, Tapscott EB. Changes in muscle mitochondrial lipid composition resulting from training and exhaustive exercise. Life Sci 17: 1075–1080, 1975. [DOI] [PubMed] [Google Scholar]
- 13.Funai K, Lodhi IJ, Spears LD, Yin L, Song H, Klein S, Semenkovich CF. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes 65: 358–370, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funai K, Song H, Yin L, Lodhi IJ, Wei X, Yoshino J, Coleman T, Semenkovich CF. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest 123: 1229–1240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg BH, Brown TJ, Simon I, Spector AA. Effect of the membrane lipid environment on the properties of insulin receptors. Diabetes 30: 773–780, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Górski J, Zendzian-Piotrowska M, de Jong YF, Niklińska W, Glatz JF. Effect of endurance training on the phospholipid content of skeletal muscles in the rat. Eur J Appl Physiol Occup Physiol 79: 421–425, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO. Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res 11: 752–759, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res 42: 1041–1048, 2001. [PubMed] [Google Scholar]
- 19.Gustavsson M, Traaseth NJ, Veglia G. Activating and deactivating roles of lipid bilayers on the Ca(2+)-ATPase/phospholamban complex. Biochemistry 50: 10367–10374, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helge JW, Dela F. Effect of training on muscle triacylglycerol and structural lipids: a relation to insulin sensitivity? Diabetes 52: 1881–1887, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. J Appl Physiol 90: 670–677, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmieć Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161: III–XIII, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 3: 321–331, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Ling J, Chaba T, Zhu LF, Jacobs RL, Vance DE. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 55: 1094–1102, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Manders RJF, Van Dijk JWM, van Loon LJC. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 42: 219–225, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell TW, Turner N, Else PL, Hulbert AJ, Hawley JA, Lee JS, Bruce CR, Blanksby SJ. The effect of exercise on the skeletal muscle phospholipidome of rats fed a high-fat diet. Int J Mol Sci 11: 3954–3964, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell TW, Turner N, Hulbert AJ, Else PL, Hawley JA, Lee JS, Bruce CR, Blanksby SJ. Exercise alters the profile of phospholipid molecular species in rat skeletal muscle. J Appl Physiol 97: 1823–1829, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mold C. Effect of membrane phospholipids on activation of the alternative complement pathway. J Immunol 143: 1663–1668, 1989. [PubMed] [Google Scholar]
- 30.Nadiv O, Shinitzky M, Manu H, Hecht D, Roberts CT, LeRoith D, Zick Y. Elevated protein tyrosine phosphatase activity and increased membrane viscosity are associated with impaired activation of the insulin receptor kinase in old rats. Biochem J 298, Part 2: 443–450, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemkov T, D'Alessandro A, Hansen KC. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 47: 2345–2357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newsom SA, Everett AC, Hinko A, Horowitz JF. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care 36: 2516–2522, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolaidis MG, Petridou A, Matsakas A, Schulz T, Michna H, Mougios V. Effect of chronic wheel running on the fatty acid composition of phospholipids and triacylglycerols in rat serum, skeletal muscle and heart. Acta Physiol Scand 181: 199–208, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest 96: 2802–2808, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paran CW, Verkerke ARP, Heden TD, Park S, Zou K, Lawson HA, Song H, Turk J, Houmard JA, Funai K. Reduced efficiency of sarcolipin-dependent respiration in myocytes from humans with severe obesity. Obesity (Silver Spring) 23: 1440–1449, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perreault L, Bergman BC, Hunerdosse DM, Playdon MC, Eckel RH. Inflexibility in intramuscular triglyceride fractional synthesis distinguishes prediabetes from obesity in humans. Obesity (Silver Spring) 18: 1524–1531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilch PF, Thompson PA, Czech MP. Coordinate modulation of d-glucose transport activity and bilayer fluidity in plasma membranes derived from control and insulin-treated adipocytes. Proc Natl Acad Sci U S A 77: 915–918, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosendal J, Knudsen J. A fast and versatile method for extraction and quantitation of long-chain acyl-CoA esters from tissue: content of individual long-chain acyl-CoA esters in various tissues from fed rat. Anal Biochem 207: 63–67, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Selathurai A, Kowalski GM, Burch ML, Sepulveda P, Risis S, Lee-Young RS, Lamon S, Meikle PJ, Genders AJ, McGee SL, Watt MJ, Russell AP, Frank M, Jackowski S, Febbraio MA, Bruce CR. The CDP-ethanolamine pathway regulates skeletal muscle diacylglycerol content and mitochondrial biogenesis without altering insulin sensitivity. Cell Metab 21: 718–730, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Senoo N, Miyoshi N, Goto-Inoue N, Minami K, Yoshimura R, Morita A, Sawada N, Matsuda J, Ogawa Y, Setou M, Kamei Y, Miura S. PGC-1α-mediated changes in phospholipid profiles of exercise-trained skeletal muscle. J Lipid Res 56: 2286–2296, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takagi A. Lipid composition of sarcoplasmic reticulum of human skeletal muscle. Biochim Biophys Acta 248: 12–20, 1971. [PubMed] [Google Scholar]
- 42.Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP. Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 292: E485–E493, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Turner N, Lee JS, Bruce CR, Mitchell TW, Else PL, Hulbert AJ, Hawley JA. Greater effect of diet than exercise training on the fatty acid profile of rat skeletal muscle. J Appl Physiol 96: 974–980, 2004. [DOI] [PubMed] [Google Scholar]
- 44.van Loon LJC, Koopman R, Stegen JHCH, Wagenmakers AJM, Keizer HA, Saris WHM. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 37: 1044–1050, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Freitas MA. MassMatrix: a database search program for rapid characterization of proteins and peptides from tandem mass spectrometry data. Proteomics 9: 1548–1555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]